 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Systemic inflammation is associated with obesity and chronic disease risk. Intake of dairy foods is associated with reduced risk of type 2 diabetes and cardiovascular disease; however, the impact of dairy foods on inflammation is not well-established. The objective of this study was to conduct a systematic review to evaluate the effect of dairy product (milk, cheese, and yogurt) and dairy protein consumption on low-grade systemic inflammation in adults without severe inflammatory disorders. A literature search was completed in September 2019 using PubMed and CENTRAL as well as inspection of reference lists from relevant review articles. The search resulted in the identification of 27 randomized controlled trials which were included in this analysis. In the 19 trials which evaluated dairy products, 10 reported no effect of the intervention, while 8 reported a reduction in at least one biomarker of inflammation. All 8 trials that investigated dairy protein intake on markers of inflammation reported no effect of the intervention. The available literature suggests that dairy products and dairy proteins have neutral to beneficial effects on biomarkers of inflammation. Additional clinical studies designed using inflammatory biomarkers as the primary outcome are needed to fully elucidate the effects of dairy intake on inflammation.
Key teaching points
Systemic inflammation is a key contributor to the progression of metabolic disorders.
The impact of dairy food consumption on systemic inflammation is unclear.
This systematic review shows that consumption of dairy products and proteins has neutral to beneficial effects on biomarkers of inflammation.
Additional studies, including clinical and prospective cohort, designed using inflammatory biomarkers as the primary outcome are warranted.
Introduction
Low-grade, systemic inflammation is considered a key contributor in the pathophysiological progression of metabolic disorders including cardiovascular disease (CVD), type 2 diabetes, and metabolic syndrome (Citation1–3). Indeed, circulating concentrations of C-reactive protein (CRP), cytokines including interleukin (IL)-6, tumor necrosis factor (TNF)-α, their receptors, and monocyte chemoattractant protein (MCP)-1, and cell adhesion molecules including intracellular adhesion molecule (ICAM)-1 and vascular cell adhesion molecule (VCAM)-1, have been positively associated with CVD risk (Citation4–11). In contrast, some cytokines have anti-inflammatory and anti-atherogenic properties, such as adiponectin and IL-10 (Citation12). Imbalance or overactivation of inflammatory pathways may contribute to the pathogenesis of chronic disease. For example, the abnormal recruitment and migration of inflammatory cells (e.g., monocytes, leukocytes, T-cells, macrophages) in the vascular endothelium can, under certain conditions, contribute to the cascade of events leading to atherosclerosis (Citation12).
A number of physiological and environmental factors are known to influence an individual’s inflammatory state and chronic disease risk, with diet being a critical modifiable factor (Citation13). In support of this concept, the Mediterranean Diet has been shown to decrease markers of inflammation (Citation14), while diets high in trans-fat or added sugars reportedly increase inflammation (Citation15,Citation16). Similarly, high intakes of saturated fat have been associated with inflammatory biomarkers in overweight subjects (Citation17,Citation18).
Dairy products are integral components of healthy dietary patterns, such as the Dietary Approaches to Stop Hypertension (DASH) and the 2015-2020 Dietary Guidelines for Americans (DGA) (Citation19,Citation20). The 2015-2020 DGA recommends that children over 9 years of age and adults consume three cup equivalents of low- or fat-free dairy products each day. However, most Americans (>2 years of age) do not meet dairy food recommendations, consuming, on average less than two cups dairy food equivalents per day (Citation19,Citation21–25). Dairy products are a major contributor of several nutrients including calcium, vitamin D, riboflavin, vitamin B12, protein, potassium, zinc, choline, magnesium, and selenium (Citation26,Citation27). Moreover, dairy products are the primary food source for three of the four nutrients of public health concern due to underconsumption (calcium, potassium, and vitamin D) as identified by the 2015 Dietary Guidelines Advisory Committee (Citation27–29). The consumption of dairy products has been attributed to the maintenance of bone health and inversely associated with a lower risk of CVD, type 2 diabetes, and metabolic syndrome (Citation30–34).
Despite being associated with reduced chronic disease risk, dairy products are often considered among foods that are associated with inflammation, most likely due to the saturated fat and lactose content of certain dairy products. Several cross-sectional studies suggest an inverse relationship between dairy product consumption and systemic inflammation (Citation35–37). While few studies have primarily examined the link between dairy and inflammation, the current evidence suggests either neutral or anti-inflammatory effects of dairy product consumption (Citation38–40). However, the role of dairy proteins on inflammation is unclear. Thus, given (i) the importance of dairy product consumption in helping achieve nutrient adequacy, (ii) the association of dairy product intake with reduced chronic disease risk, and (iii) the role of inflammation in chronic disease risk, the purpose of this study was to conduct an updated systematic review of literature, in accordance with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement (Citation41,Citation42), to evaluate the impact of both dairy product and dairy protein consumption on low-grade systemic inflammation.
Materials and methods
This systematic review was conducted in accordance with the PRISMA statement, including relevant PRISMA checklist items (see Supplemental data) (Citation41,Citation42) and for the field of nutrition (Citation41–44). An unpublished review protocol was developed and refined by all investigators prior to implementing the search strategy and reviewing the records returned. The review was registered on the International Prospective Register of Systematic Reviews (PROSPERO) as CRD42019129639.
Literature search
The comprehensive literature search was originally conducted up to December 21, 2018, and updated September 19, 2019, by one author (KMN) using two independent databases (PubMed and Cochrane Controlled Register of Trials [CENTRAL]) for relevant studies. The search term strategy included the following terms:
Dairy product/protein terms: yogurt, yoghurt, yoghourt, yogourt, yogurt, cheese, milk, dairy, milk protein, whey, casein;
Inflammation terms: inflammation, inflammatory marker, c-reactive protein, cytokine, TNF-α, tumor necrosis factor, IL-6, interleukin;
Excluded terms: pregnant, pregnancy, lactating, breast milk, human milk.
Human, clinical trials, and best match filters were applied during the PubMed search. No restrictions on publication date were imposed. The identification of studies eligible for review was performed independently by two authors (BDA and KMN) by scanning titles and abstracts using Abstrackr (Citation45), in addition to reviewing reference lists from relevant review articles (Citation38–40,Citation46–48).
Study eligibility criteria
Potentially relevant studies were exported from Abstrackr and full-text articles obtained and independently investigated by two scientists (BDA and KMN). Any disagreements were resolved by discussion, and further disagreements were resolved by a third scientist (CJC). The review included randomized controlled trials (RCT) and observational studies published in English that evaluated the effects of dairy product and/or dairy protein consumption on systemic inflammation biomarker levels. The population of interest included male and female apparently healthy adults, as described by the authors, and non-healthy adults who had a disease diagnosis which included hypercholesterolemia, hypertension, metabolic syndrome, and type 2 diabetes in the identified studies, 18 years of age, and without any diagnosis of severe inflammatory-related disorders (e.g., cancer, Crohn’s disease, rheumatoid arthritis, lupus, multiple sclerosis; ). Additionally, to be considered inclusionary, studies included dairy products (milk, yogurt, cheese) or proteins as the intervention, not solely measured as part of a dietary pattern, with intervention duration of at least 2 weeks. Studies were excluded with the following characteristics: pregnant or lactating women; human milk or non-bovine milk intervention; interventions containing only butter, cream, or ice cream; studies without an appropriate nondairy or low-dairy control group; and/or studies that did not assess an inflammatory biomarker.
Table 1. PICOS Table for Inclusion of Studies
Abstraction of data
Data were extracted from eligible studies by two scientists (KMN and BDA). Each scientist extracted data from 50% of the studies and reviewed the remaining 50% of the data extracted by the other. Twenty-eight manuscripts met inclusion criteria for extraction of data.
Data extracted from eligible studies included the following:
General information – title, authors, journal, year of publication;
Study design – country of origin, population (healthy or unhealthy); disease/condition (if unhealthy), trial type, blinding, arms, primary outcome, secondary outcome(s);
Participant characteristics – sex, age, body weight, body mass index (BMI), sample size (randomized, evaluable, male, and female);
Intervention – dairy product assignment (control or active), intervention (dairy product or dairy protein), comparator, intervention form, intervention dose, comparator dose, dairy product type, intervention energy content, comparator energy content, intervention protein content, comparator protein content, intervention duration, washout duration;
Results – sample type, results summary (difference in means);
Summary – conclusions, strengths, and limitations.
Assessment of methodological quality
To assess the risk of bias in, and quality of, individual studies, the Academy of Nutrition and Dietetics Quality Criteria Checklist was used (Citation49). The assessment tool provided several domains (inclusion/exclusion, bias, generalizability, and data collection and analysis) where potential bias could arise based on specific study designs. The authors made a judgment of the potential bias and its severity for each domain and concluded with an overall judgment rating. This process was conducted independently by two scientists (BDA and KMN), and disagreements were resolved by conferring with a third scientist (CJC). Complete results of the quality analysis can be found in the .
Results
Study selection
The initial database search retrieved 451 research articles and additional 272 articles were identified through reviewing reference lists of relevant reviews (). After duplicate articles were removed, 691 article titles and abstracts were screened of which 625 were excluded that did not meet eligibility criteria. Full-text articles were retrieved for 67 titles which were reviewed in detail resulting in identification of a total of 28 studies (27 RCT [ and ] and 1 cross-sectional study [Citation36]) for inclusion. Given the limited return of observational evidence, the remainder of the review will focus on summarizing the RCT identified in the search.
Figure 1. Flow diagram of the literature search and study selection conducted according to the PRISMA guidelines statement (41). Abbreviations: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RCT, randomized controlled trials.
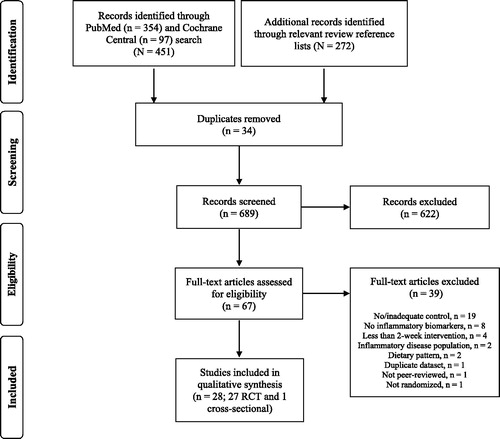
Table 2. Randomized Controlled Trials evaluating Inflammatory Biomarkers in response to a Dairy Product Intervention
Table 3. Randomized Controlled Trials evaluating Inflammatory Biomarkers in response to a Dairy Protein Intervention
Study characteristics and quality
The results from the 27 RCT are separated by those trials which evaluated the effects of dairy products (, n = 19) and dairy proteins (, n = 8) on markers of inflammation. Within each table, the studies are grouped by the study population: healthy, overweight/obese but otherwise healthy, or overweight/obese subjects with chronic disease (e.g., metabolic syndrome or type 2 diabetes). In addition, the tables include the sample size, age, trial design, intervention dose and duration, primary outcome, results for inflammatory biomarkers, and the study quality rating. Nearly, 50% of the RCT received a neutral rating (n = 14) and the remaining received a positive rating (n = 13) according to the Academy of Nutrition and Dietetics criteria (Citation49) (). There was an inherent lack of description of the method of randomization, statistical methods employed, and blinding. In many of these studies, it was not possible to employ a double-blind design due to the form of the interventions; however, the type of blinding or lack thereof was not always made clear.
Dairy product consumption in healthy adults
Two trials evaluated the effect of dairy product consumption in healthy adults on markers of inflammation (), one of which included both male and female participants (n = 176) (Citation50) and the other only female participants (n = 120) (Citation51). The first trial evaluated the effects of 2–3 servings vs 0 servings of full-fat dairy products each day over a month, while the second evaluated low-fat yogurt vs soy pudding consumption over 9 weeks. Neither study was designed with an inflammatory marker as the primary outcome. Benatar et al. (Citation50) reported no differences in CRP and tumor necrosis factor (TNF)-α receptor II (TNF-RII). In contrast, Pei et al. (Citation51) reported a significant decreased in TNF-α alone (p = 0.0219) and the TNF-α/TNF-RII ratio (p = 0.0013) following low-fat yogurt consumption, relative to the soy pudding control.
Dairy product consumption in overweight and obese but otherwise healthy adults
A total of 13 trials evaluated the effect of dairy product consumption on inflammation in overweight and obese but otherwise healthy adults (). Ten of these trials included both male and female participants with sample sizes ranging from 18 to 112 participants. The remaining three trials (Citation52–54) included only female participants and sample sizes of 31, 27, and 69, respectively. Six studies evaluated the effects of milk relative to an isocaloric beverage or no milk (Citation52–57). One study evaluated both milk and yogurt relative to isocaloric quantities of fruit juice and biscuits (Citation58,Citation59). The remaining six studies evaluating the effects of dairy servings relative to a lesser number or no servings of dairy each day. The trials ranged in duration from 28 days to 6 months and in all but two of the trials, the primary outcome was not reported or not an inflammatory biomarker.
A majority of the trials reported no significant differences in CRP, cytokines, or other inflammatory markers. Bruun et al. (Citation56) reported a significant decrease in uric acid following 6 months of low-fat milk consumption at 1 L/day relative to cola (p = 0.009). Labonte et al. (Citation60) reported decreased CRP relative to baseline in control group, and the decrease from baseline was significantly greater in the control group than the dairy group which included low- and full-fat dairy products (p = 0.04). Van Meijl et al. (Citation58,Citation59) reported no effect of low-fat dairy product consumption on CRP, IL-6, TNF-α, TNF-RI, and MCP-1 but increased TNF-RII (p = 0.020), and decreased TNF-α index (p = 0.015) which may suggest lower biological availability of TNF-α. Finally, three trials by Zemel et al. (Citation57,Citation61–63) showed decreased CRP following consumption of three servings of fat-free and low-fat dairy products vs ≤1 serving dairy/day or three servings of soy for 28 days to 24 weeks (p < 0.05 for all). In addition, decreased TNF-α, MCP-1, and IL-6, and increased adiponectin were reported in one of the trials (p < 0.01 for all) (Citation57).
Dairy product consumption in unhealthy, overweight, and obese adults
Four trials evaluated the effects of dairy product consumption in unhealthy, overweight, and obese participants. In three of these trials (Citation64–67), participants met the criteria for metabolic syndrome (n = 33, 40, and 113, respectively) and were provided 3–5 servings of dairy products per day, relative to the equivalent nondairy products or a lesser quantity of dairy product servings per day. In the fourth trial, participants had been diagnosed with type 2 diabetes (n = 25) and were provided 240 mL/day milk or soy milk (Citation68). All trials included both male and female participants, and two of the trials (Citation66,Citation68) were designed using an inflammatory biomarker as the primary outcome. Three of the four trials reported no significant differences between dairy product (fat-free, low-fat, reduced-fat, and full-fat) intake and control in CRP, cytokines, or other inflammatory markers (Citation64,Citation65,Citation67,Citation68). Stancliffe et al. (Citation66) reported decreased CRP, IL-6, TNF-α, and MCP-1, and increased adiponectin following 84 days of 3 dairy product servings daily, relative to nondairy products (p < 0.02 for all).
Dairy protein consumption in healthy adults
Two trials evaluated the effects of dairy protein consumption in healthy adults, the first of which included both male and female participants (n = 20) (Citation69), while the second included only female participants (n = 28). Neither was designed using an inflammatory biomarker as the primary outcome. Ballard et al. (Citation69) evaluated the effects of 5 g/day whey protein for 2 weeks, relative to a non-nutritive sweetener, and reported no changes in CRP, cytokines (IL-6, IL-8, TNF-α), or other inflammatory biomarkers (ICAM, VCAM, MCP-1). Steinberg et al. (Citation70) evaluated the effects of 25 g/day milk protein for 6 weeks, relative to soy, and reported no significant differences in any of the inflammatory biomarkers (ICAM, VCAM, nor nitric oxide products) assessed as well.
Dairy protein consumption in overweight and obese but otherwise healthy adults
A total of three trials evaluated the effects of daily dairy protein intake in overweight or obese but otherwise healthy adults, two of which included only female subjects (n = 35 and 34, respectively) (Citation71,Citation72), and the third trial included both male and female participants (n = 72) (Citation73). None of the trials were designed using an inflammatory biomarker as the primary outcome. The trials provided 26–67 g/day casein or milk protein for 6–16 weeks or soy protein and no significant differences were reported in any of the inflammatory biomarkers assessed (CRP, IL-6, ICAM, VCAM, homocysteine, and adiponectin).
Dairy protein consumption in unhealthy, overweight, and obese adults
Three trials evaluated the effects of dairy protein intake (casein or whey) in overweight and obese participants with hypercholesterolemia (Citation74,Citation75) or hypertension (Citation76), relative to a nondairy protein (Citation74,Citation75) or no protein (Citation76) control (n = 22–38). None of these trials were designed using an inflammatory biomarker as the primary outcome variable. After 4–12 weeks of supplementation, no significant differences were reported in any of the inflammatory biomarkers assessed (CRP, IL-6, ICAM, nor VCAM).
Discussion
Systemic inflammation contributes to the risk and progression of chronic disease, which is in turn influenced by a number of factors including diet (Citation13). This systematic review evaluated the effects of dairy product or dairy protein interventions on markers of inflammation. Overall, the results of this review show that the consumption of dairy products has no adverse effects and potentially beneficial effects and dairy proteins have no adverse effects on systemic inflammation. Additionally, the results indicate that the beneficial effects were most commonly reported in trials that evaluated overweight/obese populations with an average age ∼42 years. Specifically, of the 8 studies (Citation51,Citation56–58,Citation61,Citation62,Citation64,Citation66) that reported beneficial findings, 7 were in overweight/obese populations with mean ages of ∼39, 50, 41, 42, 31, 54, and 37 years, respectively. The differences in results between trials reporting beneficial and neutral effects could potentially be a result of less variability in or higher baseline systemic inflammation in the participants evaluated (Citation77).
To our knowledge, three systematic reviews (Citation38–40) have previously been completed evaluating the effects of dairy product consumption on inflammation, none of which evaluated the effect of dairy proteins. Labonte et al. (Citation38) completed their systematic search in 2012 and included eight trials in overweight and obese adults, all of which were included in this study. The authors similarly concluded that dairy product intake did not result in adverse effects on markers of inflammation. Bordoni et al. (Citation39) completed their extensively inclusive systematic search in 2013 and included 52 clinical studies. Studies accumulated additional points based on study characteristics (e.g. intervention type and duration, design, number of inflammatory markers changed). Based on this approach, the authors concluded that dairy products are anti-inflammatory, specifically in interventions with fermented dairy products or in trials which evaluated subjects with metabolic disorders. It is difficult to directly compare this review to this study as the authors created a scoring system (“inflammatory score”) based on the net change in inflammatory markers (null, positive, or negative). Finally, the most recent review by Ulven et al. (Citation40) was completed in 2018 and included 16 trials, 4 of which overlap with this study (Citation51,Citation55,Citation60,Citation64). The authors concluded that most studies did suggest dairy product consumption led to anti-inflammatory effects in healthy and metabolically abnormal subjects. Thus, there is consistency among the systematic reviews completed to date showing a lack of association between dairy product consumption and systemic inflammation, and in some circumstances dairy consumption may be associated with reduced inflammation.
A unique contribution of this work is the review of the relationship between dairy protein consumption and inflammation. Some studies have suggested that animal protein intake is associated with increased CVD and mortality (Citation78–80). For example, Tharrey et al. (Citation79) examined data from the Adventist Health Study-2 cohort and reported that “Meat” protein was associated with an increased hazard ratio for cardiovascular mortality. We identified eight trials which evaluated the effects of dairy proteins on markers of inflammation. Seven of the eight trials evaluated CRP, and all reported no effect of the intervention. Similarly, in the three trials that evaluated inflammatory-related cytokines and six trials that evaluated other inflammatory markers, there was no effect of the dairy protein intervention. Accordingly, the evidence reviewed suggests that dairy protein consumption is not linked with inflammation.
According to the 2015-2020 DGA, American Heart Association, and American College of Cardiology, dairy foods such as low-fat milk, cheese, and yogurt are integral components in healthy eating patterns and specifically for reduction of low-density lipoprotein cholesterol and blood pressure (Citation19,Citation81,Citation82). However, dairy products are often considered among foods that are associated with increased inflammation, mostly due to the saturated fat content (Citation83). A systematic review by Telle-Hansen et al. (Citation84) of 37 RCT suggests minor or no effects of dietary fat intake on inflammatory markers in overweight/obese subjects. Emerging evidence indicates that dairy product consumption is linked to lower risk for CVD and metabolic syndrome, and the lack of detrimental effects from intake of saturated fat can be attributed to the heterogeneity of saturated fatty acids unique to the dairy food matrix (Citation85). Specifically, a systematic review by Drouin-Chartier et al. (Citation34) concluded that neither total dairy product nor cheese consumption was associated with higher risk for coronary artery disease or CVD, and total dairy product and cheese intake were associated with lower stroke risk. Similarly, in a crossover RCT, healthy participants (>21 years of age), who consumed a modified, high-dairy fat, DASH dietary pattern for three weeks, showed similar blood pressure lowering effects, but in addition reduced very-low-density lipoprotein cholesterol and triglycerides, as compared to the standard DASH dietary pattern (Citation86). Specific to inflammation, Byrd et al. (Citation87) recently published novel dietary and lifestyle inflammation scores. Both high-fat and low-fat dairy had negative weights and, as such, were associated with lower dietary inflammation scores in this analysis. A complete mechanistic understanding of the role of dairy foods and vascular function and ultimately cardiovascular risk in humans is lacking. Studies in vitro and in vivo suggest dairy foods reportedly improve vascular function regardless of blood pressure-lowering effect by reducing oxidative status (Citation88). In addition, inclusion of dairy cheese in an 8-day high-sodium diet prevents vascular dysfunction in older adults by decreasing oxidative stress suggesting the dairy matrix and fat protect the vasculature from the effects of sodium (Citation89).
Components of dairy product matrix such as vitamin D, calcium, protein, live and active cultures in fermented dairy, and bioactive peptides appear to suppress the inflammatory response (Citation63,Citation88,Citation90) and may ultimately have vascular effects. Dairy foods may regulate immune function within the GI tract by interacting with the mucosal layer, improving intestinal barrier function, and stimulating immunocytes which can in turn affect cardiovascular health, for example, through flux of metabolites into the bloodstream (Citation88). Further, in vitro and in vivo studies suggest dairy components may beneficially modulate immune function in the GI tract via reducing lipopolysaccharide activity, Gram-negative bacteria, and bacterial translocation, increasing tight junction proteins, and improving barrier function (Citation88). In support of this, supplementation of fermented milk for 2 weeks at 400 g/day resulted in altered gut microbiota and microbial metabolites that improve barrier function in healthy men (Citation91–93). Taken together, these findings suggest the dairy food matrix may modulate the effects of dairy fat on chronic disease risk (Citation94). This notion is supported in this study where various dairy products types (fat-free, low-fat, reduced-fat, and full-fat) were utilized in the 19 trials that evaluated the effects of dairy product consumption on markers of inflammation and showed neutral to beneficial effects. This agrees with results from other studies suggesting the Mediterranean and DASH dietary patterns, which incorporate dairy products, are associated with reduced inflammation (Citation95,Citation96).
This study identified important knowledge gaps that need to be addressed in future studies. First, the majority of studies included in this systematic review were not designed using an inflammatory marker as the primary outcome and, in turn, baseline systemic inflammation of trial participants was not commonly considered in the trial design. Only four of the 27 trials reported design and completion of sample size calculations using variability associated with an inflammatory biomarker (Citation52,Citation60,Citation66,Citation68). Of these four trials, two reported no significant differences in any of the inflammatory biomarkers assessed (CRP, IL-6, TNF-α, IL-1β) (Citation52,Citation68) and two trials reported significant changes in inflammatory biomarkers including CRP (Citation60) and CRP, IL-6, TNF-α, MCP-1, and adiponectin, relative to controls (Citation66). Further, 16 of the 27 RCT were specifically designed using non-inflammatory-related outcomes as the primary outcome variable; thus, a majority of the trials included in the review may be insufficiently powered to detect differences in inflammatory biomarkers.
A second gap identified among the trials is the lack of consistency in which biomarkers of inflammation were measured. A majority of papers evaluated CRP or one or more other circulating inflammatory-related cytokines. However, some studies included cellular markers of inflammation and/or markers of tissue infiltration. This lack of consistency makes comparison across studies difficult and limits the generalizability of the results. Further, future studies should use appropriate controls to allow for a more robust understanding of the impacts of dairy foods on inflammation. For example, in several of the studies the difference between the dairy product or dairy protein interventions and the control may not have been large enough to induce change (Citation67,Citation69,Citation76,Citation97). Additionally, the control intervention employed in each study varied and included controls that did not match the macronutrient composition of dairy. Due to the complex nature of inflammation, completion of additional well-controlled clinical trials using inflammatory biomarkers as the primary outcome and systematic reviews with a consistent methodology is warranted (Citation98–100).
Additional methodological gaps include controlling for sex and intervention length. To the best of our knowledge, 21 trials evaluated both male and female populations and although 9 of these note controlling for sex in the statistical model (Citation36,Citation56,Citation60,Citation66–69,Citation75,Citation101), many of these trials do not report controlling for sex or report results by sex with the exception of the trial by Dugan et al. (Citation64,Citation65). As there is insufficient evidence to suggest males and females would respond similarly, analysis by sex in future studies would be ideal as well as longer-term interventions to allow generalizability. Of the eight studies (Citation51,Citation56–58,Citation61,Citation62,Citation64,Citation66) that reported beneficial findings, intervention lengths ranged from 28 days to 24 weeks. Thus, it is possible that treatment durations of <28 days (Citation69) were insufficient at inducing an effect. Finally, there seems to be a lack of observational studies on the relationship between dairy products or dairy proteins and inflammation, as only one observational study was identified in our search. The cross-sectional study by Panagiotakos et al. (Citation36) evaluated over 3000 overweight participants who consumed <8, 8–11, 11–14, or >14 dairy product servings/week and concluded dairy product consumption was inversely associated with inflammatory biomarker levels including CRP, IL-6, and TNF-α. Although other observational studies were identified in our search, they were subsequently excluded due to the lack of an appropriate control or relevant outcomes, thus more adequately designed observational studies using inflammatory markers as the primary outcome, could help to eliminate this knowledge gap.
Strengths of this systematic review include using the appropriate methodology for conducting nutrition-related systematic reviews and ensuring that only relevant studies were included (Citation41–44). In addition, the inclusion of studies examining the effects of dairy proteins on biomarkers of inflammation was, to our knowledge, a novel aspect to this review. Finally, we did not impose any restrictions on publication date in our search strategy to allow for a more thorough search. Limitations of this review include restricting our search strategy to two databases and inclusion of trials published in English only, which could have resulted in overlooked eligible studies. We attempted to limit overlooked trials by reviewing reference lists from relevant review articles (Citation38–40,Citation46–48). In addition, we did not conduct a quantitative analysis; however, the systematic review by Bordoni et al. (Citation39) and the recent validation study by Byrd et al. (Citation87), which both utilized inflammatory scores, came to similar conclusions. Lastly, we did not include studies that examined the role of dairy on inflammatory biomarkers in subjects with inflammatory disorders, limiting the generalizability of the findings.
The preponderance of the evidence shows that consumption of dairy products or dairy proteins does not adversely affect biomarkers of inflammation in healthy and overweight or obese individuals and potentially provides beneficial effects. The results of this study provide additional support for the role of dairy product consumption in reducing chronic disease risk. Further, research is warranted specifically on adequately and consistently designed trials and subsequent systematic review.
Author’s contributions
KMN, BDA, and CJC designed the study and wrote the manuscript. KMN developed the search strategy and conducted the search. KMN and BDA reviewed abstracts and full-text articles and completed the data extraction and risk of bias assessment. All authors read and approved the final manuscript.
Supplemental Material
Download MS Word (66 KB)Supplemental Material
Download MS Excel (119.1 KB)Disclosure statements
KMN and BDA have no relevant interests to declare. CJC is currently employed by the National Dairy Council.
Additional information
Funding
References
- Libby P, Okamoto Y, Rocha VZ, Folco E. Inflammation in atherosclerosis: transition from theory to practice. Circ J. 2010;74(2):213–20. doi:10.1253/circj.cj-09-0706.
- Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract. 2014;105(2):141–50. doi:10.1016/j.diabres.2014.04.006.
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–7. doi:10.1038/nature05485.
- Mora S, Ridker PM. Justification for the use of statins in primary prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER)-can C-reactive protein be used to target statin therapy in primary prevention? Am J Cardiol. 2006;97(2A):33A–41a. doi:10.1016/j.amjcard.2005.11.014.
- Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347(20):1557–65. doi:10.1056/NEJMoa021993.
- Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101(15):1767–72. doi:10.1161/01.CIR.101.15.1767.
- Cortez-Cooper M, Meaders E, Stallings J, Haddow S, Kraj B, Sloan G, McCully KK, Cannon JG. Soluble TNF and IL-6 receptors: indicators of vascular health in women without cardiovascular disease. Vasc Med. 2013;18(5):282–9. doi:10.1177/1358863x13508336.
- Tuomisto K, Jousilahti P, Sundvall J, Pajunen P, Salomaa V. C-reactive protein, interleukin-6 and tumor necrosis factor alpha as predictors of incident coronary and cardiovascular events and total mortality. A population-based, prospective study. Thromb Haemost. 2006;95(3):511–8. doi:10.1160/th05-08-0571.
- Basurto L, Gregory MA, Hernández SB, Sánchez-Huerta L, Martínez AD, Manuel-Apolinar L, Avelar FJ, Alonso LAM, Sánchez-Arenas R. Monocyte chemoattractant protein-1 (MCP-1) and fibroblast growth factor-21 (FGF-21) as biomarkers of subclinical atherosclerosis in women. Exp Gerontol. 2019;124:110624. doi:10.1016/j.exger.2019.05.013.
- Demerath E, Towne B, Blangero J, Siervogel RM. The relationship of soluble ICAM-1, VCAM-1, P-selectin and E-selectin to cardiovascular disease risk factors in healthy men and women. Ann Hum Biol. 2001;28(6):664–78. doi:10.1080/03014460110048530.
- Kunutsor SK, Bakker SJL, Dullaart RPF. Soluble vascular cell adhesion molecules may be protective of future cardiovascular disease risk: findings from the PREVEND prospective cohort study. J Atheroscler Thromb. 2017;24(8):804–18. doi:10.5551/jat.38836.
- Zernecke A, Weber C. Inflammatory mediators in atherosclerotic vascular disease. Basic Res Cardiol. 2005;100(2):93–101. doi:10.1007/s00395-005-0511-6.
- Calder PC, Ahluwalia N, Brouns F, Buetler T, Clement K, Cunningham K, et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br J Nutr. 2011;106 Suppl 3 (Suppl 3S):S5–S78. doi:10.1017/s0007114511005460.
- Mayr HL, Tierney AC, Thomas CJ, Ruiz-Canela M, Radcliffe J, Itsiopoulos C. Mediterranean-type diets and inflammatory markers in patients with coronary heart disease: a systematic review and meta-analysis. Nutr Res. 2018; 50:10–24. doi:10.1016/j.nutres.2017.10.014.
- Koebnick C, Black MH, Wu J, Shu YH, MacKay AW, Watanabe RM, Buchanan TA, Xiang AH. A diet high in sugar-sweetened beverage and low in fruits and vegetables is associated with adiposity and a pro-inflammatory adipokine profile. Br J Nutr. 2018;120(11):1230–9. doi:10.1017/s0007114518002726.
- Borst SE, Conover CF. High-fat diet induces increased tissue expression of TNF-alpha. Life Sci. 2005;77(17):2156–65. doi:10.1016/j.lfs.2005.03.021.
- Fernandez-Real JM, Broch M, Vendrell J, Ricart W. Insulin resistance, inflammation, and serum fatty acid composition. Diabetes Care. 2003;26(5):1362–8. doi:10.2337/diacare.26.5.1362.
- Klein-Platat C, Drai J, Oujaa M, Schlienger JL, Simon C. Plasma fatty acid composition is associated with the metabolic syndrome and low-grade inflammation in overweight adolescents. Am J Clin Nutr. 2005;82(6):1178–84. doi:10.1093/ajcn/82.6.1178.
- U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2015 – 2020 Dietary Guidelines for Americans. 8th ed. December 2015. Available from: https://health.gov/dietaryguidelines/2015/guidelines/.
- Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336(16):1117–24. doi:10.1056/NEJM199704173361601.
- U.S. Department of Agriculture, Agricultural Research Service, Beltsville Human Nutrition Research Center, Food Surveys Research Group (Beltsville, MD) and U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics (Hyattsville, MD). What We Eat in America, NHANES 2015-2016. Available from: https://data.nal.usda.gov/dataset/what-we-eat-america-wweia-database.
- Su C, Zhang B, Wang H, Wang Z, Zhang J, Jiang H, Jia X, Huang F. Milk consumption and effects on dietary calcium among Chinese aged 45 and above in 15 provinces, 2015. Wei Sheng Yan Jiu = J. Hyg. Res. 2018;47(2):194–8.
- Vandevijvere S, De Vriese S, Huybrechts I, Moreau M, Temme E, De Henauw S, De Backer G, Kornitzer M, Leveque A, Van Oyen H. The gap between food-based dietary guidelines and usual food consumption in Belgium, 2004. Public Health Nutr. 2009;12(3):423–31. doi:10.1017/s1368980008002164.
- Garriguet D. Canadians' eating habits. Health Rep. 2007;18(2):17–32.
- Doidge JC, Segal L. Most Australians do not meet recommendations for dairy consumption: findings of a new technique to analyse nutrition surveys. Aust N Z J Public Health. 2012;36(3):236–40. doi:10.1111/j.1753-6405.2012.00870.x.
- National Dairy Council. NHANES 2011-2014. Data Source: Centers for Disease Control and Prevention, National Center for Health Statistics, and National Health and Nutrition Examination Survey Data. Hyattsville, MD: U.S. Department of Health and Human Services. Available from: http://www.cdc.gov/nchs/nhanes.htm.
- U.S. Department of Health and Human Services and U.S. Department of Agriculture (USDA). Scientific Report of the 2015 Dietary Guidelines Advisory Committee. February 2015. Available from: http://health.gov/dietaryguidelines/2015-scientific-report/.
- Keast DR, Fulgoni VL, 3rd, Nicklas TA, O'Neil CE. Food sources of energy and nutrients among children in the United States: National Health and Nutrition Examination Survey 2003–2006. Nutrients. 2013;5(1):283–301. doi:10.3390/nu5010283.
- O'Neil CE, Keast DR, Fulgoni VL, Nicklas TA. Food sources of energy and nutrients among adults in the US: NHANES 2003–2006. Nutrients. 2012;4(12):2097–120. doi:10.3390/nu4122097.
- Rizzoli R. Dairy products, yogurts, and bone health. Am J Clin Nutr. 2014;99(5 Suppl):1256S–62s. doi:10.3945/ajcn.113.073056.
- Bonjour JP, Kraenzlin M, Levasseur R, Warren M, Whiting S. Dairy in adulthood: from foods to nutrient interactions on bone and skeletal muscle health. J Am Coll Nutr. 2013;32(4):251–63. doi:10.1080/07315724.2013.816604.
- Gil A, Ortega RM. Introduction and executive summary of the supplement, role of milk and dairy products in health and prevention of noncommunicable chronic diseases: a series of systematic reviews. Adv Nutr. 2019;10(suppl_2):S67–s73. doi:10.1093/advances/nmz020.
- Fontecha J, Calvo MV, Juarez M, Gil A, Martinez-Vizcaino V. Milk and dairy product consumption and cardiovascular diseases: an overview of systematic reviews and meta-analyses. Adv Nutr. 2019;10(suppl_2):S164–s189. doi:10.1093/advances/nmy099.
- Drouin-Chartier JP, Brassard D, Tessier-Grenier M, Cote JA, Labonte ME, Desroches S, Couture P, Lamarche B. Systematic review of the association between dairy product consumption and risk of cardiovascular-related clinical outcomes. Adv Nutr. 2016;7(6):1026–40. doi:10.3945/an.115.011403.
- Esmaillzadeh A, Azadbakht L. Dairy consumption and circulating levels of inflammatory markers among Iranian women. Public Health Nutr. 2010;13(9):1395–402. doi:10.1017/s1368980009992126.
- Panagiotakos DB, Pitsavos CH, Zampelas AD, Chrysohoou CA, Stefanadis CI. Dairy products consumption is associated with decreased levels of inflammatory markers related to cardiovascular disease in apparently healthy adults: the ATTICA study. J Am Coll Nutr. 2010;29(4):357–64. doi:10.1080/07315724.2010.10719852.
- Salas-Salvado J, Garcia-Arellano A, Estruch R, Marquez-Sandoval F, Corella D, Fiol M, et al. Components of the Mediterranean-type food pattern and serum inflammatory markers among patients at high risk for cardiovascular disease. Eur J Clin Nutr. 2008;62(5):651–9. doi:10.1038/sj.ejcn.1602762.
- Labonte ME, Couture P, Richard C, Desroches S, Lamarche B. Impact of dairy products on biomarkers of inflammation: a systematic review of randomized controlled nutritional intervention studies in overweight and obese adults. Am J Clin Nutr. 2013;97(4):706–17. doi:10.3945/ajcn.112.052217.
- Bordoni A, Danesi F, Dardevet D, Dupont D, Fernandez AS, Gille D, et al. Dairy products and inflammation: a review of the clinical evidence. Crit Rev Food Sci Nutr. 2017;57(12):2497–525. doi:10.1080/10408398.2014.967385.
- Ulven SM, Holven KB, Gil A, Rangel-Huerta OD. Milk and dairy product consumption and inflammatory biomarkers: an updated systematic review of randomized clinical trials. Adv Nutr. 2019;10(suppl_2):S239–s250. doi:10.1093/advances/nmy072.
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi:10.1371/journal.pmed.1000097.
- Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA, PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi:10.1186/2046-4053-4-1.
- Lichtenstein AH, Yetley EA, Lau J. Application of systematic review methodology to the field of nutrition: nutritional research series. Vol. 1. AHRQ technical reviews. Rockville (MD): Agency for Healthcare Research and Quality (US); 2009.
- Lichtenstein AH, Yetley EA, Lau J. Application of systematic review methodology to the field of nutrition. J Nutr. 2008;138(12):2297–306. doi:10.3945/jn.108.097154.
- Wallace BC, Trikalinos TA, Lau J, Brodley C, Schmid CH. Semi-automated screening of biomedical citations for systematic reviews. BMC Bioinformatics. 2010; 11:55. doi:10.1186/1471-2105-11-55.
- Da Silva MS, Rudkowska I. Dairy products on metabolic health: current research and clinical implications. Maturitas. 2014;77(3):221–8. doi:10.1016/j.maturitas.2013.12.007.
- Drouin-Chartier JP, Cote JA, Labonte ME, Brassard D, Tessier-Grenier M, Desroches S, Couture P, Lamarche B. Comprehensive review of the impact of dairy foods and dairy fat on cardiometabolic risk. Adv Nutr. 2016;7(6):1041–51. doi:10.3945/an.115.011619.
- Lamarche B. Review of the effect of dairy products on non-lipid risk factors for cardiovascular disease. J Am Coll Nutr. 2008;27(6):741S–6s. doi:10.1080/07315724.2008.10719752.
- Academy of Nutrition and Dietetics. Evidence analysis manual: steps in the Academy Evidence Analysis Process. Appendix 8: Quality criteria checklist - primary research. Chicago (IL): Academy of Nutrition and Dietetics; 2016.
- Benatar JR, Jones E, White H, Stewart RA. A randomized trial evaluating the effects of change in dairy food consumption on cardio-metabolic risk factors. Eur J Prev Cardiolog. 2014;21(11):1376–86. doi:10.1177/2047487313493567.
- Pei R, DiMarco DM, Putt KK, Martin DA, Gu Q, Chitchumroonchokchai C, White HM, Scarlett CO, Bruno RS, Bolling BW. Low-fat yogurt consumption reduces biomarkers of chronic inflammation and inhibits markers of endotoxin exposure in healthy premenopausal women: a randomised controlled trial. Br J Nutr. 2017;118(12):1043–51. doi:10.1017/s0007114517003038.
- Beavers KM, Serra MC, Beavers DP, Cooke MB, Willoughby DS. Soymilk supplementation does not alter plasma markers of inflammation and oxidative stress in postmenopausal women. Nutr Res. 2009;29(9):616–22. doi:10.1016/j.nutres.2009.09.002.
- Drouin-Chartier JP, Gagnon J, Labonte ME, Desroches S, Charest A, Grenier G, Dodin S, Lemieux S, Couture P, Lamarche B. Impact of milk consumption on cardiometabolic risk in postmenopausal women with abdominal obesity. Nutr J. 2015; 14:12. doi:10.1186/1475-2891-14-12.
- Rosado JL, Garcia OP, Ronquillo D, Hervert HD, Caamano MC, Martinez G, Gutierrez J, Garcia S. Intake of milk with added micronutrients increases the effectiveness of an energy-restricted diet to reduce body weight: a randomized controlled clinical trial in Mexican women. J Am Diet Assoc. 2011;111(10):1507–16. doi:10.1016/j.jada.2011.07.011.
- Gjevestad GO, Ottestad I, Biong AS, Iversen PO, Retterstol K, Raastad T, Skalhegg BS, Ulven SM, Holven KB. Consumption of protein-enriched milk has minor effects on inflammation in older adults-a 12-week double-blind randomized controlled trial. Mech Ageing Dev. 2017; 162:1–8. doi:10.1016/j.mad.2017.01.011.
- Bruun JM, Maersk M, Belza A, Astrup A, Richelsen B. Consumption of sucrose-sweetened soft drinks increases plasma levels of uric acid in overweight and obese subjects: a 6-month randomised controlled trial. Eur J Clin Nutr. 2015;69(8):949–53. doi:10.1038/ejcn.2015.95.
- Zemel MB, Sun X, Sobhani T, Wilson B. Effects of dairy compared with soy on oxidative and inflammatory stress in overweight and obese subjects. Am J Clin Nutr. 2010;91(1):16–22. doi:10.3945/ajcn.2009.28468.
- van Meijl LE, Mensink RP. Low-fat dairy consumption reduces systolic blood pressure, but does not improve other metabolic risk parameters in overweight and obese subjects. Nutr Metab Cardiovasc Dis. 2011;21(5):355–61. doi:10.1016/j.numecd.2009.10.008.
- van Meijl LE, Mensink RP. Effects of low-fat dairy consumption on markers of low-grade systemic inflammation and endothelial function in overweight and obese subjects: an intervention study. Br J Nutr. 2010;104(10):1523–7. doi:10.1017/s0007114510002515.
- Labonte ME, Cyr A, Abdullah MM, Lepine MC, Vohl MC, Jones P, Couture P, Lamarche B. Dairy product consumption has no impact on biomarkers of inflammation among men and women with low-grade systemic inflammation. J Nutr. 2014;144(11):1760–7. doi:10.3945/jn.114.200576.
- Zemel MB, Richards J, Mathis S, Milstead A, Gebhardt L, Silva E. Dairy augmentation of total and central fat loss in obese subjects. Int J Obes. 2005;29(4):391–7. doi:10.1038/sj.ijo.0802880.
- Zemel MB, Richards J, Milstead A, Campbell P. Effects of calcium and dairy on body composition and weight loss in African-American adults. Obes Res. 2005;13(7):1218–25. doi:10.1038/oby.2005.144.
- Zemel MB, Sun X. Dietary calcium and dairy products modulate oxidative and inflammatory stress in mice and humans. J Nutr. 2008;138(6):1047–52. doi:10.1093/jn/138.6.1047.
- Dugan CE, Aguilar D, Park YK, Lee JY, Fernandez ML. Dairy consumption lowers systemic inflammation and liver enzymes in typically low-dairy consumers with clinical characteristics of metabolic syndrome. J Am Coll Nutr. 2016;35(3):255–61. doi:10.1080/07315724.2015.1022637.
- Dugan CE, Barona J, Fernandez ML. Increased dairy consumption differentially improves metabolic syndrome markers in male and female adults. Metab Syndr Relat Disord. 2014;12(1):62–9. doi:10.1089/met.2013.0109.
- Stancliffe RA, Thorpe T, Zemel MB. Dairy attentuates oxidative and inflammatory stress in metabolic syndrome. Am J Clin Nutr. 2011;94(2):422–30. doi:10.3945/ajcn.111.013342.
- Wennersberg MH, Smedman A, Turpeinen AM, Retterstol K, Tengblad S, Lipre E, et al. Dairy products and metabolic effects in overweight men and women: results from a 6-mo intervention study. Am J Clin Nutr. 2009;90(4):960–8. doi:10.3945/ajcn.2009.27664.
- Miraghajani MS, Esmaillzadeh A, Najafabadi MM, Mirlohi M, Azadbakht L. Soy milk consumption, inflammation, coagulation, and oxidative stress among type 2 diabetic patients with nephropathy. Diabetes Care. 2012;35(10):1981–5. doi:10.2337/dc12-0250.
- Ballard KD, Bruno RS, Seip RL, Quann EE, Volk BM, Freidenreich DJ, et al. Acute ingestion of a novel whey-derived peptide improves vascular endothelial responses in healthy individuals: a randomized, placebo controlled trial. Nutr J. 2009;8(1):34. doi:10.1186/1475-2891-8-34.
- Steinberg FM, Guthrie NL, Villablanca AC, Kumar K, Murray MJ. Soy protein with isoflavones has favorable effects on endothelial function that are independent of lipid and antioxidant effects in healthy postmenopausal women. Am J Clin Nutr. 2003;78(1):123–30. doi:10.1093/ajcn/78.1.123.
- Anderson JW, Fuller J, Patterson K, Blair R, Tabor A. Soy compared to casein meal replacement shakes with energy-restricted diets for obese women: randomized controlled trial. Metab Clin Exp. 2007;56(2):280–8. doi:10.1016/j.metabol.2006.10.013.
- Greany KA, Nettleton JA, Wangen KE, Thomas W, Kurzer MS. Consumption of isoflavone-rich soy protein does not alter homocysteine or markers of inflammation in postmenopausal women. Eur J Clin Nutr. 2008;62(12):1419–25. doi:10.1038/sj.ejcn.1602885.
- Rebholz CM, Reynolds K, Wofford MR, Chen J, Kelly TN, Mei H, Whelton PK, He J. Effect of soybean protein on novel cardiovascular disease risk factors: a randomized controlled trial. Eur J Clin Nutr. 2013;67(1):58–63. doi:10.1038/ejcn.2012.186.
- Frota KdMG, dos Santos Filho RD, Ribeiro VQ, Arêas JAG. Cowpea protein reduces LDL-cholesterol and apolipoprotein B concentrations, but does not improve biomarkers of inflammation or endothelial dysfunction in adults with moderate hypercholesterolemia. Nutr Hosp. 2015;31(4):1611–9. doi:10.3305/nh.2015.31.4.8457.
- Jenkins DJ, Srichaikul K, Wong JM, Kendall CW, Bashyam B, Vidgen E, et al. Supplemental barley protein and casein similarly affect serum lipids in hypercholesterolemic women and men. J Nutr. 2010;140(9):1633–7. doi:10.3945/jn.110.123224.
- Lee YM, Skurk T, Hennig M, Hauner H. Effect of a milk drink supplemented with whey peptides on blood pressure in patients with mild hypertension. Eur J Nutr. 2007;46(1):21–7. doi:10.1007/s00394-006-0625-8.
- Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–72. doi:10.2337/db06-1491.
- Richter CK, Skulas-Ray AC, Champagne CM, Kris-Etherton PM. Plant protein and animal proteins: do they differentially affect cardiovascular disease risk? Adv Nutr. 2015;6(6):712–28. doi:10.3945/an.115.009654.
- Tharrey M, Mariotti F, Mashchak A, Barbillon P, Delattre M, Fraser GE. Patterns of plant and animal protein intake are strongly associated with cardiovascular mortality: the Adventist Health Study-2 cohort. Int J Epidemiol. 2018;47(5):1603–12. doi:10.1093/ije/dyy030.
- Chen Z, Glisic M, Song M, Aliahmad HA, Zhang X, Moumdjian AC, et al. Dietary protein intake and all-cause and cause-specific mortality: results from the Rotterdam Study and a meta-analysis of prospective cohort studies. Eur J Epidemiol. 2020;35(5):411–29. doi:10.1007/s10654-020-00607-6.
- Van Horn L, Carson JA, Appel LJ, Burke LE, Economos C, Karmally W, et al. Recommended dietary pattern to achieve adherence to the American Heart Association/American College of Cardiology (AHA/ACC) Guidelines: a scientific statement from the American Heart Association. Circulation. 2016;134(22):e505–e529. doi:10.1161/cir.0000000000000462.
- Fekete ÁA, Givens DI, Lovegrove JA. Can milk proteins be a useful tool in the management of cardiometabolic health? An updated review of human intervention trials. Proc Nutr Soc. 2016;75(3):328–41. doi:10.1017/S0029665116000264.
- Melnik BC. Milk–the promoter of chronic Western diseases. Med Hypotheses. 2009;72(6):631–9. doi:10.1016/j.mehy.2009.01.008.
- Telle-Hansen VH, Christensen JJ, Ulven SM, Holven KB. Does dietary fat affect inflammatory markers in overweight and obese individuals?—a review of randomized controlled trials from 2010 to 2016. Genes Nutr. 2017; 12:26. doi:10.1186/s12263-017-0580-4.
- Unger AL, Torres-Gonzalez M, Kraft J. Dairy fat consumption and the risk of metabolic syndrome: an examination of the saturated fatty acids in dairy. Nutrients. 2019;11(9):2200. doi:10.3390/nu11092200.
- Chiu S, Bergeron N, Williams PT, Bray GA, Sutherland B, Krauss RM. Comparison of the DASH (Dietary Approaches to Stop Hypertension) diet and a higher-fat DASH diet on blood pressure and lipids and lipoproteins: a randomized controlled trial. Am J Clin Nutr. 2016;103(2):341–347. doi:10.3945/ajcn.115.123281.
- Byrd DA, Judd SE, Flanders WD, Hartman TJ, Fedirko V, Bostick RM. Development and validation of novel dietary and lifestyle inflammation scores. J Nutr. 2019;149(12):2206–2218. doi:10.1093/jn/nxz165.
- Hirahatake KM, Bruno RS, Bolling BW, Blesso C, Alexander LM, Adams SH. Dairy foods and dairy fats: new perspectives on pathways implicated in cardiometabolic health. Adv Nutr. 2020;11(2):266–279. doi:10.1093/advances/nmz105.
- Alba BK, Stanhewicz AE, Dey P, Bruno RS, Kenney WL, Alexander LM. Controlled feeding of an 8-d, high-dairy cheese diet prevents sodium-induced endothelial dysfunction in the cutaneous microcirculation of healthy, older adults through reductions in superoxide. J Nutr. 2020;150(1):55–63. doi:10.1093/jn/nxz205.
- Da Silva MS, Rudkowska I. Dairy nutrients and their effect on inflammatory profile in molecular studies. Mol Nutr Food Res. 2015;59(7):1249–1263. doi:10.1002/mnfr.201400569.
- Burton KJ, Pimentel G, Zangger N, Vionnet N, Drai J, McTernan PG, Pralong FP, Delorenzi M, Vergères G. Modulation of the peripheral blood transcriptome by the ingestion of probiotic yoghurt and acidified milk in healthy, young men. PLoS One. 2018;13(2):e0192947. doi:10.1371/journal.pone.0192947.
- Burton KJ, Rosikiewicz M, Pimentel G, Bütikofer U, von Ah U, Voirol M-J, et al. Probiotic yogurt and acidified milk similarly reduce postprandial inflammation and both alter the gut microbiota of healthy, young men. Br J Nutr. 2017;117(9):1312–1322. doi:10.1017/S0007114517000885.
- Pimentel G, Burton KJ, von Ah U, Bütikofer U, Pralong FP, Vionnet N, Portmann R, Vergères G. Metabolic footprinting of fermented milk consumption in serum of healthy men. J Nutr. 2018;148(6):851–860. doi:10.1093/jn/nxy053.
- Mozaffarian D. Dairy foods, obesity, and metabolic health: the role of the food matrix compared with single nutrients. Adv Nutr. 2019;10(5):917S–923s. doi:10.1093/advances/nmz053.
- Schwingshackl L, Hoffmann G. Mediterranean dietary pattern, inflammation and endothelial function: a systematic review and meta-analysis of intervention trials. Nutr Metab Cardiovasc Dis. 2014;24(9):929–939. doi:10.1016/j.numecd.2014.03.003.
- Soltani S, Chitsazi MJ, Salehi-Abargouei A. The effect of dietary approaches to stop hypertension (DASH) on serum inflammatory markers: a systematic review and meta-analysis of randomized trials. Clin Nutr. 2018;37(2):542–550. doi:10.1016/j.clnu.2017.02.018.
- Thompson WG, Rostad Holdman N, Janzow DJ, Slezak JM, Morris KL, Zemel MB. Effect of energy-reduced diets high in dairy products and fiber on weight loss in obese adults. Obes Res. 2005;13(8):1344–1353. doi:10.1038/oby.2005.163.
- Calder PC. Biomarkers of immunity and inflammation for use in nutrition interventions: International Life Sciences Institute European Branch work on selection criteria and interpretation. Endocr Metab Immune Disord Drug Targets. 2014;14(4):236–244. doi:10.2174/1871530314666140709091650.
- Calder PC, Ahluwalia N, Albers R, Bosco N, Bourdet-Sicard R, Haller D, et al. A consideration of biomarkers to be used for evaluation of inflammation in human nutritional studies. Br J Nutr. 2013;109 Suppl (1S1):S1–S34. doi:10.1017/s0007114512005119.
- Minihane AM, Vinoy S, Russell WR, Baka A, Roche HM, Tuohy KM, et al. Low-grade inflammation, diet composition and health: current research evidence and its translation. Br J Nutr. 2015;114(7):999–1012. doi:10.1017/s0007114515002093.
- Turner KM, Keogh JB, Meikle PJ, Clifton PM. Changes in lipids and inflammatory markers after consuming diets high in red meat or dairy for four weeks. Nutrients. 2017;9(8):886. doi:10.3390/nu9080.
- Ottestad I, Lovstad AT, Gjevestad GO, Hamarsland H, Benth JS, Andersen LF, et al. Intake of a protein-enriched milk and effects on muscle mass and strength. A 12-week randomized placebo controlled trial among community-dwelling older adults. J Nutr Health Aging. 2017;21(10):1160–1169. doi:10.1007/s12603-016-0856-1.
- Maersk M, Belza A, Stodkilde-Jorgensen H, Ringgaard S, Chabanova E, Thomsen H, et al. Sucrose-sweetened beverages increase fat storage in the liver, muscle, and visceral fat depot: a 6-mo randomized intervention study. Am J Clin Nutr. 2012;95(2):283–289. doi:10.3945/ajcn.111.022533.
- Turner KM, Keogh JB, Clifton PM. Red meat, dairy, and insulin sensitivity: a randomized crossover intervention study. Am J Clin Nutr. 2015;101(6):1173–1179. doi:10.3945/ajcn.114.104976.
- Van Loan MD, Keim NL, Adams SH, Souza E, Woodhouse LR, Thomas A, et al. Dairy foods in a moderate energy restricted diet do not enhance central fat, weight, and intra-abdominal adipose tissue losses nor reduce adipocyte size or inflammatory markers in overweight and obese adults: a controlled feeding study. J Obes. 2011; 2011:989657. doi:10.1155/2011/989657.
- Centers for Disease Control and Prevention. Defining adult overweight and obesity. Available from: https://www.cdc.gov/obesity/adult/defining.html.
