Abstract
Azadirachta indica, used in antidiabetic herbal drugs, was reported to contain α-glucosidase inhibitor. Bioassay guided purification characterized the inhibitor as nimbidiol (a diterpenoid), present in root and stem-bark of the tree. Nimbidiol inhibited intestinal (mammalian) maltase-glucoamylase, sucrase-isomaltase, lactase, trehalase and fungal α-glucosidases. Nimbidiol showed a mixed competitive inhibition on intestinal carbohydrases. IC50, Ki and Ki′ (µM) were 1.35 ± 0.12, 0.08 ± 0.01, 0.25 ± 0.11, respectively, for maltase-glucoamylase (maltotetraose as substrate). Nimbidiol was more potent inhibitor of isomaltase (IC50 0.85 ± 0.035 µM), lactase (IC50 20 ± 1.33 µM) and trehalase (IC50 30 ± 1.75 µM) than acarbose, voglibose, salacinol, kotalanol and mangiferin. Ki and Ki′ values (µM) for intestinal sucrase were 0.7 ± 0.12 and 1.44 ± 0.65, respectively. Development of nimbidiol as an antidiabetic drug appears to be promising because of broad inhibition spectrum of intestinal glucosidases and easy synthesis of the molecule.
Introduction
One of the most direct and beneficial types of therapy for non-insulin dependent diabetes is lowering of blood glucose level by delaying glucose absorption in blood after taking a carbohydrate meal. Inhibition or lowering of activities of some intestinal membrane bound carbohydrases like maltase-glucoamylase and sucrase-isomaltase by suitable inhibitors, slow-down absorption of glucose into the blood stream. During the last 40 years, a large number of glucosidase inhibitors have been isolated from plants and micro-organismsCitation1 and some of these were developed as drugs to control type II or non-insulin dependent diabetesCitation2. Different inhibitors isolated from plants and microorganisms include diverse types of compounds like acarbose, isoacarbose, cyclodextrins, acarviosine-glucose and hibiscus acidCitation3–6. Although the list of α-glucosidase inhibitors isolated from various biological sources are long, yet the bioactive molecules of traditional antidiabetic herbal drugs characterized are few in number. We recently reported that the medicinal plant Tinospora cordifolia, which according to old ayurveda system of medicine contains antidiabetic agents, has α-glucosidase inhibitory activityCitation7. The inhibitor molecule present in the plant was purified and identified to be saponarinCitation8. Neem (Azadirachta indica) tree is native to India and Burma; it grows in much of Southeast Asia and West Africa. Neem is a fast growing tropical evergreen tree and lives for long years. Neem has been used extensively in ayurvedic medicine for various purposes like: twigs for cleaning teeth, juice of leaves in healing skin disorders, as a tonic, as insect repellent etc. Various parts of the plant are used for the treatment of fever and infections. Hypoglycaemic effect of Azadirachta indica leaf extracts and seed oil was observed in normal and alloxan induced diabetic rabbitsCitation9. We reported earlier in a patent that Azadirachta indica extract blocked intestinal α-glucosidase activityCitation10. The present study reports the bioassay guided purification of the inhibitor molecule as nimbidiol from the stem-bark and root of the tree. The molecule reversibly inhibits the activities of maltase-glucoamylase and sucrase-isomaltase of mammalian (rat) intestine. The molecule also inhibits the activities of intestinal lactase and trehalase and microbial α-glucosidases. The inhibitor may serve as an important lead molecule for the treatment of type II diabetes.
Materials
Chemicals
Soluble starch (potato), maltose, sucrose, maltotriose, maltotetraose and isomaltose were purchased from Sigma chemicals, USA. Lactose and dinitrosalicylic acid (DNSA) were purchased from Merck, India. Trehalose was a product from British Drug House. Silica gel (60–120 mesh) was purchased from Merck, India. All other chemicals used were of chemically pure grade. Fungal maltase (Aspergillus niger), fungal invertase (Baker’s yeast) and pancreatic amylase (porcine) were obtained from Sigma chemicals. Rat intestinal acetone powder (I-1630), used as a source of intestinal glucosidases, was purchased from Sigma chemicals.
Methods
Preparation of plant extracts
Aqueous extract
A 100 gm of fresh and healthy root collected, was washed in tap water and crushed into small pieces. The mass was blended with 100 mL of water for 3–4 min to obtain a paste. The paste obtained was squeezed through a nylon cloth and filtered under suction to obtain 90 mL of clear liquid. The clear liquid (90 mL) was passed through PM10 KDa membrane filter. The filtrate (80 mL) collected was lyophilized to dryness (3.0 gm). The dry powder was resuspended in 10 mL of water and used as a crude inhibitor extract. The same extraction methodology was followed using 100 gm of fresh stem-bark.
Methanolic extract
A 100 gm of fresh root was collected and dried in a hot air oven at 50–60°C for 4–5 h to obtain 23.75 gm of dry mass. The dry mass was crushed, immersed in 1 L of methanol and kept for 48 h at room temperature. The methanolic extract was filtered and evaporated in a rotary evaporator to obtain approximately 1.1 gm of dry solid. The dry solid was further suspended in 10 mL of 80% (v/v) methanol. The solution was used as a crude inhibitor sample. The same process was repeated using 100 gm of fresh stem-bark.
Determination of enzyme inhibition by the extracts
The potency of crude inhibitor extract to inhibit pancreatic amylase, intestinal glucosidases (maltase, sucrase, lactase, trehalase, maltase-glucoamylase and sucrase-isomaltase) and fungal glucosidases (maltase, sucrase) was studied. Pancreatic amylase activity was determined using 0.1 M phosphate buffer, pH 7.0 at 37°C. The incubation mixtures (2 mL) containing 0.5 U of enzyme in buffer was preincubated with requisite amount of inhibitor extract for 10 min at room temperature. The reaction was initiated by adding 1 mg/mL of starch and incubation lasted for 20 min. Amount of reducing sugars formed was estimated by dinitrosalicylic acid reagent according to the method of Sengupta et al.Citation11. One unit of enzyme activity was taken as the amount of enzyme which could liberate one µmol of reducing sugar per min under the experimental conditions. Maltase, sucrase, isomaltase, lactase and trehalase activities were determined by estimating the amount of glucose liberated from maltose, sucrose, isomaltose, lactose, trehalose respectively by glucose oxidase–peroxidase (GOD-POD) methodCitation12. One unit of enzyme activity was taken as the amount of enzyme which could liberate one µmol of glucose per min from the substrates under the experimental conditions. Intestinal maltase, sucrase, isomaltase, lactase and trehalase activities were determined in 0.1 M phosphate buffer pH 6.8 at 37°C. Fungal maltase was assayed in 0.1 M sodium acetate buffer pH 5.0 at 50°C. Fungal sucrase was assayed in 0.1 M sodium acetate buffer pH 4.5 at 50°C. The reaction mixtures (0.5 mL) containing enzyme (10 milli units) and requisite amount of inhibitor extracts were preincubated for 10 min. The reaction was started by addition of substrate (1 mg/mL). The reaction continued for 20 min and was terminated by keeping the tubes in boiling water bath for 3–4 min. Liberated glucose was estimated by GOD-POD reagent (Span Diagnostic limited, India). The concentration of inhibitor required to inhibit 50% of enzyme activity (substrate concentration 1 mg/mL) under the above-mentioned conditions was taken as IC50 value. Intestinal glucoamaylase activities were determined similarly by GOD-POD method using maltotriose and maltotetraose (1 mg/mL), as mentioned by Breitmeier et al.Citation13. Inhibitory activities of root extract were compared with that of stem-bark extract. Inhibitory activities of the root and stem-bark extracts (both aqueous and methanolic) were expressed in terms of inhibitory unit (IU). One IU was considered as the amount of crude extract, which could inhibit the enzyme activity by 50%; the substrate concentration being 1 mg/mL.
Isolation and purification of nimbidiol from stem-bark and root of Azadirachta indica
It is to be mentioned that the methanolic root and stem-bark extract was more potent against intestinal disaccharidases than the aqueous extracts. Thus, bioassay-guided isolation and purification of nimbidiol was carried out using methanolic extracts with respect to its inhibitory activity on intestinal glucosidases. Nimbidiol was isolated from dry root and stem-bark of Azadirachta indica according to the process described in the patentCitation14. Air-dried and milled root (850 gm) of Azadirachta indica was percolated with methanol (3 L) for 30 h at room temperature. The extract was concentrated to a small volume (100 mL) under reduced pressure at 50°C. The entire process was repeated two times more to ensure complete extraction. The combined concentrates (300 mL) was further concentrated to 75 mL, diluted with water (500 mL) with stirring and extracted with ethyl acetate (300 mL × 3). The organic extract was evaporated to dryness under reduced pressure at 50°C to get a brown residue (20 gm), which was fractionated by chromatography over a column of silica gel followed by bioassay studies of the individual fractions. Fractionation of the combined active fractions was repeated two more times in an identical manner. The combined active fractions thus obtained were then chromatographed over diaion HP-20. Elution with methanol:water (3:1 and 1:1) gave the most active four fractions. These fractions were combined and rechromatographed over silica gel. The resultant combined active fraction was subjected to preparative TLC to obtain pure nimbidiol. 1H and 13C NMR studies were done. The data obtained were compared with that available in literatureCitation15,Citation16. Nimbidiol obtained was also compared with pure nimbidiol isolated by TLC according to the method of AraCitation17. Air-dried and milled stem-bark (750 gm) of Azadirachta indica were percolated with methanol (2.5 L) for 24 h at room temperature. The entire process was repeated thrice to ensure complete extraction. The whole extract was concentrated to a small volume (100 mL) under reduced pressure at 50°C. The concentrates (100 mL) was diluted with water (400 mL) with stirring and extracted four times with 250 mL of ethyl acetate. The organic extract (1000 mL) was washed with 250 mL of 4% (w/v) of sodium carbonate solution and then twice with 500 mL of water. Ethyl acetate extract was concentrated (50 mL) under reduced pressure at 50°C. The concentrated extract was adsorbed on charcoal column and active fraction was eluted with methanol – benzene (1:1, v/v). The fraction was dried and the solid obtained (150 mg) was extracted with hexane. Hexane soluble fraction was subjected to preparative thick layer chromatography using CHCl3-MeOH (97:3) as solvent system. The preparative thick layer chromatography yielded pure nimbidiol. The compound isolated in both the ways gave identical UV absorption spectrum as reported by Majumder et al.Citation16. The same process was carried out for purification of the inhibitor molecule from dry stem-bark.
Determination of kinetics of enzyme inhibition by nimbidiol
Inhibition kinetics of nimbidiol on intestinal and fungal glucosidases was studied. The reaction mixture (0.5 mL) contained different amounts of substrate in respective buffer, 0.010 U of enzyme and 0.05–10 µM nimbidiol. The reaction mixtures containing enzyme and inhibitor were preincubated for 10 min and the reaction was started by addition of substrate (0.5–6 mM). Enzyme activities were determined as mentioned earlier. The concentration of inhibitor required to inhibit 50% of enzyme activity at a substrate concentration of 1 mg/mL under the above-mentioned conditions was taken as IC50 value. Ki and Ki′ values were determined from 1/V vs I (Dixon plot) and S/V vs I plots respectively.
Results
Inhibitory activity of crude bark extract on enzyme activities
shows the inhibitory activities present in the aqueous and methanolic extract of stem and root-bark. The extracts inhibited glucosidases (mammalian intestinal and fungal origin) very strongly, while little inhibition was observed in case of pancreatic amylase (with soluble starch and dextrin as substrate). The crude methanolic extract was more active and potent than the aqueous extract. The crude methanolic root extract showed IC50 value of 7 ± 1.5 µM and 8.5 ± 1.0 µM for intestinal maltase-glucoamylase with maltotetraose and matotriose as substrates, respectively, and IC50 value of 52 ± 2 µM with maltose as substrate. The IC50 value of the methanolic root extract was much lower for isomaltase (6 ± 0.75 µM) than intestinal sucrase (28 ± 2.5 µM). The methanolic root extract was also a potent inhibitor of intestinal lactase (IC50 68 ± 2.5 µM) and trehalase (IC50 83 ± 2.5 µM). Both methanolic and aqueous extract also inhibited fungal maltase and sucrase activities ().
Table 1. Inhibitory potency of aqueous and methanolic extract of Azadirachta indica towards intestinal and fungal glucosidases.
Nimbidiol purification
Nimbidiol (C17H22O3), a modified diterpenoid (), was purified and isolated as mentioned in the text. Melting point was found to be 226°C. The purification method yielded 15 mg of pure compound {[α]20D = +3.4(c1,CHCl3)}, the yield being about 0.00176% (w/w). Reference compound for comparison, was also isolated by known method as mentioned in the text. The compound displayed characteristic UV absorption [λEtOH max 210, 238, 282, 323 nm] as described by Majumder et al.Citation16. 13C NMR (CDCl3) δ 37.9 (C-1), 18.9 (C-2), 41.3 (C-3), 33.2 (C-4), 49.8 (C-5), 36.0 (C-6), 200.11 (C-7), 123.8 (C-8), 152.4 (C-9), 37.8 (C-10), 110.1 (C-11), 151.2 (C-12), 142.0 (C-13), 113.5 (C-14), 32.5 (C-15), 21.3 (C-16), 23.2 (C-17). MS: m/z 274 [M]+ (100%), 259 (97), 245 (5), 217 (23), 203 (13), 191 (70), 189 (71), 177 (44), 163 (19), 151 (6), 137 (6), 115 (8), 83 (6), 77 (6), 69 (27), 55 (11) and 41 (20). The NMR and MS data obtained were compared with previously reported valuesCitation16. 13C NMR spectra were recorded on a Bruker DRX 300 (USA) NMR spectrophotometer and mass spectra recorded on a JEOL JMS600 instrument.
Inhibition of various α-glucosidases by nimbidiol
and show the IC50 and inhibitory constants of nimbidiol and other α-glucosidase inhibitors. Ki and Ki′ values for nimbidiol were calculated using Dixon plot and S/V vs I plots respectively (). IC50 and Ki values of nimbidiol for intestinal maltase (12 ± 1.23 µM/1.22 ± 0.344 µM) were almost in range with those of salacinol (9.58 µM/0.95 µM) but higher than those of kotalanol (6.58 µM/0.54 µM), acarbose (2.0 µM/0.18 µM) and voglibose (1.19 µM/0.11 µM). Similarly, IC50 and Ki of nimbidiol on intestinal sucrose (6.75 ± 0.80 µM/0.7 ± 0.12 µM) were almost similar to those for salacinol (2.51 µM/0.95 µM), but higher than those for kotalanol (1.37 µM/0.42 µM), acarbose (1.7 µM/0.57 µM) and voglibose (0.22 µM/0.067 µM). Nimbidiol inhibited maltase-glucoamylase strongly when maltotriose and maltotetraose were used as substrates. The compound also inhibited intestinal lactase and trehalase with IC50 value of 20 ± 1.3 and 30 ± 1.75, respectively (). IC50, Ki and Ki′ value (µM) of nimbidiol for fungal glucosidases were 14.23 ± 1.40, 1.8 ± 0.20 and 4.25 ± 0.54 respectively for maltase and 10.85 ± 0.75, 1.068 ± 0.080 and 2.66 ± 0.78 for sucrase ().
Table 2. Comparison of IC50 values of nimbidiol and other glucosidase inhibitors.
Table 3. Comparison of inhibitor constants of nimbidiol and other glucosidase inhibitors.
Figure 2. Determination of Ki of nimbidiol on maltase and sucrase. Dixon plot (1/V vs I) for inhibition of nimbidiol on activities of intestinal maltase (a), intestinal sucrase (b), fungal maltase (c) and fungal sucrase (d). Nimbidiol (0.5–8 µM) was added against different concentrations (0.5–6 mM) of maltose and sucrose [S]. Enzyme activities were determined as mentioned in the text.
![Figure 2. Determination of Ki of nimbidiol on maltase and sucrase. Dixon plot (1/V vs I) for inhibition of nimbidiol on activities of intestinal maltase (a), intestinal sucrase (b), fungal maltase (c) and fungal sucrase (d). Nimbidiol (0.5–8 µM) was added against different concentrations (0.5–6 mM) of maltose and sucrose [S]. Enzyme activities were determined as mentioned in the text.](/cms/asset/e2fa31b4-1aba-40d8-a93e-9849f5fd1cd8/ienz_a_694877_f0002_b.gif)
Figure 3. Determination of Ki′ of nimbidiol on maltase and sucrase. S/V vs I plot for inhibition of nimbidiol on activities of intestinal maltase (a), intestinal sucrase (b), fungal maltase (c) and fungal sucrase (d). nimbidiol (0.5–8 µM) was added against different concentrations (0.5–6 mM) of maltose and sucrose [S]. Enzyme activities were determined as mentioned in the text.
![Figure 3. Determination of Ki′ of nimbidiol on maltase and sucrase. S/V vs I plot for inhibition of nimbidiol on activities of intestinal maltase (a), intestinal sucrase (b), fungal maltase (c) and fungal sucrase (d). nimbidiol (0.5–8 µM) was added against different concentrations (0.5–6 mM) of maltose and sucrose [S]. Enzyme activities were determined as mentioned in the text.](/cms/asset/db6dd22c-0de5-4fc4-a79f-ee4c93a7be07/ienz_a_694877_f0003_b.gif)
Figure 4. Determination of Ki and Ki′ of nimbidiol on intestinal glucoamylase. Dixon plot (1/V vs I) for inhibition of nimbidiol on activities of intestinal glucoamylase using maltotetraose as substrate (a), and maltotriose as substrate (b). S/V vs I plot for inhibition of nimbidiol on intestinal glucoamylase using maltotetraose as substrate (c) and maltotriose as substrate (d). Nimbidiol (0.05–0.8 µM) was added against different concentrations (0.5–6 mM) of maltotetraose and maltotriose. [S]. Enzyme activities were determined as mentioned in the text.
![Figure 4. Determination of Ki and Ki′ of nimbidiol on intestinal glucoamylase. Dixon plot (1/V vs I) for inhibition of nimbidiol on activities of intestinal glucoamylase using maltotetraose as substrate (a), and maltotriose as substrate (b). S/V vs I plot for inhibition of nimbidiol on intestinal glucoamylase using maltotetraose as substrate (c) and maltotriose as substrate (d). Nimbidiol (0.05–0.8 µM) was added against different concentrations (0.5–6 mM) of maltotetraose and maltotriose. [S]. Enzyme activities were determined as mentioned in the text.](/cms/asset/3ee480b2-5f00-4b08-956d-9c1d392b65a9/ienz_a_694877_f0004_b.gif)
Figure 5. Determination of Ki and Ki′ of nimbidiol on intestinal isomaltase. Dixon plot (1/V vs I) for inhibition of nimbidiol on activities of intestinal isomaltase (a) and S/V vs I plot for inhibition of nimbidiol on intestinal isomaltase (b). Nimbidiol (0.05–0.8 µM) was added against different concentrations (0.5–6 mM) of isomaltose [S]. Enzyme activities were determined as mentioned in the text.
![Figure 5. Determination of Ki and Ki′ of nimbidiol on intestinal isomaltase. Dixon plot (1/V vs I) for inhibition of nimbidiol on activities of intestinal isomaltase (a) and S/V vs I plot for inhibition of nimbidiol on intestinal isomaltase (b). Nimbidiol (0.05–0.8 µM) was added against different concentrations (0.5–6 mM) of isomaltose [S]. Enzyme activities were determined as mentioned in the text.](/cms/asset/3871b08c-1e47-4ad5-b75d-e3f3e9f582ed/ienz_a_694877_f0005_b.gif)
Discussion
In mammalian digestive system, six enzyme activities (two α-amylases and four α-glucosidases) are involved in the complete digestion of starch into glucose. Salivary and pancreatic α-amylases (EC 3.2.1.1) are endohydrolases that cleave the internal α-1,4 bonds of amylose and amylopectin, and bypasses the α-1,6 branch points, thus resulting into shorter linear and branched dextrin chains. The resultant mixture is further hydrolyzed into glucose by two small-intestinal brush-border exohydrolases maltase-glucoamylase (EC 3.2.1.20 and 3.2.1.3) and sucrase-isomaltase (EC 3.2.148 and 3.2.10) before being absorbed into the bloodstream. These intestinal enzymes are crucial for the transport of glucose monosaccharides and control blood sugar level. Maltase-glucoamylase has very little activity on starch but rapidly hydrolyses α-amylase pretreated starchCitation18.
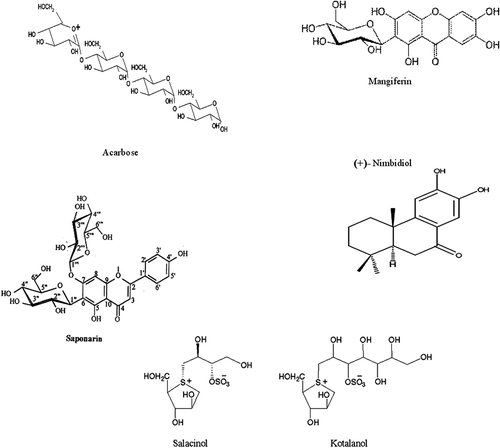
Attempts in developing antidiabetic drug using α-glucosidase inhibitor started since the end of last century. Valiolamine, produced by Streptomyces hygroscopicus var linoneus was reported as a potent inhibitor of pig intestinal maltase and sucraseCitation19. Acarbose, produced by Actinoplanes sp. was introduced in USA under the name “Precose.” 1-deoxynojirimycin, a potent inhibitor of α-glucosidase was isolated from the roots of mulberry trees and from many strains of Bacillus and StreptomycesCitation20,Citation21. Among the large number of derivatives of 1-deoxynojirimycin prepared, a selected derivative Migilitol was found to be highly active under in vivo conditionCitation22. Salacinol and kotalanol have been identified as α-glucosidase inhibitors present in water-soluble fraction of the roots and stems of Salacia reticulateCitation23,Citation24. We reported earlier in a patent that crude methanolic extract of Azadirachta indica contained a potent inhibitor of mammalian intestinal and fungal glucosidases. The root extract was most potent followed by stem-bark, leaves and seed extractCitation10. shows the inhibitory activities of the methanolic extract (both stem-bark and root) to be higher than the aqueous extract, indicating the active compound to be more soluble in methanol. The crude extracts did not inhibit pancreatic amylase but inhibited hydrolysis of malto-oligosaccharides (maltotetraose, maltotriose) strongly indicating possible inhibition of intestinal maltase-glucoamylase complex. The crude extracts inhibited intestinal maltase, sucrase, isomaltase, lactase and trehalase and could also inhibit the activities of fungal α-glucosidases. The ratio of IC50 values for methanolic extracts towards different enzymes was calculated and was compared with that of aqueous extracts. Comparative study showed that the degree of accuracy of the method used for determination of IC50 value for most of the glycosidase activities varies from 85–100% using aqueous or methanolic extracts (). However, the values vary more than 50% when trehalase or lactase activities were determined. It must be mentioned here that each enzyme has its own Km value and thus requires a specific substrate concentration. However, IC50 value must be determined at a uniform substrate concentration for all enzymes. Thus, this may probably be the reason for the method being less accurate towards intestinal lactase and trehalase. In the assessment of crude extracts from root and stem-bark of Azadirachta indica, inhibitory unit (IU) present in crude extracts were about 55000 ± 500 and 47000 ± 2000 for intestinal maltase-glucoamylase using maltotetraose and maltotriose as substrate respectively (data not given). The apparent higher inhibitory value of the crude methanolic root extract indicates that root and stem-bark might contain either other inhibitors or some factor(s) that synergistically stimulate the activity of nimbidiol. shows the structure of various α-glucosidase inhibitors of microbial and plant origin. Although nimbidiol was purified from root and stem-bark of the tree with a poor yield of 0.00176%, yet the compound could easily be synthesizedCitation25. 13C NMR data of the purified compound was compared with earlier reportsCitation16 which confirmed the isolated compound to be nimbidiol. Nimbidiol has been isolated and purified earlier by Majumder et al.Citation16 by extracting the air dried root-bark with CHCl3 and subjecting it to silica gel column chromatography. The petrol-EtOAc (5:1) fraction gave a gummy residue mainly containing nimbidiol which was further purified by repeated chromatography. It may be mentioned that present bioefficacy guided purification protocol recovered lower amount of nimbidiol from the plant, compared to yields reported by other workers from the same sourceCitation16. However, the comparison is insignificant as many easy routes for the synthesis of the compound were reported subsequentlyCitation25. Though the IC50 and Ki values of nimbidiol for intestinal maltase and sucrase are higher than that of acarbose, the compound (nimbidiol) more strongly inhibits intestinal maltase-glucoamylase when malto-oligosaccharides are used as substrates (). Breitmeier et al.Citation13 reported the Ki value of acarbose and 1-deoxynojirimycin for intestinal maltase glucoamylase complex using malto-oligosaccharides (maltopentaose, maltotetraose, maltotriose) to be 0.4 and 0.3 µM respectively which is about four times and three times higher than that for nimbidiol () respectively. It has also been reported that acarbose at a concentration of 4 µM inhibited the activities of glucoamylase by 98%Citation26 while nimbidiol showed a IC50 value of 1.56 ± 0.24 µM with maltotetraose as substrate. Interestingly, the IC50 and Ki values of nimbidiol for isomaltase were much lower than that of acarbose, voglibose, salacinol, kotalanol and mangiferin (). It has been reported that acarbose at 200 µM concentration could inhibit only 28% of intestinal isomaltase activity. The IC50 values of acarbose, voglibose, salacinol and kotalanol for intestinal trehalase were also much higher than that for nimbidiol ( and ). Nimbidiol also showed potent inhibitory activity towards intestinal lactase (IC50 20 ± 1.33 µM) while acarbose has been reported to show negligible inhibition towards the sameCitation26. Though 1-deoxynojirimycin and its derivatives were potent inhibitors of intestinal maltase and sucrase, but unlike acarbose they did not inhibit α-amylase activityCitation26. However, 1-deoxynojirimycin and its derivatives at high concentrations (20–200 µM) inhibited trehalase and lactase activities considerablyCitation26. Mangiferin appeared a much less potent inhibitor of intestinal disaccharidases than nimbidiol (, ). It appears that nimbidiol may prove to be a broad spectrum intestinal carbohydrase inhibitor useful for non-insulin dependent diabetes. It must be mentioned here that IC50 and Ki values (µM) of saponarin (isolated from T.cordifolia leaves) were 48 ± 3.55 and 8 ± 0.15 (for intestinal maltase) and 35 ± 1.95 and 6 ± 1.33 (for intestinal sucrase)Citation8. Three active ellagitannins, identified as chebulanin, chebulagic acid and chebulinic acid isolated from dried Terminalia chebula fruits were reported to possess intestinal maltase inhibitory activity but with quite high IC50 values of 690, 97 and 36 µM, respectivelyCitation27. Chebulagic acid was a reversible and noncompetitive inhibitor of intestinal maltase and a more potent inhibitor of maltase-glucoamylase complex than that of sucrase-isomaltase complexCitation28. Compounds like 3,4-dicaffeoylquinic acid, 3,5-dicaffeoylquinic acid and 4,5-dicaffeoylquinic acid isolated from the flower buds of Tussilago farfara L. also inhibited intestinal maltase activity but with IC50 value in mM rangeCitation29. The Dixon plots and S/v vs I plots showed that nimbidiol inhibited various glucosidases in a mixed competitive fashion (). Saponarin, isolated from Tinospora cordifolia also showed mixed competitive inhibition towards various glucosidasesCitation8. It is to be mentioned here that salacinol and kotalanol showed a fully competitive type of inhibition on each α-glucosidaseCitation30, while acarbose showed a full competitive inhibition towards intestinal sucraseCitation31 and maltaseCitation13 but noncompetitive type of inhibition towards pancreatic amylaseCitation32. Flavonoids isolated from the roots of Sophora flavescens showed a noncompetitive type of inhibition for α-glucosidasesCitation33. It was reported that natural circuminoid (bisdemethoxycurcumin) inhibited α-glucosidase in a noncompetitive fashionCitation34. Inhibitors from plant sources like mangiferin with xanthinone aglycon and salacinol or kotalanol with thiosugar moieties have received attention for the development of antidiabetic drugCitation30. Tadera et al.Citation35 extensively studied inhibitory activity of flavonoids on α-glucosidase activity and reported relatively poor inhibition of rat intestinal enzymes by the flavonoids. Recently, it was reported that catechin analogues with alkyl side chains were potential antioxidant molecules with α-glucosidase inhibitor activitiesCitation36. Diterpenoids have also gained much importance as α-glucosidase inhibitors. Recently, a labdane type diterpene (spicatanol) isolated from the rhizomes of Hedychium spicatum showed α-glucosidase inhibitory activities but with a higher IC50 value of 34.1 µMCitation37. Another new diterpenoid, 15-angeloyloxy-16,17-epoxy-19-kauronic acid along with its known metabolite 16-kauren-19-oic acid isolated from the roots of Chromoleana odorata, exhibited strong α-glucosidase inhibitory activitiesCitation38. Two new icetexane diterpenes-8,11,13-icetexatriene-10-hydroxy,11,12,16-triacetoxyl and 8,11,13-icetexatriene-7,10,11-dihydroxy-12,13-dihydrofuran isolated from the roots of Premna tomentosa displayed intestinal α-glucosidase inhibitory activitiesCitation39. Thus, nimbidiol appears to have broad inhibitory activities on almost all glucosidases involved not only with digestion of starch but also other dietary sugars like sucrose and lactose.
Conclusion
Nimbidiol, which could be synthesized easilyCitation25, is a broad spectrum inhibitor of all intestinal glucosidases (maltase-glucoamylase, sucrase-isomaltase, lactase and trehalase). Thus, the plant bioactive molecule shows much promise to be developed as an antidiabetic drug.
Declaration of interest
The authors declare no conflicts of interest. The authors alone are responsible for the content and writing of the article.
References
- Naoki A. Glycosidase inhibitors: Update and perspectives on practical use. Glycobiology 2003;13:93–104.
- Clissold SP, Edwards C. Acarbose. A preliminary review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential. Drugs 1988;35:214–243.
- Kim MJ, Lee SB, Lee HS, Lee SY, Baek JS, Kim D et al. Comparative study of the inhibition of α-glucosidase, α-amylase, and cyclomaltodextrin glucanosyltransferase by acarbose, isoacarbose, and acarviosine-glucose. Arch Biochem Biophys 1999;371:277–283.
- Hansawasdi C, Kawabata J, Kasai T. α-amylase inhibitors from roselle (Hibiscus sabdariffa Linn.) tea. Biosci Biotechnol Biochem 2000;64:1041–1043.
- Nahoum V, Roux G, Anton V, Rougé P, Puigserver A, Bischoff H et al. Crystal structures of human pancreatic α-amylase in complex with carbohydrate and proteinaceous inhibitors. Biochem J 2000;346 Pt 1:201–208.
- Qian M, Nahoum V, Bonicel J, Bischoff H, Henrissat B, Payan F. Enzyme-catalyzed condensation reaction in a mammalian α-amylase. High-resolution structural analysis of an enzyme-inhibitor complex. Biochemistry 2001;40:7700–7709.
- Sengupta S, Charkroborty A, Mukherjee A, Sarkar T. A process for the isolation of an α-glycosidase inhibitor, useful for non-insulin dependent diabetes treatment. C.S.I.R. Patent Application No. 0339NF, Delhi, India, 2005.
- Sengupta S, Mukherjee A, Goswami R, Basu S. Hypoglycemic activity of the antioxidant saponarin, characterized as α-glucosidase inhibitor present in Tinospora cordifolia. J Enzyme Inhib Med Chem 2009;24:684–690.
- Khosla P, Bhanwra S, Singh J, Seth S, Srivastava RK. A study of hypoglycaemic effects of Azadirachta indica (Neem) in normaland alloxan diabetic rabbits. Indian J Physiol Pharmacol 2000;44:69–74.
- Sengupta S, Mukherjee A. A process for the characterization of the bioactive component present in the Bark and root of Azadirachta indica (Neem) used in herbal remedies for the treatment of diabetes. Indian Patent Journal No. 45/2008. Indian Patent Application No. 617/kol/2007, Kolkata, India, 2008.
- Sengupta S, Jana ML, Sengupta D, Naskar AK. A note on the estimation of microbial glycosidase activities by dinitrosalicylic acid reagent. Appl Microbiol Biotechnol 2000;53:732–735.
- Bergmeyer HU, Brent E, Schmidt E, Stork H. (1974). Enzymatic analysis of glucose. In: Bergmeyer HU, ed. Methods of Enzymatic Analysis. London: Academic Press London, 1196–1201.
- Breitmeier D, Günther S, Heymann H. Acarbose and 1-deoxynojirimycin inhibit maltose and maltooligosaccharide hydrolysis of human small intestinal glucoamylase-maltase in two different substrate-induced modes. Arch Biochem Biophys 1997;346:7–14.
- Sengupta S, Mukherjee A. Characterization of nimbidiol as an α-glucosidase inhibitor present in neem (Azadirachta indica), useful for non-insulin dependent diabetes treatment. Indian Patent Application No. 768/KOl/2011, Kolkata, India, 2011.
- Buckingham J. (1994). Dictionary of natural products. London, UK: Chapman and Hall, 1794.
- Majumder PL, Maiti DC, Kraus W, Bokel M. Nimbidiol, a modified diterpenoid of the root-bark of Azadirachta indica. Phytochemistry 1987;26:3021–3023.
- Ara I. (1989). Studies in the chemical constituents of Azadirachta indica (neem). PhD Thesis. Karachi, Pakistan: University of Karachi/H.E.J Research Institute of Chemistry, 149–151.
- Sim L. (2010). Structural and inhibition studies of human intestinal glucosidases. PhD Thesis. Toronto, Canada: Graduate Department of Medical Biophysics, University of Toronto, 8–12.
- Kameda Y, Asano N, Yoshikawa M, Takeuchi M, Yamaguchi T, Matsui K et al. Valiolamine, a new α-glucosidase inhibiting aminocyclitol produced by Streptomyces hygroscopicus. J Antibiot 1984;37:1301–1307.
- Yagi M, Kouno T, Aoyagi Y, Murai H. The structure of maranoline, a piperidine alkaloid from Morus species. Nippon Nogei Kagaku Koishi 1976;50:571–572.
- Schmidt DD, Frommer W, Müller L, Truscheit E. [Glucosidase inhibitors from Bacilli]. Naturwissenschaften 1979;66:584–585.
- Osonoi T, Saito M, Mochizuki K, Fukaya N, Muramatsu T, Inoue S et al. The a-glucosidase inhibitor miglitol decreases glucose fluctuations and inflammatory cytokine gene expression in peripheral leukocytes of Japanese patients with type 2 diabetes mellitus. Metab Clin Exp 2010;59:1816–1822.
- Yoshikawa M, Murakami T, Shimada H, Matsuda H, Yamahara J, Tanabe G, Muraoka O. Salacinol, potent antidiabetic principle with unique thiosugar sulfonium sulfate structure from the Ayurvedic traditional medicine Salacia reticulata in Sri Lanka and India. Tetrahedron Lett 1997;38: 8367–8370.
- Yoshikawa M, Murakami T, Yashiro K, Matsuda H. Kotalanol, a potent α-glucosidase inhibitor with thiosugar sulfonium sulfate structure, from antidiabetic ayurvedic medicine Salacia reticulata. Chem Pharm Bull 1998;46:1339–1340.
- Zambrano JL, Rosales V, Nakano T. First formal synthesis of (+)-nimbidiol. Synthesis, X-ray structure and anticancer activity of a novel ring C aromatic diterpene: Dimethyl (+)-podocarpa-8,11,13-triene-12,13-dicarboxylate. Tetrahedron Lett 2003;44:1859–1862.
- Samulitis BK, Goda T, Lee SM, Koldovský O. Inhibitory mechanism of acarbose and 1-deoxynojirimycin derivatives on carbohydrases in rat small intestine. Drugs Exp Clin Res 1987;13:517–524.
- Gao H, Huang YN, Xu PY, Kawabata J. Inhibitory effect on á-glucosidase by the fruits of Terminalia chebula. Retz Food Chem 2007;105:628–634.
- Gao H, Huang YN, Gao B, Kawabata J. Chebulagic acid is a potent α-glucosidase inhibitor. Biosci Biotechnol Biochem 2008;72:601–603.
- Gao H, Huang YN, Gao B, Xu PY, Inagaki C, Kawabata J. α-Glucosidase inhibitory effect by the flower buds of Tussilago farfara L. Food Chem 2008;106:1195–1120.
- Matsuda H, Morikawa T, Yoshikawa M. Anti-diabetogenic constituents from several natural medicines. Pure Appl Chem 2002;74:1301–1308.
- Hanozet G, Pircher HP, Vanni P, Oesch B, Semenza G. An example of enzyme hysteresis. The slow and tight interaction of some fully competitive inhibitors with small intestinal sucrase. J Biol Chem 1981;256:3703–3711.
- Schmidt DD, Frommer W, Junge B, Müller L, Wingender W, Truscheit E et al. α-Glucosidase inhibitors. New complex oligosaccharides of microbial origin. Naturwissenschaften 1977;64:535–536.
- Kim JH, Ryu YB, Kang NS, Lee BW, Heo JS, Jeong IY et al. Glycosidase inhibitory flavonoids from Sophora flavescens. Biol Pharm Bull 2006;29:302–305.
- Du ZY, Liu RR, Shao WY, Mao XP, Ma L, Gu LQ et al. α-glucosidase inhibition of natural curcuminoids and curcumin analogs. Eur J Med Chem 2006;41:213–218.
- Tadera K, Minami Y, Takamatsu K, Matsuoka T. Inhibition of α-glucosidase and α-amylase by flavonoids. J Nutr Sci Vitaminol 2006;52:149–153.
- Hakamata W, Nakanishi I, Masuda Y, Shimizu T, Higuchi H, Nakamura Y et al. Planar catechin analogues with alkyl side chains: A potent antioxidant and an α-glucosidase inhibitor. J Am Chem Soc 2006;128:6524–6525.
- Prabhakar Reddy P, Tiwari AK, Ranga Rao R, Madhusudhana K, Rama Subba Rao V, Ali AZ et al. New Labdane diterpenes as intestinal α-glucosidase inhibitor from antihyperglycemic extract of Hedychium spicatum (Ham. Ex Smith) rhizomes. Bioorg Med Chem Lett 2009;19:2562–2565.
- Wafo P, Kamdem RS, Ali Z, Anjum S, Begum A, Oluyemisi OO et al. Kaurane-type diterpenoids from Chromoleana odorata, their X-ray diffraction studies and potent a-glucosidase inhibition of 16-kauren-19-oic acid. Fitoterapia 2011;82:642–646.
- Ayinampudi SR, Domala R, Merugu R, Bathula S, Janaswamy MR. New icetexane diterpenes with intestinal a-glucosidase inhibitory and free-radical scavenging activity isolated from Premna tomentosa roots. Fitoterapia 2012;83:88–92.
- Miyahara C, Miyazawa M, Satoh S, Sakai A, Mizusaki S. Inhibitory effects of mulberry leaf extract on postprandial hyperglycemia in normal rats. J Nutr Sci Vitaminol 2004;50:161–164.