Abstract
Carbonic anhydrases (CA) catalyze activated ester hydrolysis in addition to the hydration of CO2 to bicarbonate. They also show phosphatase activity with 4-nitrophenyl phosphate as substrate but not sulfatase with the corresponding sulfate. Here we prove that the enzyme is catalyzing the synthesis of cyclic diols from sulfate esters. 5-, 6- and 8-membered ring cyclic sulfates incorporating a neighboring secondary alcohol moiety were treated with CA II and yielded the corresponding cyclic diols. Inhibitory properties of obtained cyclic and original sulfate esters were then investigated on human carbonic anhydrase I (hCA I), hCA II, hCA IV and hCA VI (h = human isoform). KI-s of these compounds ranged between 32.7–423 μM against hCA I, 2.13–32.4 μM against hCA II, 13.7–234 μM against hCA IV and 76–278 μM against CA VI, respectively. The sulfatase activity of CA with such esters is amazing considering the fact that 4-nitrophenyl-sulfate is not a substrate of these enzymes.
Introduction
Mammals possess 16 different carbonic anhydrase (CA, EC 4.2.1.1) isoforms, which are involved in many crucial physiological processes connected with respiration and transport of CO2/HCO3−, pH and CO2 homeostasis, electrolyte secretion in a variety of tissues/organs, biosynthetic reactions, bone resorption, etcCitation1–6. Some of the isozymes are cytosolic (CA I, CA II, CA III, CA VII and CA XIII), two are mitochondrial (CA VA and CA VB), one is secreted (CA VI), and others are membrane-bound (CA IV, CA IX, CA XII and CA XIV)Citation1–11. In a recent preliminary work from our group, we investigated the interaction between natural phenolic compound, antioxidant phenolic compounds, hydroxy/metoxy organic compounds and salicylic acid derivatives with two cytosolic catalytically active isoforms (CA I and II) of the metalloenzyme CACitation1–8. Indeed, phenol 12 binds to CA in a diverse manner compared to the classical inhibitors of the sulfonamides/sulfamates/sulfamides, which coordinate to the Zn2+ ion from the enzyme active site by substituting the fourth, non-protein ligand, a water molecule or hydroxide ionCitation12–15. Recently, Christianson’s group then reported the X-ray crystal structure for the adduct of human carbonic anhydrase II (hCA II) with phenolCitation12, showing indeed this inhibitor to bind to hCA II by anchoring its OH moiety to the zinc-bound H2O/hydroxide ion of the enzyme through a hydrogen bond as well as to the NH amide of Thr199, an amino acid conserved in all α-CAs and critically important for the catalytic cycle of these enzymesCitation4–6,Citation12–15. Furthermore, the phenyl moiety of this inhibitor was found to lay in the hydrophobic part of the hCA II active site, where presumably CO2, the physiologic substrate of the CAs, binds in the precatalytic complex, explaining thus the behaviour of phenol as a unique CO2 competitive inhibitorCitation1–3,Citation13–16.
Inhibitory effects of different phenols, metoxyphenol derivatives, anions, metal ions and drugs have been investigated up to now against many mammalian, fish, bacterial and fungal CAsCitation12–20. CA II is the physiologically most relevant isoenzyme. CA inhibitors (CAIs) are used for several applications, in particular for the treatment of glucoma, epilepsy, as diuretics etc. Other compounds, targeting isoforms IX and XII, have applications as antitumor agents/diagnostic toolsCitation1–11. Therefore, discovery of novel CAIs targeting various isoenzymes has gained attention nowadaysCitation1–10. Cyclic diols were developed treatments of type II diabetes, Gaucher disease and also as an anti-HIV drugCitation21–24. In the current study, we aimed to synthesize cyclic diols 5, 7 and 9 using carbonic anhydrase enzyme and determine the inhibitory effects of cyclic diols and sulfate esters on four α-CA isozymes, hCA I, hCA II, hCA IV and hCA VI.
Results and discussion
Chemistry
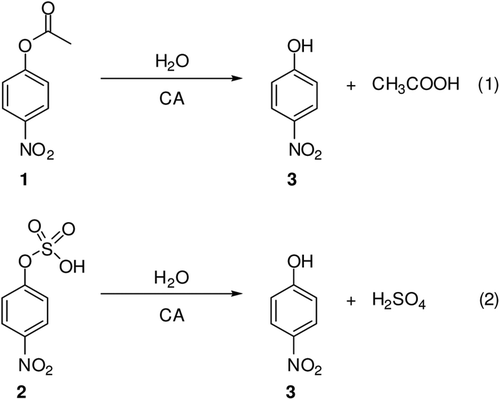
It is known that carboxylate/phosphate esters are hydrolyzed by α-CAs, although 4-nitrophenylsulfate was shown not to be a substrate for the cytosolic isoforms hCA I, II and XIIICitation1–6. Recently, one of our groups reported kinetic study on the hydrolysis of 4-nitrophenyl acetate 1 and phosphate in the presence of three cytosolic CA isozymes, hCA I, hCA II and hCA XIIICitation1–6. In solution, these esters are hydrolyzed by the nucleophilic attack of water (or hydroxide ions) to the central atom (carbonyl CO for acetate 1, phosphorus for phosphate) with formation of a transition state from which the 4-nitrophenoxide is released. Considering the fact that CAs contain the equivalent of a strong base (hydroxide ions, HO− coordinated to the zinc ion) at neutral pH, due to the powerful activation of H2O by the zinc ion from the active site cavity and the hydrophobic environment of the protein, in principle, hydrolytic reactions 1–2 of should have the same mechanism as the hydrolysis catalyzed by bases in solution. In these studies, Innocenti et al.Citation1,Citation2 showed that the hydrolytic processes described by Eqs. 1–2 of , involve the active site Zn2+(OH)− functionality of the enzyme, that is, the same one responsible of the CO2 hydration activity of α-CAs. Probably, compounds 4, 6 and 8 are hydrolysed by CA II in the same way in the current study. It is interesting to note here that the aliphatic, cyclic sulfates investigated here, unlike the aromatic activated one (ester 2) indeed act as substrates for CAs. The sulfatase activity of this enzyme has been in fact discovered earlier with a cyclic sulfate ester as substrate, by Kaiser and LoCitation3.
Scheme 1. Reactions 1 and 2 catalyzed by α-carbonic anhydrases (CAs). Whereas the 4-nitrophenyl acetate hydrolysis occurs easily, the corresponding sulfate 2 is not a substrate for CAsCitation1–3.
Furthermore, we report here an inhibition study of the four catalytically active human isoforms hCA I, II, IV and VI with compounds 4–11. They incorporate sulfate esters or cyclis diols in their molecules and scaffolds representing thus an interesting starting point for different chemotypes belonging to the CAIs. In fact, in an earlier studyCitation25 we reported micromolar/submicromolar inhibitors of the cytosolic isoforms hCA I and II with a library of organic nitrates.
CA purification, assay and inhibition
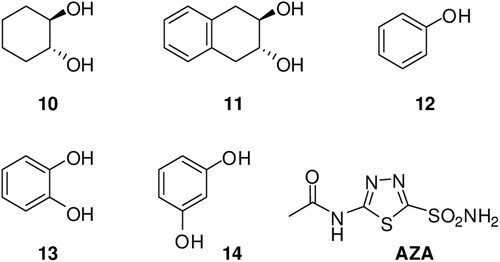
The purification of hCA isozymes was performed with a simple one step method by a Sepharose-4B-aniline-sulfanilamide affinity columnCitation9–11. Inhibitory effects of compounds trans-(1R(S),6R(S))-6-Hydroxycyclohex-3-enyl hydrogen sulfate (4), (1R,2R)-cyclohexane-1,2-diol (5), trans-(1R(S),8R(S),Z)-8-Hydroxycyclooct-4-enyl hydrogen sulfate (6), (2R,3R)-1,2,3,4-tetrahydronaphthalene-2,3-diol (7), 9(R(S))-Hydroxy-1,2,3,4-tetrahydro-1,4-methanonaphthalen-2(R(S))-yl sulfate (8), 9(R(S))-Hydroxy-1,2,3,4-tetrahydro-1,4-methanonaphthalen-2(R(S))-diol (9), trans-(1R(S),2R(S))-cyclohexane-1,2-diol (10) and trans-(2R(S),3R(S))-1,2,3,4-Tetrahydronaphthalene-2,3-diol (11) on these isoenzyme activities were tested under in vitro conditions; KI values were calculated from Lineweaver-Burk plots and are given in Citation25–29.
Table 1. KI values obtained from regression analysis graphs for hCA I, hCA II, hCA IV, and hCA VI in the presence of different inhibitors concentrations (μM).
The CA isozymes play important roles in different tissuesCitation1–9,Citation28–32. It is known that CA has been purified many times from different organisms and the effects of various chemicals, pesticides, anions, metal ions and drugs have been investigated on its activityCitation8–20,Citation28–32. We report here a study on the inhibitory effects of organic sulfates, diols and some phenolic compounds on the CA esterase activity of isoforms hCA I, II, IV and VI. Data of show the following, regarding inhibition of hCA I, II, IV and VI with compounds 4–11 and with positive controls 12, 13, 14, Acetazolamide (AZA):
Against the slow cytosolic isozyme hCA I, compounds 5, 7, 9–10 behave as weak, micromolar inhibitors, with KI values the range of 135–188 μM. Compound 11 was an ineffective hCA I inhibitor (KI of 423 μM). A second group of derivative, including 4, 6 and 8, showed better inhibitory activity as compared to the previously mentioned compounds 5 and 7, with KI values of 32.7–77.2 μM, (). Compound 11 showed KI value of 423 μM, and various substutions patterns such as the introduction of the hydroxyl and sulfate- moieties lead to minor changes in activity, compounds 5, 7, 9–11 behaving as rather weak hCA I inhibitors (). The same effect is observed when the inhibition constants are calculated () by means of Lineweaver-Burk plots, these compounds showing KI-s in the range of 135–423 μM.
These compounds incorporate moieties leading to an acidification of the OH groups form the organic sulfates and cyclic diols scaffold (such as OSO3H or OH group in the 2-or 3-position in compound 4 and 5), as well as the bulkier scaffolds present in 6, 7 and especially 8, 9, 11. These were among the best inhibitors in this series of organic sulfates and cyclic diols. Data of also show that similarly to phenolic compoundsCitation9–15, most of the investigated organic sulfates (4, 6 and 8), act as competitive inhibitors with 4-NPA as substrate, i.e. they bind in the same regions of the active site cavity as the substrate. However, the binding site of 4-nitrophenylacetate (NPA) itself is unknown, but it is presumed to be in the same region as that of CO2, the physiological substrate of this enzymeCitation12–15. Similarly to salicylic acid derivatives and phenolic compounds investigated earlier by us, the investigated compounds act as competitive inhibitors with 4-NPA as substrate, that is, they bind in same regions of the active site cavity as compared to the substrate.
A better inhibitory activity has been observed with compounds 4–6, 10 investigated here for the inhibition of the rapid cytosolic isozyme hCA II (). Four derivatives, i.e. 7–9, 11 showed moderate hCA II inhibitory activity with KI-s in the range of 10.4–32.4 μM (), whereas the remaining four derivatives were quite effective hCA II inhibitors, with KI-s in the range of 2.13–5.41 μM (). Structure-activity relationship (SAR) is thus quite sharp for this small series of tetralin scaffold compounds (8, 9 and 11) are ineffective leads. The best hCA II inhibitor in this series of derivatives 4.
Compound 11, and some of its congeners such as compounds 8 and 9 are also weak inhibitors of CA IV, with KI-s of 77.9–234 μM. However, again compound 7 is medium potency inhibitor (KI of 53.8 μM), and compounds 4–6 and 10 show a higher affinity for this isozyme, with inhibition constant in the range of 13.7–23.6 μM, AZA with KI of 5.64 μM ().
Phenol 12 and some of its congeners such as 13 and 14 are also weak inhibitors of the secreted isozyme hCA VI, with KI-s of 208–550 μM13. However, again the compounds 8 and 9 are medium potency inhibitors (KI of 221–278 μM), and derivatives 4–7, 10 and 11 show higher affinity for this isozyme, with inhibition constants in the range of 76.2–145 μM ().
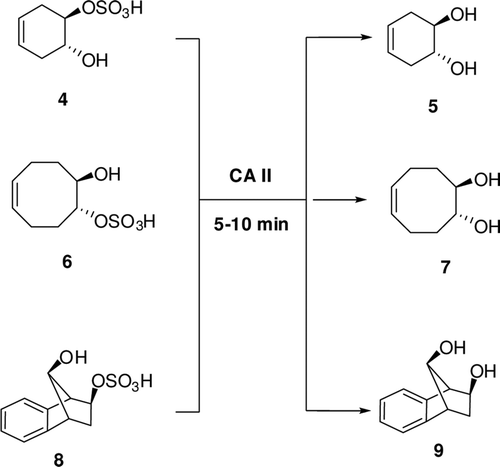
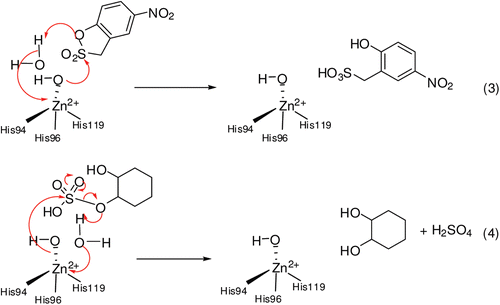
We hypothesize that CAs (which as we show above, possess esterase activity against several substrates), hydrolyses these organic sulfates leading to sulfuric acid and cyclic diols, as illustrated in . Previously, studies showed the hydrolysis reaction of 2-Hydroxy-5-nitro-α-toluenesuIfonic acid sultone ()Citation1–3.
Scheme 2. The hydrolysis reaction of compound 4, 6 and 8 with carbonic anhydrase II (CA II) isoenzyme.
Scheme 3. The hydrolysis reaction of 2-Hydroxy-5-nitro-α-toluenesuIfonic acid sultoneCitation3 (3) and cyclic sulfate esters (4).
In recent studies, it was reported that phenols and natural phenolic compounds act as CAIs, and could represent the starting point for a new class of inhibitors that may have advantages for patients with sulfonamide allergies (thioxolone acts as a prodrug)Citation8–16.
Cyclic diols or cyclitols are receiving considerable attention as chemotherapeutic agents against diabetes, cancer, and viral infectionsCitation26–29. Several multiple sulfated compounds have been found in biologically active compounds and marine organismsCitation28. For instance, sulfated sterols have exhibited effects such as anti-HIV, antiviral activity, and inhibition of protein tyrosine kinasesCitation26–28. Recent studies also showed that sulfo-containing salicylic acid derivatives have inhibitory effects on CA I and II isoenzymesCitation9. However, it is critically important to explore further classes of potent CAIs in order to detect compounds with a different inhibition profile as compared to the sulfonamides and their bioisosteres and to find novel applications for the inhibitors of these widespread enzymes, in particular tumor-related isoforms.
Conclusions
Cyclic diols 5, 7 and 9 were synthesized from sulfate esters 4, 6 and 8 using CA II isoenzyme. Cyclic diols and sulfate esters 4–11 affect the activity of CA isozymes due to the presence of the functional group (OH) in their cyclic aliphatic scaffold. Our findings indicate thus another class of possible CAIs of interest, in addition to the well-known sulfonamides/sulfamates/sulfamides, although their mechanism of CA inhibition remains rather elusive at this moment. We have reported several inhibitors of both CA and other proteins so farCitation30–44 and discovery of novel inhibitors still requires further investigations. Indeed, some cyclic diols investigated here showed effective CA I, II, IV and VI inhibitory activity, in the micromolar range, by the esterase assay method. These findings point out that substituted cyclic diols may be used as leads for generating more potent CAIs eventually targeting other isoforms which have not been assayed yet for their interactions with such agents.
Experimental
Chemicals
Sepharose 4B, protein assay reagents, p-nitrophenol, 4-nitrophenylacetate and chemicals for electrophoresis were purchased from Sigma-Aldrich Co. All other chemicals were of analytical grade and obtained from Merck.
Purification of CA isozymes from human blood by affinity chromatography
Fresh citrated human whole blood obtained from the Blood Center of the Research Hospital at Atatürk University. Cells were washed three times by centrifugation at 1000xg at 4 ± 6°C, for 20 min in four volumes of 25 mM Na2HPO4 (pH 7,4) buffer. Supernatant and fluffy coat were removed. The erythrocytes were lysed in 10 volumes of 5 mM Na2HPO4 (pH 7.4) buffer, containing 1 mM EDTA. After 20 min, the haemolysate was centrifuged at 10,000g for 60 min. The particulate fraction was washed four times in the same buffer. The membranes were centrifuged down at 15,000g for 60 min. pH was adjusted to 8.3 with solid Tris. Sepharose-4B-aniline-sulfanilamide affinity column equilibrated with 25 mM Tris-HCl/0.1 M Na2SO4 (pH 8.3). The affinity gel was washed with 25 mM Tris-HCl/25 mM Na2PO4 (pH 8.3). Finally, human carbonic anhydrase IV (hCA IV) isozyme was eluted with 25 mM Tris-HCl/0.5 M NaClO4 (pH 7.4)Citation45,Citation46. Fresh non-citrated human whole blood obtained from the Blood Center of the Research Hospital at Atatürk University. The blood samples were centrifuged at 5000 rpm for 15 min and precipitant were removed. The serum was isolated. The pH was adjusted to 8.7 with solid Tris. Sepharose-4B-aniline-sulfanilamide affinity column equilibrated with 25 mM Tris-HCl/0.1 M Na2SO4 (pH 8.7). The affinity gel was washed with 25mM Tris-HCl/22mM Na2SO4 (pH 8.7). The hCA-VI isozyme was eluted with 0.25 M H2NSO3H/25 mM Na2HPO4 (pH 6.7). All procedures were performed at 4°CCitation16,Citation47.
Enzyme mediated synthesis of cyclic diols
The reactions were performed in the presence of hCA II in water at pH 7.5. A 10-fold excess of the starting sulphate esters 4, 6, 8 was used to limit side reactions. Three reactions were performed with and without enzyme in a sodium phosphate solution at pH 7.4 (20 mM phosphate buffer). Stock solution in dimethyl sulfoxide of three sulphate esters (10 mM) were added to three aqueous solutions in order to reach the final concentration of 0.08 mM. The clear mixture was incubated at 25°C for 5 min, for 4 and 6 and for 10 min, for 8. The sulphate esters yielded the corresponding diols in 100% yield in 5–10 min although 4 and 6 hydrolyzed without enzyme solution in approximately 150 min whereas 8 was not hydrolyzed without the enzyme (). Prior to analyzing the products, the mixture was left for 2 h to be separated from CA by decantation. The thermal denaturation of the enzyme (2 min at 80°C) was also tested to ensure the release from casting site of some possible tightly bound ligands.
Table 2. Time-% conversion rate with buffer or H2O medium (rt, 1 atm).
CA inhibition
CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spectrophotometer (Shimadzu UV-VIS) according to the method described by Verpoorte et al.Citation48 The enzymatic reaction, in a total volume of 3.0 mL, contained 1.4 mL 0.05M Tris-SO4 buffer (pH 7.4), 1 mL, 3 mM NPA, 0.5 mL H2O and 0.1 mL enzyme solution. A reference measurement was obtained by preparing the same cuvette without enzyme solution. The inhibitory effects of compounds 4–11 were examined. All compounds were tested in triplicate at each concentration used. Different inhibitor concentrations were used. Control cuvette activity in the absence of inhibitor was taken as 100%. For each inhibitor an Activity%- [Inhibitor] graph was drawn. To determine KI values, three different inhibitor concentrations were tested. In these experiments, NPA was used as substrate at five different concentrations (0.15–0.75 mM). The Lineweaver-Burk curves were drawnCitation49.
Protein determination
Protein during the purification steps was determined spectrophotometrically at 595 nm according to the Bradford method, using bovine serum albumin as the standardCitation50.
Sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis
SDS polyacrylamide gel electrophoresis was performed after purification of the enzymes. It was carried out in 10% and 3% acrylamide for the running and the stacking gel, respectively, containing 0.1% SDS according to LaemmliCitation51.
Synthesis of sulfate esters
Detailed synthetic procedures for the preparation of all derivatives can be found in: Ref 37.
Declaration of interest
This study was financed by Turkish Republic Prime Ministry State Planning Organization (DPT), (Project no: 2010K120440) for Murat Şentürk. Work from Supuran lab was financed by an FP 7 EU grant (Metoxia project).
References
- Innocenti A, Scozzafava A, Parkkila S, Puccetti L, De Simone G, Supuran CT. Investigations of the esterase, phosphatase, and sulfatase activities of the cytosolic mammalian carbonic anhydrase isoforms I, II, and XIII with 4-nitrophenyl esters as substrates. Bioorg Med Chem Lett 2008;18:2267–2271.
- Innocenti A, Supuran CT. Paraoxon, 4-nitrophenyl phosphate and acetate are substrates of α- but not of ß-, γ- and ζ-carbonic anhydrases. Bioorg Med Chem Lett 2010;20:6208–6212.
- Kaiser ET, Lo KW. The carbonic anhydrase catalyzed hydrolysis of 2-hydroxy-5-nitro-α-toluenesuIfonic acid sultone. J Am Chem Soc 1969;91:4912–4918.
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–181.
- Supuran CT, Scozzafava A. Carbonic anhydrases as targets for medicinal chemistry. Bioorg Med Chem 2007;15:4336–4350.
- Sly WS, Hu PY. Human carbonic anhydrases and carbonic anhydrase deficiencies. Annu Rev Biochem 1995;64:375–401.
- Parkkila S, Parkkila AK. Carbonic anhydrase in the alimentary tract. Roles of the different isozymes and salivary factors in the maintenance of optimal conditions in the gastrointestinal canal. Scand J Gastroenterol 1996;31:305–317.
- Pastorekova S, Parkkila S, Pastorek J, Supuran CT. Carbonic anhydrases: current state of the art, therapeutic applications and future prospects. J Enzyme Inhib Med Chem 2004;19:199–229.
- Bayram E, Senturk M, Kufrevioglu OI, Supuran CT. In vitro inhibition of salicylic acid derivatives on human cytosolic carbonic anhydrase isozymes I and II. Bioorg Med Chem 2008;16:9101–9105.
- Sentürk M, Gülçin I, Beydemir S, Küfrevioglu OI, Supuran CT. In Vitro Inhibition of Human Carbonic Anhydrase I and II Isozymes with Natural Phenolic Compounds. Chem Biol Drug Des 2011;77:494–499.
- Alp C, Ekinci D, Gültekin MS, Sentürk M, Sahin E, Küfrevioglu OI. A novel and one-pot synthesis of new 1-tosyl pyrrol-2-one derivatives and analysis of carbonic anhydrase inhibitory potencies. Bioorg Med Chem 2010;18:4468–4474.
- Nair SK, Ludwig PA, Christianson DW. Two-site binding of phenol in the active site of human carbonic anhydrase II: structural implications for substrate association. J Am Chem Soc 1994;116:3659–3660.
- Innocenti A, Vullo D, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors: interactions of phenols with the 12 catalytically active mammalian isoforms (CA I-XIV). Bioorg Med Chem Lett 2008;18:1583–1587.
- Durdagi S, Sentürk M, Ekinci D, Balaydin HT, Göksu S, Küfrevioglu ÖI, et al. Kinetic and docking studies of phenol-based inhibitors of carbonic anhydrase isoforms I, II, IX and XII evidence a new binding mode within the enzyme active site. Bioorg Med Chem 2011;19:1381–1389.
- D’Ambrosio K, Vitale RM, Dogné JM, Masereel B, Innocenti A, Scozzafava A et al. Carbonic anhydrase inhibitors: bioreductive nitro-containing sulfonamides with selectivity for targeting the tumor associated isoforms IX and XII. J Med Chem 2008;51:3230–3237.
- Ozturk Sarikaya SB, Topal F, Senturk M, Gulcin I, Supuran CT. In vitro inhibition of α-carbonic anhydrase isozymes by some phenolic compounds. Bioorg Med Chem Lett 2011;21:4259–4262.
- Hilvo M, Baranauskiene L, Salzano AM, Scaloni A, Matulis D, Innocenti A et al. Biochemical characterization of CA IX, one of the most active carbonic anhydrase isozymes. J Biol Chem 2008;283:27799–27809.
- Supuran CT, Scozzafava A, Casini A. Carbonic anhydrase inhibitors. Med Res Rev 2003;23:146–189.
- Ceyhun SB, Sentürk M, Ekinci D, Erdogan O, Ciltas A, Kocaman EM. Deltamethrin attenuates antioxidant defense system and induces the expression of heat shock protein 70 in rainbow trout. Comp Biochem Physiol C Toxicol Pharmacol 2010;152:215–223.
- Ceyhun SB, Senturk M, Yerlikaya E, Erdogan O, Kufrevioglu OI, Ekinci D. In vitro and in vivo effects 321 of some pesticides on carbonic anhydrase enzyme from rainbow trout (Oncorhynchus 322 mykiss) gills. Pestic Biochem Physiol 2010;97:177–181.
- Winchester BG. Iminosugars: from botanical curiosities to licensed drugs. Tetrahedron 2009;20:645–651.
- Scott LJ, Spencer CM. Miglitol: a review of its therapeutic potential in type 2 diabetes mellitus. Drugs 2000;59:521–549.
- Yu Z, Sawkar AR, Whalen LJ, Wong CH, Kelly JW. Isofagomine- and 2,5-anhydro-2,5-imino-D-glucitol-based glucocerebrosidase pharmacological chaperones for Gaucher disease intervention. J Med Chem 2007;50:94–100.
- Saracoglu N, Talaz O, Azizoglu A, Watson WH, Balci M. Synthesis and structure of cyclopropano-annelated homosesquinorbornene derivatives containing pyramidalized double bonds: evidence for the sterical effect of a cyclopropyl group on the degree of C=C double-bond pyramidalization. J Org Chem 2005;70:5403–5408.
- Ekinci D, Cavdar H, Talaz O, Sentürk M, Supuran CT. NO-releasing esters show carbonic anhydrase inhibitory action against human isoforms I and II. Bioorg Med Chem 2010;18:3559–3563.
- Hughes AB, Rudge AJ. Deoxynojirimycin: synthesis and biological activity. Nat Prod Rep 1994;11:135–162.
- Heightman TD, Vasella AT. Recent insights into inhibition, structure, and mechanism of configuration-retaining glycosidases. Angew Chem Int Ed 1999;38:750–770.
- Kornprobst JM, Sallenave C, Barnathan G. Sulfated compounds from marine organisms. Comp Biochem Physiol B, Biochem Mol Biol 1998;119:1–51.
- Cavdar H, Saracoglu N. Synthesis of new beta-hydroxy nitrate esters as potential glycomimetics or vasodilators. Eur J Org Chem 2008;4615–4621.
- Senturk M, Ekinci D, Goksu S, Supuran CT. Effects of dopaminergic compounds on carbonic anhydrase isozymes I, II, and VI. J Enzyme Inhib Med Chem 2011; doi:10.3109/14756366.2011.591290.
- Ekinci D, Ceyhun SB, Sentürk M, Erdem D, Küfrevioglu OI, Supuran CT. Characterization and anions inhibition studies of an a-carbonic anhydrase from the teleost fish Dicentrarchus labrax. Bioorg Med Chem 2011;19:744–748.
- Ekinci D, Ceyhun SB, Aksakal E, Erdogan O. IGF and GH mRNA levels are suppressed upon exposure to micromolar concentrations of cobalt and zinc in rainbow trout white muscle. Comp Biochem Physiol C Toxicol Pharmacol 2011;153:336–341.
- Erdogan O, Ceyhun SB, Ekinci D, Aksakal E. Impact of deltamethrin exposure on mRNA expression levels of metallothionein A, B and cytochrome P450 1A in rainbow trout muscles. Gene 2011;484:13–17.
- Ekinci D, Sentürk M, Beydemir S, Küfrevioglu OI, Supuran CT. An alternative purification method for human serum paraoxonase 1 and its interactions with sulfonamides. Chem Biol Drug Des 2010;76:552–558.
- Aksakal E, Ceyhun SB, Erdogan O, Ekinci D. Acute and long-term genotoxicity of deltamethrin to insulin-like growth factors and growth hormone in rainbow trout. Comp Biochem Physiol C Toxicol Pharmacol 2010;152:451–455.
- Ceyhun SB, Aksakal E, Ekinci D, Erdogan O, Beydemir S. Influence of cobalt and zinc exposure on mRNA expression profiles of metallothionein and cytocrome P450 in rainbow trout. Biol Trace Elem Res 2011; doi:10.1007/s12011-011–9068-z.
- Cakmak R, Durdagi S, Ekinci D, Sentürk M, Topal G. Design, synthesis and biological evaluation of novel nitroaromatic compounds as potent glutathione reductase inhibitors. Bioorg Med Chem Lett 2011;21:5398–5402.
- Aksakal E, Ekinci D, Erdogan O, Beydemir S, Alim Z, Ceyhun SB. Increasing stocking density causes inhibition of metabolic-antioxidant enzymes and elevates mRNA levels of heat shock protein 70 in rainbow trout. Livest Sci 2011;141:69–75.
- Siktar E, Ekinci D, Siktar E, Beydemir S, Gülçin I, Günay M. Protective role of l-carnitine supplementation against exhaustive exercise induced oxidative stress in rats. Eur J Pharmacol 2011;668:407–413.
- Balaydin HT, Durdagi S, Ekinci D, Senturk M, Goksu S, Menzek A. Inhibition of human carbonic anhydrase isozymes I, II and VI with a series of bisphenol, metoxy and bromophenol compounds. J Enzyme Inhib Med Chem 2011; doi:10.3109/14756366.2011.596836.
- Ceyhun SB, Sentürk M, Yerlikaya E, Erdogan O, Küfrevioglu OI, Ekinci D. Purification and characterization of carbonic anhydrase from the teleost fish Dicentrarchus labrax (European seabass) liver and toxicological effects of metals on enzyme activity. Environ Toxicol Pharmacol 2011;32:69–74.
- Senturk M, Ekinci D, Alici HA, Beydemir S. Paraoxonase-1, an organophosphate detoxifier and cardioprotective enzyme, is inhibited by anesthetics: an in vitro and in vivo insight. Pestic Biochem Physiol 2011; doi:10.1016/j.pestbp.2011.09.007.
- Ekinci D, Beydemir S. Risk assessment of pesticides and fungicides for acid-base regulation and salt transport in rainbow trout tissues. Pestic Biochem Physiol 2010;97:66–70.
- Ekinci D, Beydemir S. Purification of PON1 from human serum and assessment of enzyme kinetics against metal toxicity. Biol Trace Elem Res 2010;135:112–120.
- Wistrand PJ, Carter ND, Conroy CW, Mahieu I. Carbonic anhydrase IV activity is localized on the exterior surface of human erythrocytes. Acta Physiol Scand 1999;165:211–218.
- Whitney PL, Briggle TV. Membrane-associated carbonic anhydrase purified from bovine lung. J Biol Chem 1982;257:12056–12059.
- Kivelä J, Parkkila S, Waheed A, Parkkila AK, Sly WS, Rajaniemi H. Secretory carbonic anhydrase isoenzyme (CA VI) in human serum. Clin Chem 1997;43:2318–2322.
- Verpoorte JA, Mehta S, Edsall JT. Esterase activities of human carbonic anhydrases B and C. J Biol Chem 1967;242:4221–4229.
- Lineweaver H, Burk D. The determination of enzyme dissocation constants. J Am Chem Soc 1934;56:658–666.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–254.
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970;227:680–685.
- Cavdar H, Saracoglu N. Ring opening of epoxides with NaHSO4: isolation of beta-hydroxy sulfate esters and an effective synthesis for trans-diols. Tetrahedron 2009;65:985–989.