Abstract
Background: Ageing people show increasing morbidity, dependence and vulnerability.
Objectives: To compare the relationships of different measures of multimorbidity with dependence (operationalized as disability) and vulnerability (operationalized as frailty).
Method: A cross-sectional analysis within the BELFRAIL cohort (567 subjects aged ≥ 80). Multimorbidity was measured using a disease count (DC), the Charlson comorbidity index (CCI) and the cumulative illness rating scale (CIRS), respectively. Associations with disability (based on activities of daily living) and frailty (defined by the Fried frailty criteria) were assessed using bivariable and multivariable analyses. Net reclassification improvement (NRI) values were calculated to compare the abilities of the DC, CCI and CIRS to identify patients with disability or frailty.
Results: Disability was associated with the DC (crude odds ratio, OR: 2.1; 95% confidence interval, CI: 1.4–3.4), CCI (crude OR: 1.8; 95% CI: 1.2–2.7) and CIRS (crude OR: 4.0; 95% CI: 2.5–6.5); only the association with CIRS was independent of age, sex, chronic inflammation, impaired cognition and frailty (adjusted OR: 3.2; 95% CI: 1.7–5.8). Frailty was associated with CCI (crude OR: 2.4; 95% CI: 1.2–4.6) and CIRS (crude OR: 2.6; 95% CI: 1.3–5.3); adjusted for age, sex, chronic inflammation, impaired cognition and disability. These associations were not statistically significant. The NRIs demonstrated a similar ability of the DC, CCI, and CIRS to identify patients with disability or frailty, respectively.
Conclusion: The associations of different measures of multimorbidity with disability and frailty differ but their ability to identify patients with disability or frailty is similar. Generally, multimorbidity scores incompletely reflect dependence and vulnerability in this age group.
Keywords::
Patients with multiple chronic diseases, patients with disability and frail patients represent partly overlapping but distinct groups.
Multimorbidity, measured by the cumulative illness rating scale, is independently associated with disability, but not with frailty.
Different measures of multimorbidity show similarly limited ability to identify patients with disability or frailty.
INTRODUCTION
In ageing populations, the prevalence of chronic disease(s), vulnerability and dependence is increased. Providers caring for older patients are especially challenged by multimorbidity, which is, however, difficult to define (Citation1–3). The number of tools proposed to measure multimorbidity illustrates this difficulty (Citation4,Citation5). Multimorbidity has an impact on different health-related outcomes, but few studies have compared the relationships of different measures of multimorbidity with these outcomes (Citation4,Citation6). For the prediction of mortality, Perkins et al. have demonstrated that a simple disease count (DC) is of equal value to more complicated multimorbidity measures but no comparative studies have assessed the relationships of different measures of multimorbidity with vulnerability and dependence (Citation7).
To define the condition of older people who ‘show great medical complexity and vulnerability,’ the frailty concept has been introduced and disability is used to operationalize dependence (Citation8,Citation9). Frailty and disability have been described as both important outcome variables and proven predictors of adverse outcomes (Citation8,Citation10).
Fried et al. demonstrated that multimorbidity, disability and frailty are distinct but overlapping concepts that are likely causally related (Citation11). However, they used a relatively restricted measure of multimorbidity, including only nine chronic diseases. More comprehensive measures of multimorbidity might show a closer relationship with disability and frailty. The aim of this study was to investigate the relationship of multimorbidity with disability and frailty in a population aged 80 years and older and to examine whether this relationship is different for different measures of multimorbidity.
METHODS
Study design and population
The BELFRAIL study is a prospective, population-based cohort study. The study design and the characteristics of the cohort have been previously described in detail (Citation12). The Biomedical Ethics Committee of the Université Catholique de Louvain (UCL), Belgium (B40320084685), approved the study protocol. In brief, general practitioners (GPs) in three Belgian areas were invited to include patients aged 80 years and older. Only three exclusion criteria were used: severe dementia (known mini-mental state examination (MMSE) score < 15/30) (Citation13), palliative care and medical emergency. All participants gave informed consent.
Multimorbidity measures
All measures of multimorbidity were based on morbidity data reported by the participants’ GPs. At the time of inclusion, the GP was asked to record both the medical history and the current medical problems of the patient to provide a comprehensive problem list for each participant. Additionally, the GP filled out a structured questionnaire on the presence or absence of 22 chronic conditions (listed in the next paragraph). Multimorbidity was measured using a disease count (DC), the Charlson comorbidity index (CCI) and the cumulative illness rating scale (CIRS), respectively (Citation3–5,Citation14,Citation15).
The DC was only based on the structured questionnaire, which included questions on hypertension, lipid disorders, angina pectoris, cardiomyopathy, myocardial infarction (MI), transient ischaemic attack (TIA), stroke, peripheral arterial disease (PAD), episodes of decompensated heart failure, episodes of atrial fibrillation, known valvular disease, thyroid disease, respiratory impairment (either asthma or chronic obstructive pulmonary disease, (COPD)), Parkinson's disease, arthritis, osteoarthritis, documented osteoporosis, cancer, depression, renal insufficiency, locomotor sequelae of stroke and diabetes.
To compose the CCI and the CIRS, the problem lists reported by the GPs were assessed and encoded by two researchers (PB and OD). In the case of discrepancy between the first and the second researcher, the problem list of the patient was discussed with a third researcher (BV) to reach a consensus on which problems to encode for that patient. This approach eventually elicited 58 different chronic conditions (listed in Appendix 1, web only) reported within the study sample. The CIRS categorizes all of these chronic conditions within different body systems (Appendix 1, web only) and counts the number of body systems affected by at least one chronic disease (Citation15). The CCI only includes 19 chronic diseases (Appendix 1, web only), which are selected and weighted based on the strength of their association with mortality (Citation14).
There is no consensus in the literature on cut-off values to define multimorbidity using these measures. The cut-off values used in this study were based on common sense and the distribution of each measure in the sample; they are reported in and .
Table 1. Study sample characteristics of the BELFRAIL cohort (Citation12).
Table 2. Crude and adjusted ORs for the associations of multimorbidity measures (CCI, CIRS and DC) with disability and frailty (n = 567).
Outcome measures
Disability
The lowest sex-specific quintile of activities of daily living (ADL) was used to operationalize disability (Citation9). The ADL assessment, performed by a clinical research assistant (CRA), includes six questions on self-reported difficulties in climbing stairs, walking outdoors for five minutes without resting, getting up from and sitting down in a chair, dressing and undressing oneself, using one's own transportation or public transportation and self-care of toenails. The answers vary from 1 ‘no, I cannot do this’ to 5 ‘yes, without any problems’. The total score ranges between 6 and 30 and is calculated by adding the scores of all activities.
Frailty
Frailty was defined by the Fried criteria, which categorizes individuals as frail or non-frail based on the presence or absence of five measurable characteristics: recent unintentional weight loss (as reported by the GP), weakness (measured grip strength in the lowest sex-specific quintile), poor endurance and energy (self-report of exhaustion on item three on the geriatric depression scale, (GDS)), slowness (slowest sex-specific quintile in a test of timed walking speed) and a low physical activity level (longitudinal aging study Amsterdam physical activity questionnaire (LAPAQ) score in the lowest sex-specific quintile) (Citation16,Citation17). Assessments of grip strength, GDS scores, timed walking speed and LAPAQ scores were all performed by the CRA. Individuals with three or more of these components were classified as frail (Citation8,Citation16,Citation17).
Analyses
Patients with and without disability and patients with and without frailty were compared using independent-sample t-tests for continuous variables (age and crude high-sensitivity C-reactive protein (hCRP) levels) and chi-square tests for categorical variables (gender, categories of multimorbidity and impaired cognition). Chronic inflammation (measured by the level of hCRP) and impaired cognition (defined as a score under 25 on the MMSE) were included based on their established relationships with disability and frailty (Citation8,Citation18).
The associations of the different multimorbidity measures with disability and frailty were assessed using crude odds ratios (ORs). Subsequently, one series of logistic regression models was used to study the relationships of the different measures of multimorbidity (independent variables) with disability (dependent variable). A second series of logistic regression models was used to study the relationships of the different measures of multimorbidity (independent variables) with frailty (dependent variable). All models were adjusted for age, sex, impaired cognition and chronic inflammation. Based on the close relationship of dependence and vulnerability, the disability models were additionally adjusted for frailty, and the frailty models were additionally adjusted for disability. These analyses were performed using SPSS 19.0 (SPSS Inc., Chicago, IL, USA).
To further compare the relationships of the CCI score, CIRS score and DC with disability and frailty, the ability of the different measures in identifying patients with disability or frailty was compared by measuring the net reclassification improvement (NRI) (Citation19). First, the ability of the CCI in identifying patients with disability or frailty was defined and subsequently compared with the ability of the DC using the NRI values. Second, the CIRS and DC and the CIRS and CCI were compared in the same way. Throughout all analyses, a P-value < 0.05 was considered statistically significant.
RESULTS
Characteristics of the study sample
The BELFRAIL study included 567 participants with a mean age of 84.7 years (SD: 3.7). The population characteristics are reported in . We observed a high morbidity burden: for each participant, at least one chronic disease was reported, and 95.9% suffered from more than one disease. The median ADL score within this sample was 25 (interquartile range: 6), and 41 (7.2%) patients were frail (meeting > 3/5 Fried frailty criteria).
Multimorbidity, disability and frailty
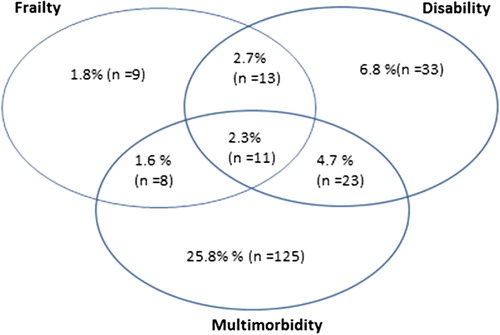
illustrates the overlap between multimorbidity (defined here as > 5 chronic diseases reported by the GP), disability (defined as the lowest sex-specific quintile of ADL) and frailty (defined as meeting > 3/5 Fried frailty criteria). One in five patients (20.3%) with more than five chronic diseases had disability, and 11.4% of these patients were frail. Of the patients with disability, 30% were frail, and 42.5% had more than five chronic diseases. Of all frail patients, 58.5% had disability, and 46.3% had more than five chronic diseases.
Associations of multimorbidity measures with disability and frailty
illustrates that disability is related to age, inflammatory status, cognitive function, and multimorbidity, defined using a DC, the CCI and the CIRS. Frailty was related to age and multimorbidity, defined using the CCI and CIRS; no (significant) relationship was observed between frailty and the DC (). Crude ORs are reported in . After adjustment for age, sex, chronic inflammation, a low MMSE score and frailty, only the relationship between CIRS and disability was statistically significant (adjusted OR: 3.2; 95% CI: 1.7–5.8) (). For frailty, the multiple logistic regression analysis adjusted for age, sex, chronic inflammation, a low MMSE score and disability could not show a statistically significant independent association between any measure of multimorbidity and frailty ().
Comparison of the ability of the CCI, CIRS and DC to identify people with disability or frailty
The NRI values reported in show that none of the measures of multimorbidity was significantly superior in identifying people with disability or frailty.
Table 3. Comparison of the ability of different measures of multimorbidity to define patients with disability or frailty: NRI values.
DISCUSSION
Main findings
In this population of patients aged 80 years and older, multimorbidity, disability and frailty were partly overlapping but distinct concepts. A DC was related to disability, but not to frailty. The CCI and CIRS scores were both related to disability and frailty, but the multivariable analyses could only demonstrate a statistically significant independent association between the CIRS and disability. All measures demonstrated a similar ability to identify people with disability or frailty.
Strengths and limitations
The current is the first large study to examine the relationship of multimorbidity with frailty and disability in a primary-care population of patients aged 80 years and older. The strength of this study is that the assessment of multimorbidity was based on morbidity data collected by GPs because this provides a comprehensive and objective view on the health status of the patients. However, defining multimorbidity remains a challenge (Citation3,Citation4,Citation20,Citation21). A comparison of different measures of multimorbidity strengthens the reliability of our results. However, the lack of consensus in the literature required choices that may be subject to criticism. First, to assess multimorbidity cut-off values were defined (Citation3). For the DC and CIRS, the cut-off was set at > 3 because a lower limit is not sensible in a population with a high prevalence of co-existing chronic diseases. For the CCI, the cut-off was set at > 5 because the CCI accounts for age, which implies a minimum score of four for every patient aged 80 years or older. Second, although a DC is the most commonly used measure of multimorbidity, there is no consensus on which conditions to include in these lists but the fact that we included more than 12 diseases ensures that we unlikely underestimate multimorbidity (Citation3,Citation4).
Frailty and disability were defined by means of validated and standardized measures. However, these measures are only proxies for the complex biopsychosocial concepts of human functioning and vulnerability (Citation8,Citation22,Citation23).
Additionally, the limited sample size and the low number of frailty cases require cautious interpretation of the multivariable analyses, also because of the high number of confounders in the model. This may explain why the multivariate analyses could not demonstrate a statistically significant relationship between the CCI and CIRS and frailty.
Comparison with existing literature
In comparison with Fried et al. this study shows a higher overlap between multimorbidity and disability (20.3% compared to 10.6%) and a similar overlap between multimorbidity and frailty (11.4% compared to 9.6%) (Citation11). The higher overlap with disability in our sample might be explained by the fact that our study included older patients but this does not explain for the similar overlap with frailty. Another explanation may be the fact that the current study used a more comprehensive assessment of multimorbidity. This may indicate that measures of multimorbidity, which include more diseases show higher overlap with disability but not with frailty.
Only few studies have investigated associations between multimorbidity and disability or frailty because most work has focused on the relationships with individual diseases (Citation8,Citation24,Citation25). As for disability, others have demonstrated the number of diseases to be explanatory for functional disabilities for people aged 65–84 years, but not for people over 85 years (Citation28). Whereas our study indicated frailty to be independent of the number of chronic conditions, De Groot et al. demonstrated an increasing prevalence of frailty in people with more diseases (Citation5). Again all comparisons are severely hampered by the heterogeneous definition of multimorbidity.
Our multivariable analyses seem to indicate that the performance of different measures is not equal across outcomes, so we cannot simply confirm the findings of Perkins et al., which demonstrated a simple DC to be of equal value to more comprehensive measures of multimorbidity for the prediction of mortality (Citation7). However, cautious interpretation is required as in terms of absolute numbers, no measure was found to be significantly superior to identify people with disability or frailty.
Implications for future research and clinical practice
Further research should assess the validity of different multimorbidity measures across different outcomes via both cross-sectional and prospective studies. However, measuring multimorbidity will likely remain a challenge, and it is questionable whether these measures, being simple or complicated, are suitable to capture the complexity of multimorbidity. The European General Practice Research Network recently defined multimorbidity beyond a combination of diseases and included both biopsychosocial and somatic risk factors. In this definition disability and frailty were included as main outcomes of multimorbidity, but several modifiers of the effects of multimorbidity were defined, as biopsychosocial factors, the burden of individual diseases, the patients’ personal coping strategies and the organization of the health care system (Citation27). Further research in older people suffering from multiple chronic diseases would likely benefit from redirecting attention from a disease-oriented care to the search for comprehensive measures that address the entire patient (Citation28).
Conclusion
Patients with multimorbidity, disability and frailty represent partly overlapping but distinct groups. More comprehensive measures of multimorbidity seem to be more closely associated with disability but not with frailty. However, the ability to identify patients with disability or frailty is similar for CCI, CIRS and DC. Generally, the results indicate that scores on multimorbidity measures do not reflect well the dependence or vulnerability of patients aged 80 years and older.
SUPPLEMENTARY MATERIAL AVAILABLE ONLINE
igen_a_914167_sm2219.pdf
Download PDF (25.9 KB)ACKNOWLEDGEMENTS
This study was possible thanks to the participating GPs and the patients.
FUNDING
The BELFRAIL study (B40320084685) was supported by an unconditional grant from the Foundation Louvain. The Foundation Louvain is the support unit of the Université catholique de Louvain and is responsible for developing the education and research projects of the university by soliciting gifts from corporations, foundations and alumni. PB is a fellow of the Research Foundation Flanders (FWO).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper
REFERENCES
- Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: A cross-sectional study. Lancet 2012;380:37–43.
- Starfield B. Threads and yarns: Weaving the tapestry of comorbidity. Ann Fam Med. 2006;4:101–3.
- Fortin M, Stewart M, Poitras ME, Almirall J, Maddocks H. A systematic review of prevalence studies on multimorbidity: Toward a more uniform methodology. Ann Fam Med. 2012;10:142–51.
- Huntley AL, Johnson R, Purdy S, Valderas JM, Salisbury C. Measures of multimorbidity and morbidity burden for use in primary care and community settings: A systematic review and guide. Ann Fam Med. 2012;10:134–41.
- de Groot V, Beckerman H, Lankhorst G, Bouter L. How to measure comorbidity: A critical review of available methods. J Clin Epidemiol. 2003;56:221–9.
- Wolff JL, Starfield B, Anderson G. Prevalence, expenditures, and complications of multiple chronic conditions in the elderly. Arch Intern Med. 2002;162:2269–76.
- Perkins AJ, Kroenke K, Unutzer J, Katon W, Williams JW Jr, Hope C, et al. Common comorbidity scales were similar in their ability to predict health care costs and mortality. J Clin Epidemiol. 2004;57:1040–8.
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–56.
- McWhinnie JR. Disability assessment in population surveys— results of the OECD common development effort. Rev Epidemiol Sante Publique 1981;29:413–9.
- Scott WK, Macera CA, Cornman CB, Sharpe PA. Functional health status as a predictor of mortality in men and women over 65. J Clin Epidemiol. 1997;50:291–6.
- Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: Implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59:255–63.
- Vaes B, Pasquet A, Wallemacq P, Rezzoug N, Mekouar H, Olivier PA, et al. The BELFRAIL (BFC80+) study: A population-based prospective cohort study of the very elderly in Belgium. BMC Geriatr. 2010;10:39.
- Tombaugh TN, McIntyre NJ. The mini-mental-state-examination—a comprehensive review. J Am Geriatr Soc. 1992;40:922–35.
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–83.
- Hudon C, Fortin M, Soubhi H. Abbreviated guidelines for scoring the cumulative illness rating scale (CIRS) in family practice. J Clin Epidemiol. 2007;60:212.
- Lyness JM, Noel TK, Cox C, King DA, Conwell Y, Caine ED. Screening for depression in elderly primary care patients. A comparison of the Center for epidemiologic studies-depression scale and the geriatric depression scale. Arch Intern Med. 1997;157:449–54.
- Stel VS, Smit JH, Pluijm SMF, Visser M, Deeg DJH, Lips P. Comparison of the LASA physical activity questionnaire with a 7-day diary and pedometer. J Clin Epidemiol. 2004;57:252–8.
- Barzilay JI, Blaum C, Moore T, Xue QL, Hirsch CH, Walston JD, et al. Insulin resistance and inflammation as precursors of frailty: The cardiovascular health study. Arch Intern Med. 2007;167:635–41.
- Pencina MJ, D’Agostino RB Sr, D’Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–72.
- Valderas JM, Starfield B, Sibbald B, Salisbury C, Roland M. Defining comorbidity: Implications for understanding health and health services. Ann Fam Med. 2009;7:357–63.
- Ritchie C. Health care quality and multimorbidity: The jury is still out. Med Care 2007;45 :477–9.
- Karunananthan S, Bergman H, Wolfson C, Quail J, Weiss D, Zhu B. Frdata: Examining candidate components to define frailty in the elderly using three Canadian databases. Am J Epidemiol. 2005;161:S1–S.
- Markle-Reid M, Browne G. Conceptualizations of frailty in relation to older adults. J Adv Nurs. 2003;44:58–68.
- Shlipak MG, Stehman-Breen C, Fried LF, Song X, Siscovick D, Fried LP, et al. The presence of frailty in elderly persons with chronic renal insufficiency. Am J Kidney Dis. 2004;43:861–7.
- Wilhelm-Leen ER, Hall YN, Deboer IH, Chertow GM. Vitamin D deficiency and frailty in older Americans. J Intern Med. 2010;268:171–80.
- Hogan DB, MacKnight C, Bergman H, Steering Committee, Canadian Initiative on Frailty and Aging. Models, definitions, and criteria of frailty. Aging Clin Exp Res. 2003;15:1–29.
- Le Reste JY, Nabbe P, Manceau B, Lygidakis C, Doerr C, Lingner H, et al. The European General Practice Research Network presents a comprehensive definition of multimorbidity in family medicine and long term care, following a systematic review of relevant literature. J Am Med Dir Assoc. 2013;14:132–33.
- Mangin D, Heath I, Jamoulle M. Beyond diagnosis: Rising to the multimorbidity challenge. Br Med J. 2012;344:e3526.

