Abstract
This article describes the photodynamic inactivation of mosquito iridescent virus (MIV) Aedes flavescens in the presence of water-soluble C60 fullerenes. It has been observed that the photodynamic inactivation of MIV for about 1 h reduces the infectious titre of the virus in large wax-moth larvae Galleria mellonella to 4.5 lg ID50/mL. The influence of the C60 concentration on its anti-viral activity was tested in the concentration range from 1 to 0.001 mg/mL. It has been found that C60 is able to inactivate the iridovirus even in low concentrations. Consequently, the findings of this work suggest that photoexcited C60 fullerenes can be successfully used for the inactivation of iridoviruses in biological systems.
Introduction
Fullerenes belong to the third natural allotropic form of carbon. C60 fullerene is the most studied member of fullerene family. It is a highly stable icosahedral molecule (with size of ∼0.7 nm) consisting of 60 carbon atomsCitation1. In recent years, much attention was paid to a comprehensive investigation of C60 influence in biological systems in vitro and in vivoCitation2,Citation3. It was shown that the addition of fullerenes in some gel-like medicinal forms may significantly increase their wound healing propertiesCitation4. Fullerenes and their derivatives are able to penetrate through cell membranesCitation5 and they have strong anti-oxidantCitation6, anti-viralCitation3,Citation7,Citation8 as well as anti-microbialCitation9–11 properties. It is also interesting that under irradiation, C60 can be activated and reactive oxygen species are generated, which can cause destruction of nucleic acids, proteins and lipidsCitation3,Citation12,Citation13. There are several reports in literature regarding studies on photodynamic inactivation of viruses from Orthomyxoviridae, Rhabdoviridae, Togaviridae and Leviviridae families. It has been reported that the viruses lose their infectivity by more than 7 lg ID50 during a few hours of photoinactivation with C60 fullerenesCitation14–16. A general concept of applying fullerenes in the photodynamic therapy is discussed more detail in paperCitation17.
Iridoviruses are large DNA viruses (with sizes of ∼180–200 nm) that can be replicated in the cytoplasm of infected cells. Iridoviruses have been found to infect invertebrates and poikilothermic vertebrates, including amphibians, reptiles and fishesCitation18. Iridoviruses that infect mosquitoes and midges belong to the genus ChloriridovirusCitation19. Mosquito iridescent viruses (MIVs) are widely distributed in natural reservoirs and play role as regulators of the insect quantity.
Iridoviruses not only infect mosquitoes and other insects but also cause mass mortality of fish that leads to significant economic losses in industrial aquaculture. Consequently, the isolation of these emerging iridoviruses is closely associated with an increase of the world aquaculture production ratesCitation20.
Thus, the purpose of this work was to study the effect of photoactivated C60 on infectious titre of iridoviruses. For this study, virions of MIV, isolated from the mosquito larvae Aedes flavescens in natural reservoirs of the Kyiv region (Ukraine) were used.
Materials and methods
Highly stable water colloid suspension of C60 fullerenes (maximum concentration 1 mg/mL) was prepared by transfer of C60 (purity >99.5%) from toluene to water using ultrasound sonicationCitation21.
It is important to note that the considered water solution of C60 fullerenes does not show a cytotoxic effect at concentrations below 1 mg/mLCitation22.
For the characterisation of C60 films deposited from water solutions (C60 concentration in water was 1 mg/mL) on Au(111) surface, scanning tunnelling microscopy (STM; NT-MDT Moscow, Russia) images using a low-current scanning head were taken. The Pt-Ir (80:20) tip was mechanically cut from a wire (0.25 mm in diameter). Typical imaging conditions were in a range from 0.01 to 0.1 nA and from 0.1 to 0.8 V. Reconstructed Au(111) substrates of 150-nm thickness were prepared by vacuum deposition onto a freshly cleaved mica surfaces heated at ∼600 K followed by a careful annealing in a gas flame (propane). During ∼10 min after flaming, the substrate revealed reconstruction lines in air. Exposition in air during 1-h lifted reconstruction. A droplet of the water solution of C60 fullerenes was deposited on these gold substrates.
MIV Ae. flavescens was propagated in multi-host wax-moth larvae G. mellonella. The wax-moth larvaes were infected by intraperitoneal injection and incubated at room temperature (20–22°C).
Fourteen days after injection of the MIV Ae. flavescens, it was purified from the infected wax-moth larvae G. mellonella by the differential centrifugation method. Briefly, after homogenisation of infected larvae G. mellonella, cell debris was separated by centrifugation at 3000g for 5 min at 10°C. The pellet was discarded and the supernatant centrifuged in a ultracentrifuge (Beckman L5-50B in a rotor SW-40) for 40 min at 70,500g at 4°C. A characteristic blue pellet confirmed the presence of the mosquito iridescent virions. The virus pellet was suspended in TNE (50-mM Tris-HCl, 150-mM NaCl, 1-mM disodium ethylene diaminetetraacetic acid [EDTA], pH 7.5) and centrifuged at 1100g for 5 min at 10°C. The suspension was layered onto a 10−50% (w/w) linear sucrose gradient in TNE. After centrifugation at 70,500g for 40 min at 4°C, one visible band near the bottom of the tube was collected, diluted in fresh TNE and centrifuged at 70,500g for 40 min at 4°C. The virus pellet was placed into TNE at a final protein concentration of 1.7 mg/mL. The infectious titre of MIV Ae. flavescens in the wax-moth larvae G. mellonella was determined by the Reed and Munich methodCitation23. The infectious titre of virus in larvae G. mellonella was 11.2 lg ID50/mL.
For the photodynamic inactivation of MIV, a 400-W halogen lamp (intensity of light radiation was 10Citation20 photons/m2s) was used.
For the experiments, various solutions with constant concentration of virus (0.17 mg/mL) and different concentrations of C60 (1, 0.1, 0.01 and 0.001 mg/mL) were used. The photodynamic inactivation of MIV was performed at 400–850-nm wavelengths in a glass tube. The distance between the source of light and virus was 10 cm. The exposure time was 2.5, 10, 30 and 60 min. The MIV without C60 fullerenes and MIV with C60 fullerenes but without photodynamic inactivation were used as controls.
After exposure, the material from all variants was diluted in the sterile distilled water in an interval of 10−1 to 10−12. The wax-moth larvae G. mellonella were infected by the material from every dilution. The infected larvae were incubated at temperature 20–22°C. Fourteen days after injection, the titers of MIV were determined by the methods of blue pellets.
Results and discussion
The STM images of a submonolayer C60 film deposited from water solution on Au(111) surface are shown in . An almost random arrangement of C60 clusters (with sizes up to ∼2.8 nm) ( can be seen. It is well known that C60 molecules form highly ordered hexagonal structures on reconstructed Au(111) in vacuumCitation24. The absence of such ordering in our case can be attributed to the degradation in water solution. However, nucleation of islands occurs along the preferential direction <112> (see . Despite of the high mobility of C60 molecules on Au(111) at room temperature, we were able to observe single C60 molecules (. The immobilisation of the C60 fullerene is due to the lifting of the reconstruction by water.
Figure 1. (A) Large-scale scanning tunnelling microscopy image of submonolayer C60 fullerene film deposited from water solution (C60 fullerene concentration in water was 1 mg/mL) on Au(111) surface. Some C60 fullerene clusters are aligned along preferential direction. (B) Single C60 fullerenes. Lateral size is increased because of shape of the tip. Inset: Cross-section along line AB. Scanning parameters: It = 40 pA, Ut = 0.7 V.
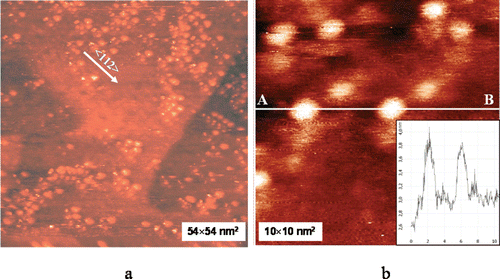
The above-obtained results are in a good agreement with previously predicted theoretical calculationsCitation25,Citation26, which show that the water colloid solution of C60 contains single C60 molecules, their clusters and crystalline phase (with sizes of ∼0.7−4 nm in dependence of C60 concentration in water) in the hydrated state. Analysis of the UV/VIS spectroscopic dataCitation21 showed that C60 fullerenes in water are characterised by two intense broad absorption bands with maximums at 265 and 345 nm and also by narrow spectral lines at 450 and 622 nm.
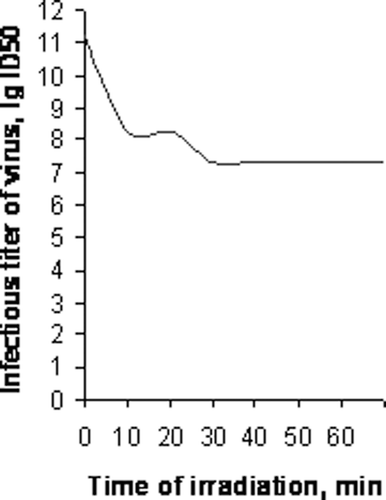
The most interesting and important finding of this study is possibility for the photodynamic inactivation of MIV Aе. flavescens by using an aqueous solution of C60 (concentration 1 mg/mL). It has been found that after a treatment of about 2.5 min, the infectious titre of virus can be decreased by 2 lg ID50/mL units (). In the control group, the infectious titre of MIV was 11.2 lg ID50/mL. The infectious titre of MIV in the G. mellonella after handling for about 2.5 min was decreased to 9.0 lg ID50/mL. Further handling for 10 and 30 min leads to decrease of the infectious titre of the virus by 2.93 and 3.88 lg ID50/mL units, respectively. Interestingly, the indexes of infectious titre of MIV after the photodynamic inactivation for 1 h were not significantly different from those obtained from photodynamic inactivation for 30 min. Infectious titre of MIV Aе. flavescens after the photodynamic inactivation during 1 h was 7.15 lg ID50/mL.
Figure 2. Photodynamic inactivation of mosquito iridescent virus Ae. flavescens by C60 fullerenes (maximum concentration 1 mg/mL).
Our results showed that after photodynamic inactivation for 30 min, C60 in a concentration of 0.1 mg/mL reduced the infectious titre of MIV Ae. flavescens by 4.0 lg ID50/mL units. In a concentration of 0.01 mg/mL, C60 reduced the infectious titre of the MIV by 4.5 lg ID50/mL units. The use of C60 in a concentration of 0.001 mg/mL actually did not influence the infectious titre of MIV Ae. flavescens. The infectious titre of MIV Ae. flavescens was 10.7 lg ID50/mL, that is only 0.5 lg ID50/mL less than in the control group (). The presence of C60 in a viral suspension, but without the photodynamic inactivation, as well as irradiation of viral suspension in the absence of C60 did not influence the infectious titre of MIV.
Table 1. Photodynamic inactivation of mosquito iridoviruses Ae. flavescens by С60.
It is important to note that the effectiveness of reducing the infectious titre of MIV on the C60 concentration and exposure time significantly related to fullerene aggregation in waterCitation26: formation of fullerene clusters (aggregates) with single molecules leads to a significant restructuring of their electronic structure, which determines the mechanism of their photoactivationCitation17.
Conclusion
The photodynamic inactivation of viruses by using C60 and their derivatives can be considered as a promising treatment, and thus, it can be successfully applied in medicine. Recently, a lot of works involved in investigation of anti-viral and anti-bacterial properties of C60 were reported in the literatureCitation9,Citation27. In spite of the fact that the most of the investigations were involved in interactions of fullerenes with either the human immunodeficiency virusCitation28 or hepatitis C virusCitation29, in this work we studied for first time the interaction of C60 and MIV Aе. flavescens. The obtained results demonstrated the anti-viral properties of C60 fullerenes during photoactivation. Namely, during the photodynamic inactivation the C60 fullerenes (30 min) in a most efficient concentration of 0.01 mg/mL interact with the virions of MIV Aе. flavescens causing a reduce of the infectious titre of virus by 4.5 lg ID50/mL units.
Furthermore, the effect of the concentration of C60 fullerenes on the infectious titre of virus was also investigated. In photodynamic inactivation, C60 fullerenes were found to be active against virions of MIV Aе. flavescens even at very low concentration (in the concentration range from 0.1 to 0.01 mg/mL). Our results are in agreement with previous published reportsCitation27,Citation30.
Declaration of interest
This work was partly supported by BMBF grant.
References
- Kroto H, Heath J, O’Brien S, Curl R, Smalley R. C60: Buckminsterfullerene. Nature 1985;318:162–163.
- Bakry R, Vallant RM, Najam-ul-Haq M, Rainer M, Szabo Z, Huck CW et al. Medicinal applications of fullerenes. Int J Nanomedicine 2007;2:639–649.
- Cataldo F, Da Ros T, (Eds.). Medicinal chemistry and pharmacological potential of fullerenes and carbon nanotubes. Series. Carbon Materials: Chemistry and Physics. Elsevier, Springer, Netherlands. 2008. pp. 145–162.
- Da Ros T, Spalluto G, Prato M. Biological Applications of Fullerene Derivatives: A Brief Overview. Croat Chem Acta 2001;74:743–755.
- Foley S, Crowley C, Smaihi M, Bonfils C, Erlanger BF, Seta P et al. Cellular localisation of a water-soluble fullerene derivative. Biochem Biophys Res Commun 2002;294:116–119.
- Prylutska SV, Grynyuk II, Matyshevska OP, Prylutskyy YuI, Ritter, U, Scharff P. Anti-oxidant Properties of C60 Fullerenes in vitro. Fullerenes, Nanotubes, and Carbon Nanostruct 2008;16:698–705.
- Sirotkin AK, Zarubaev VV, Poznyiakova LN, Dumpis MA, Muravieva TD., Krisko TK, Belousova IM, Kiselev OI, Piotrovsky LB. Pristine Fullerene C60: Different Water Soluble Forms-Different Mechanisms of Biological Action. Fullerenes, Nanotubes, and Carbon Nanostruct 2006;14:327–333.
- Ji H, Yang Z, Jiang W, Geng C, Gong M, Xiao H et al. Antiviral activity of nano carbon fullerene lipidosome against influenza virus in vitro. J Huazhong Univ Sci Technol Med Sci 2008;28:243–246.
- Tsao N, Luh TY, Chou CK, Chang TY, Wu JJ, Liu CC et al. In vitro action of carboxyfullerene. J Antimicrob Chemother 2002;49:641–649.
- Li Q, Mahendra S, Lyon DY, Brunet L, Liga MV, Li D et al. Antimicrobial nanomaterials for water disinfection and microbial control: potential applications and implications. Water Res 2008;42:4591–4602.
- Spesia MB, Milanesio ME, Durantini EN. Synthesis, properties and photodynamic inactivation of Escherichia coli by novel cationic fullerene C60 derivatives. Eur J Med Chem 2008;43:853–861.
- Bosi S, Da Ros T, Spalluto G, Prato M. Fullerene derivatives: an attractive tool for biological applications. Eur J Med Chem 2003;38:913–923.
- Burlaka A PSidorik, E P, Prylutska SV, Matyshevska OP, Golub A A, Prylutskyy Yul, Scharff P. Catalytic system of the reactive oxygen species on the C60 fullerene basis. Exp Oncol 2004;26:326–327.
- Käsermann F, Kempf C. Photodynamic inactivation of enveloped viruses by buckminsterfullerene. Antiviral Res 1997;34:65–70.
- Zarubaev VV, Belousova IM, Kiselev OI, Piotrovsky LB, Anfimov PM, Krisko TC, Muraviova TD, Rylkov VV, Starodubzev AM, Sirotkin AC. Photodynamic inactivation of influenza virus with fullerene C60 suspension in allantoic fluid. Photodiagnosis and Photodynamic Therapy 2007;4:31–35.
- Badireddy AR, Hotze EM, Chellam S, Alvarez P, Wiesner MR. Inactivation of bacteriophages via photosensitization of fullerol nanoparticles. Environ Sci Technol 2007;41:6627–6632.
- Mroz P, Tegos GP, Gali H, Wharton T, Sarna T, Hamblin MR. Photodynamic therapy with fullerenes. Photochem Photobiol Sci 2007;6:1139–1149.
- Buchatskyy LP. Viral infections of marine and freshwater animals. Kiev: Noosphera 1994 (in Russian).
- Chinchar VG, Essbauer S, He JG, Hyatt A, Miyazaki T, Seligy V, Williams T. Eighth report of the international committee on taxonomy of viruses. San Diego: Elsevier, Academic Press. 2005. pp. 150–162.
- Williams T, Barbosa-Solomieu V, Chinchar VG. A decade of advances in iridovirus research. Adv Virus Res 2005;65:173–248.
- Scharff P, Risch K, Carta-Abelmann L, Dmytruk IM, Bilyi MM, Golub OA, Khavryuchenko AV, Buzaneva EV, Aksenov VL, Avdeev MV, Prylutskyy YuI Durov, SS. Structure of C60 fullerene in water: Spectroscopic data. Carbon 2004;42:1203–1206.
- Prylutska SV, Matyshevska OP, Golub AA, Prylutskyy YuI Potebnya, GP, Ritter U, Scharff P. Study of С60 fullerenes and С60-containing composites cytotoxicity in vitro. Mater Sci Engineer C 2007;27:1121–1124.
- Reed L, Muench H. A simple method of estimating fifty percent endpoints. Am J Hy 1938;27:493–497.
- Altman EI, Colton RJ. Determination of the orientation of C60 adsorbed on Au(111) and Ag(111) Phys Rev, b Condens Matter 1993;48:18244–18249.
- Prilutski YuI Durov, SS, Yashchuk VN, Ogul’chansky TYu, Pogorelov VE, Astashkin YuA Buzaneva, EV, Kirghizov YuD Andrievsky, GV, Scharff P. Theoretical predictions and experimental studies of self-organized C60 nanoparticles in water solution and on the support. Eur Phys J D 1999;9:341–343.
- Bulavin L, Adamenko I, Prylutskyy Yu Durov, S, Graja A, Bogucki A, Scharff P. Structure of fullerene C60 in aqueous solution. Phys Chem Chem Phys 2000;2:1627–1629.
- Zarubaev VV, Belousova I, Rylkov V, Slita A, Sirotkin A, Anfimov P, Muraviova T, Starodubtsev A. Photodynamic inactivation of enveloped viruses by fullerene: Study of efficacy and safety, medicinal chemistry and pharmacological potential of fullerenes and carbon nanotubes. Carbon Mat Chem Phys 2008;1:107–121.
- Marchesan S, Da Ros T, Spalluto G, Balzarini J, Prato M. Anti-HIV properties of cationic fullerene derivatives. Bioorg Med Chem Lett 2005;15:3615–3618.
- Mashino T, Shimotohno K, Ikegami N, Nishikawa D, Okuda K, Takahashi K et al. Human immunodeficiency virus-reverse transcriptase inhibition and hepatitis C virus RNA-dependent RNA polymerase inhibition activities of fullerene derivatives. Bioorg Med Chem Lett 2005;15:1107–1109.
- Kumar A, Menon SK. Fullerene derivatized s-triazine analogues as antimicrobial agents. Eur J Med Chem 2009;44:2178–2183.
