Abstract
Dairy is one of the main sources for high quality protein in the human diet. Processing may, however, cause denaturation, aggregation, and chemical modifications of its amino acids, which may impact protein quality. This systematic review covers the effect of milk protein modifications as a result of heating, on protein digestion and its physiological impact. A total of 5363 records were retrieved through the Scopus database of which a total of 102 were included. Although the degree of modification highly depends on the exact processing conditions, heating of milk proteins can modify several amino acids. In vitro and animal studies demonstrate that glycation decreases protein digestibility, and hinders amino acid availability, especially for lysine. Other chemical modifications, including oxidation, racemization, dephosphorylation and cross-linking, are less well studied, but may also impact protein digestion, which may result in decreased amino acid bioavailability and functionality. On the other hand, protein denaturation does not affect overall digestibility, but can facilitate gastric hydrolysis, especially of β-lactoglobulin. Protein denaturation can also alter gastric emptying of the protein, consequently affecting digestive kinetics that can eventually result in different post-prandial plasma amino acid appearance. Apart from processing, the kinetics of protein digestion depend on the matrix in which the protein is heated. Altogether, protein modifications may be considered indicative for processing severity. Controlling dairy processing conditions can thus be a powerful way to preserve protein quality or to steer gastrointestinal digestion kinetics and subsequent release of amino acids. Related physiological consequences mainly point towards amino acid bioavailability and immunological consequences.
1. Introduction
Milk is an important source for high quality protein in the human diet. The high nutritional quality of milk proteins originates from both its high level of essential amino acids, as well as it high bioavailability. This high bioavailability of milk proteins compared to plant proteins is due to its high digestibility, which is partly due to the absence of anti-nutritional factors and different ways of processing (Schaafsma Citation2012). Industrial dairy processing can however change the structure of milk proteins in several ways, depending on the conditions under which it has been processed. The main protein modifications occurring during processing are denaturation and aggregation of the protein and chemical modifications of its amino acids. These processing-induced protein modifications may change digestion and the overall physiological impact the consumption of these proteins have. The most studied physiological consequences of the effect of heating on proteins are its digestibility and bioavailability. However, the protein modifications may also cause changes along the gastrointestinal tract (e.g. related to microbiota, epithelial physiology and immune responses) or have other physiological consequences, which could be either locally or systemic. gives an overview of the different processes and their potential consequences.
Figure 1. Schematic overview of the reactions that can occur to milk proteins upon heating and how the state of the protein may have physiological consequences through differences in digestion.
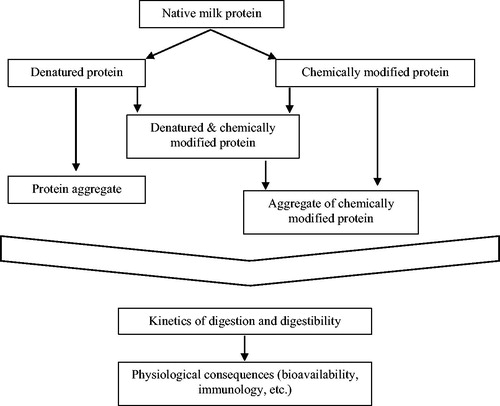
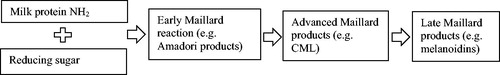
Aggregation of protein can be a consequence of heat-induced denaturation, where internal disulfide bridges within protein molecules are broken followed by intermolecular disulfide bridge reformation, although also other chemical modifications may lead to protein aggregation. These chemical modifications may occur during heating of milk, and will have an impact on the protein as such, as well as increase the tendency of the protein to aggregate. Most previous research has focused on glycation, also known as the Maillard reaction, which is the reaction between reducing sugars and the primary amines (NH2) of a protein. In milk, the Maillard reaction mainly occurs between the reducing sugar lactose and the NH2-group of the essential amino acid lysine, which is abundant in all milk proteins. This reaction proceeds through multiple stages, with specific early, advanced and late products often used as marker, as schematically depicted in . Besides the chemical modification of the lysine residues, the Maillard reaction can also lead to Maillard-induced aggregation of protein (Van Boekel Citation1998).
Figure 2. Stages of the Maillard reaction between milk proteins and reducing sugars and commonly used markers to monitor each stage.
Next to the Maillard reaction, other chemical modifications can occur during heating of milk, for example oxidation, cyclization and racemization of amino acids (Liardon and Hurrell Citation1983; Meltretter et al. Citation2007).
Several reviews already describe the effect of heating on food proteins in general (Friedman Citation1999; Gerrard et al. Citation2012; Mauron Citation1990). However, with milk being one of the main protein sources in the Western diet (EFSA Citation2012), and the sole source of protein for formula fed infants, it is important to specifically understand the role of industrial (heat) processing on milk proteins. Another reason to specifically focus on milk proteins is that the major class of milk proteins, the caseins, respond differently to heating than most other food proteins, as will be explained in more detail below.
Existing reviews on milk protein’s response to industrial (heat) processing mainly focus on the direct physico-chemical effects on the milk protein itself (Ferrer et al. Citation1999; Mehta and Deeth Citation2016; Pellegrino, Cattaneo, and De Noni Citation2011), and only give a limited perspective on the physiological relevance. This review will focus more specifically on the effect of dairy protein modifications, as a result of industrial heating, on its digestion and the overall physiological relevance of these modifications.
1.1. Dairy proteins
Milk proteins are classified as the casein fraction, which represents approximately 80% of all protein in bovine milk, and the whey protein fraction, representing approximately 20% of all protein in bovine milk (Walstra, Wouters, and Geurts Citation2006). These proteins have different physico-chemical properties and therefore behave differently upon heating.
The casein fraction of bovine milk consists of four different casein molecules, αs1-, αs2-, β-, and κ-casein. The individual casein molecules lack a tertiary structure, but occur in milk as large (approximately 100–200 nm) structures called casein micelles consisting of 1000’s of individual casein molecules. Because of a lack of tertiary structure, caseins don’t show the typical denaturation and aggregation upon heating. Caseins are however sensitive to processing-induced chemical modifications and aggregation (Pellegrino, Cattaneo, and De Noni Citation2011). When looking at the upper part of , the caseins thus only follow the right part of the figure with chemical modifications, and the associated aggregation.
The whey proteins, on the other hand, both denature and aggregate upon heating and can be chemically modified. They can thus follow all pathways indicated in . An exception is casein macropeptide (CMP), a casein-derived peptide in cheese whey that does not denature. Besides forming aggregates amongst each other, whey proteins also aggregate with casein micelles. The ratio between free whey protein aggregates and casein-whey aggregates depends on the pH, with the majority of the whey protein binding to the casein micelle surface at the natural pH of milk (Vasbinder and de Kruif Citation2003). The interaction between whey proteins amongst each other and between whey proteins and casein micelles is mainly driven by disulfide bridge formation. With the main whey protein, β-lactoglobulin, having a free SH-group within its tertiary structure, this protein is important in driving milk protein aggregation upon heating. Above a critical temperature (approximately 75 °C) irreversible conformational changes occur as a result of initiated aggregation processes (Loveday et al. Citation2014). The binding between whey proteins and casein micelles is mainly through interaction between the SH-groups of β-lactoglobulin and κ-casein (Donato and Guyomarc’h Citation2009).
1.2. Dairy processing
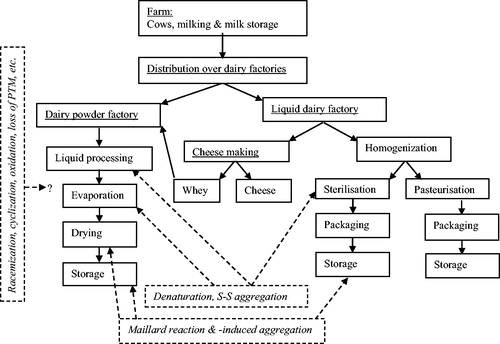
The ratio in which different reactions (denaturation, aggregation, chemical modification) occur, depends to a large extent on the exact processes to which the milk is subjected (Pellegrino, Cattaneo, and De Noni Citation2011; Walstra, Wouters, and Geurts Citation2006). This causes liquid and dry dairy products to differ in their degree of protein modifications. It is also important to consider that dairy products may be heated multiple times depending on the specific product, possibly combining liquid and dry dairy processes. The extent to which these processing induced modifications, including denaturation, aggregation, glycation, oxidation, etc. occur and their overall impact on digestion and physiological outcomes are thus determined by differences in (and combinations thereof) processing. A schematic overview of dairy processing steps and their effects on milk protein modification is given in .
Figure 3. Schematic overview of different type of dairy processes and the ensuing dairy protein modifications occurring during these processes. Product flow indicated with solid arrows, protein modifications indicated with dotted boxes and dotted arrows. PTM, post-translation modifications.
1.2.1. Liquid dairy processing
For industrial heat processing of liquid milk and dairy products, a distinction is commonly made between pasteurization and sterilization conditions, the two heat treatments that are most commonly applied (Walstra, Wouters, and Geurts Citation2006).
Pasteurization can be further subdivided in low and high pasteurization. Low pasteurization refers to a very mild heat treatment of 72 °C for around 15 seconds or 63 °C for 30 minutes, which both hardly affect milk proteins. Only some enzymes are denatured and thereby inactivated, but the major milk proteins remain in their native state. High pasteurization is less well defined, but is usually both at higher temperature (>80 °C) and longer (up to several minutes) compared to low pasteurization. Such more intense heating can lead, depending on the precise heat load, to partial or full denaturation of the whey proteins (Walstra, Wouters, and Geurts Citation2006). Under such circumstances, the whey proteins mainly aggregate on the casein micelles, where the linkage between β-lactoglobulin and κ-casein forms the basis, as discussed above. Caseins are not directly affected by pasteurization conditions, besides the abovementioned binding of whey proteins to the casein micelle (Pellegrino, Cattaneo, and De Noni Citation2011; Walstra, Wouters, and Geurts Citation2006).
When it comes to sterilization, different conditions can be distinguished, with the main distinction being the relative long in-package sterilization and UHT sterilization. With in-package sterilization, the cumulative heat load is very high, leading to full whey protein denaturation as well as extensive chemical modification of the amino acids, especially glycation of lysine. The most apparent result of this glycation is the discoloration and specific flavor of the finished product. UHT sterilization on the other hand is done at higher temperature (above 130 °C instead of 110 °C for in-package sterilization), but for much shorter heating times (seconds). At these conditions, the whey proteins also denature, but the level of chemical modification is much more limited, making this currently the most popular mode of sterilization (Pellegrino, Cattaneo, and De Noni Citation2011; Walstra, Wouters, and Geurts Citation2006).
1.2.2. Dry dairy processing
The reduced water activity during production of dried dairy products leads to a different balance between the protein modifications that occur. Dry heating enhances chemical modifications of amino acids, like glycation and oxidation, while at the same time, the rate of denaturation is lower than in liquid dairy products (Mehta and Deeth Citation2016; Pellegrino, Cattaneo, and De Noni Citation2011).
Dry dairy products have usually undergone prior heat treatments in a liquid state, which can range from low pasteurization to intense sterilization, to ensure safety and/or achieve specific functionality of the end product. When it comes to protein denaturation in dried dairy products, the main driver is thus the heating intensity of the liquid process, with limited or no further denaturation occurring during the drying process itself (Morgan et al. Citation1999; Mehta and Deeth Citation2016; Singh and Creamer Citation1991).
While denaturation happens mostly during the liquid processing, chemical modifications mainly occur during the drying process itself. Especially the effect of drying parameters on glycation has been studied in detail (Guyomarc’h et al. Citation2000). The protein modification in the final dried dairy products thus depends on a combination of the intensity of the heating of the liquid process combined with the parameters of the drying process.
1.2.3. Storage
Besides processing-induced modifications, changes may also occur during storage of the final product. Especially sterilized or dry dairy products stored for a prolonged period at high ambient temperatures are sensitive towards changes in their milk protein. Most research on storage-induced changes in protein modification has been done on dried dairy products and to a lesser extent sterilized products (Erbersdobler Citation1989; Ferrer et al. Citation1999; Mehta and Deeth Citation2016; Rutherfurd and Moughan Citation2008). Protein denaturation is not affected by storage, but protein aggregation and chemical modification of amino acids may occur during storage. The most apparent change that occurs is glycation, that also leads to browning of dairy products stored for prolonged times. The extent of modification during storage is a combination of storage conditions (time, temperature) and product conditions (predominantly water activity) (Guyomarc’h et al. Citation2000).
1.3. Consequences for digestion
The different states of the protein after heat processing, as described in , can have a large impact on their digestion. This is modulated through both the structure of the protein and the chemical modifications, which may both influence the accessibility of the amino acid bonds to the proteolytic enzymes. Generally, the unfolding of proteins increases accessibility of amino acids to proteolytic enzymes, whereas aggregation and chemical modifications, depending on the specific amino acid being modified, reduces the accessibility to proteolytic enzymes. Differences in product and processing conditions may thus lead to different effects on protein digestion.
1.4. Physiological consequences
Modification of milk proteins, and a consequentially altered digestion, may have further physiological consequences to the consumer. Different kinetics of protein digestion can, for example, lead to different postprandial absorption kinetics of amino acids. Also, the presence of peptides of different size, and different chemical modifications of these sequences, may have effects on e.g. the gastro-intestinal tract itself or the immune system (Nowak-Wegrzyn and Fiocchi Citation2009). This is especially relevant in early life for formula fed infants, where bovine milk proteins are typically the sole source of food proteins at a period where, amongst others, the digestive and immune system are not yet fully developed.
1.5. Aim of this review
As milk proteins play an important role in human nutrition, and can undergo changes during industrial processing, it is important to better understand how this may influence the digestion of milk proteins and their subsequent overall physiological activity. As bovine milk is the main milk industrially processed around the world, this review focuses on this milk specifically. This systematic review will provide an overview of the current state-of-the-art in this field, as well as indicate gaps that could be the focus for future research.
Better understanding of the relation between processing of dairy proteins, its digestibility, and subsequent physiological impact is also relevant for application in industry, where it may be applicable for designing optimal processes with a minimum impact on protein quality.
2. Methods
A systematic review on the effect of protein modifications on protein digestion in dairy was performed using several terms as tested in a search query. All searches were done using the Scopus database. Ultimately, four terminology groups were defined in relation to the research question, as specified in . Other terms that have been assessed for their inclusion were: protein, process*, modifi*, CML, Amadori, hydroly*, lysine, cross-link* and transport*. Those terms were excluded because they were either too general (protein, process*, hydroly*), did not include new publications (Amadori) or did not include additional publications relevant to the research question (modify*, CML, lysine, cross-link* and transport*). Since a substantial number of animal studies relevant to the research question were published between 1990 and 2000, a publication date within the last 30 years was chosen for the final search.
Table 1. Search terms.
The final search query was defined as (TITLE-ABS-KEY (milk* OR dairy OR (infant AND formula) OR whey OR casein* OR lact*) AND TITLE-ABS-KEY (glycat* OR Maillard* OR heat* OR furosine OR brown* OR oxidat* OR therm* OR aggregat* OR denat*) AND TITLE-ABS-KEY (digest* OR absor* OR proteoly*) AND TITLE-ABS-KEY (gastr* OR intestin* OR availab* OR metabol* OR (in AND vitro) OR allerg* OR grow*)) AND PUBYEAR > 1987. On 24 September 2018, the final search was completed using Scopus.
The identified articles were screened on their eligibility. Because of limited information available in titles, articles were included when they could be associated with at least two out of the four grouped terms. First, the titles were screened for their eligibility by one of the authors. Subsequently, abstracts of included titles were screened by all authors independently using the below described criteria.
Studies were included when they compared differently processed dairy proteins. Only the major bovine dairy proteins were included, being α-lactalbumin, β-lactoglobulin, α-caseins, β-casein and κ-casein. Both isolated proteins and proteins in a dairy matrix were included. Studies were included when digestion was involved that is (partly) representative for gastrointestinal digestion in humans, either in vivo or in vitro. Outcome measures related to protein digestibility and absorption that were included were nitrogen balance calculations, protein hydrolysis, SDS-PAGE, LC/MS-MS data, or possible physiological consequences like amino acid bioavailability, growth, and allergenicity/immunology.
Articles not in English were excluded. In addition, non-experimental publications were excluded, including conference papers, editorials and reviews. Some reviews were used as part of the introduction. Studies that focus on non-bovine milk (e.g. human milk) or hydrolyzed protein were also excluded. Furthermore, studies were excluded when no (simulation of a) dairy heat process was used, e.g. homogenization and enzymatic cross-linking, or when no comparison was made between differently heated samples. When no human-like gastrointestinal digestion or no outcome measure related to digestion or physiology was used, studies were excluded as well. Lastly, studies were excluded when the focus was on development of a new analytical methodology.
After screening for abstracts, full-text articles were grouped based on four major topics; glycation, denaturation and aggregation, other modifications and physiological effects. In turn, topics were assigned to different authors and full-text articles were assessed for eligibility according to the in- and exclusion criteria described above. For introduction purposes, and for further discussion of overall physiological relevance of findings from individual studies, a number of references were added manually.
3. Results and discussion
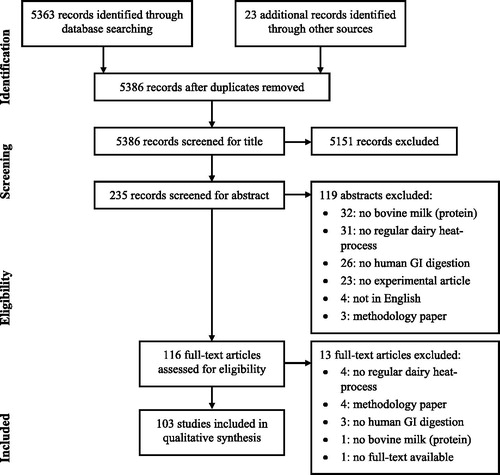
In total, 5386 records were identified through database searching and through manual addition. After screening for title, 235 abstracts were included, of which 116 were selected to assess full-text eligibility. Ultimately, 103 articles were included based on the criteria described in section 2. An overview of the process is given in . No quantitative comparison of individual studies was done as only a limited number of studies quantified both modification of the product and differences in protein hydrolysis. Moreover, those studies varied in their methods and outcome measures used, making it unfeasible to combine data in a quantitative synthesis.
Figure 4. Database search results according the PRISMA statement (Moher et al. Citation2009).
A limitation of this systematic review is that search terms were already biased towards glycation, denaturation, and aggregation related modifications, despite inclusion of heating in general. In addition, the number of search terms related to physiological consequences was limited and therefore, relative to other areas of interest, it appeared necessary to manually include articles to complement the discussion.
Included studies mainly involved in vitro and animal digestion studies. A limited number of studies included products that were not heat processed but processed in another manner (e.g. homogenized). In those studies, only the data and discussion on non-heated versus heated study arms were used for this review. Most studies investigated the effect of glycation, denaturation and aggregation, or a combination thereof, on protein hydrolysis. A limited number of studies explored the effect of other modifications on digestion and/or overall physiological effect. Those topics were discussed separately.
Several methods are used to study the effect of heat-induced modifications on protein digestion, either in humans, animals or in vitro. A single study assessed the effect of heat treatment on postprandial kinetics in humans using intrinsically labeled milk proteins. In animal studies, nitrogen balance methodology is often used, in which a diet containing either heated or unheated protein is fed after which nitrogen absorption is measured. Both labeled and non-labeled nitrogen are used. Absorption can be measured at three levels. First, nitrogen is analyzed in feces to calculate apparent digestibility. Second, a correction for endogenous protein secretion can be done to calculate true digestibility. Third, a possible effect of colonic fermentation can be excluded by measuring nitrogen in the ileum to calculate (true or apparent) ileal digestibility. A few animal studies do not use nitrogen balance methodology, but determine protein hydrolysis in samples taken from the stomach or intestine. In in vitro studies, gastrointestinal digestion is simulated, mostly based on adult or infant conditions as discussed in literature. In vitro experiments simulate GI digestion in a static or dynamic manner. In case of static experiments, the food is incubated with simulated digestive juices and samples are taken at the end of gastric and/or intestinal digestion. Dynamic models include the gradual addition of enzymes and acid, and gastric emptying of the food. This enables investigation of different gastric behavior and emptying of dairy products after heating. After simulated digestion, protein hydrolysis is characterized by analytical methods like available amino-group measurements, gel electrophoresis or mass spectrometry.
For clarification purposes, we defined protein hydrolysis as the reaction where proteins or peptides are cleaved into smaller peptides or amino acids. Protein hydrolysis is sometimes used to calculate protein digestibility. Protein digestibility is defined as the amount of nitrogen that becomes available for absorption compared to the total amount of nitrogen ingested. Finally, when peptides or amino acids become available for human metabolism after both digestion and absorption in the gut, this is defined as bioavailability.
3.1. Glycation
Glycation of milk proteins as a result of heat treatment, drying in particular, is suggested to impair protein quality. Studies investigating an effect of glycation on protein digestibility are listed in . A wide variation of products is used with a diverse range of heat processes and temperatures to induce glycation. Most studies use whey protein, casein or isolated milk proteins together with lactose or glucose. A few studies use GOS and FOS, being a mixture of (reducing) carbohydrates, while others use pectin and dextran to demonstrate effects specifically of larger carbohydrates. In addition to extreme heating conditions, methylglyoxal or 3-deoxyglucosone are used to obtain more advanced glycation products. In general, unheated and heated carbohydrate-protein mixture are compared. Several studies use mixtures heated at various temperatures or water activities to cover a range of process variations. Ultimately, heating temperature, timing, the ratio of carbohydrate and protein, and the water activity together determine the extent of the Maillard reaction in the product, as explained in section 1.2 (Guyomarc’h et al. Citation2000).
Table 2. Literature overview of studies investigating the impact of glycation on dairy protein digestibility.
Different methods are used to characterize the Maillard reaction products (MRPs), with the most common markers shown in , although some studies do not characterize the Maillard reaction at all. Methods used include a shift in molecular weight, a decrease in available amino-groups, or a higher browning after heating. This limits information about the different MRPs formed, their amount and therefore the exact stage of the Maillard reaction. Other studies directly quantify MRP-related parameters like unmodified lysine, also referred to as available or reactive lysine, modified lysine, also referred to as blocked lysine, furosine, and carboxymethyllysine (CML). This enables a better understanding of the MRPs that differ between the tested products and the exact stage of the Maillard reaction. When amino acid analyses are used to calculate digestibility, it is of major importance to distinguish between total and reactive lysine levels as standard methodology may not always distinguish between both, leading to an overestimated lysine content that could result in an underestimated lysine digestibility coefficient (Moughan and Rutherfurd Citation1996). The complicated reaction pathways, and the many resulting MRPs, likely explains why no study was found that investigates a possible quantitative relation between glycation and digestibility.
3.1.1. The effect of glycation on protein digestion
The overall result, taking all studies in together, is that glycation decreases protein digestibility. From 24 studies, only a single study does not demonstrate an effect of glycation on milk protein digestion, 3 studies show an increase and decrease in protein digestibility depending on protein or carbohydrate source, and 20 studies show a decrease. Three possible mechanisms are mentioned that could explain the observed decrease in protein digestibility: 1) direct blocking of lysine residues, preventing it from recognition and cleavage by digestive proteases; 2) indirect blocking by glycation of lysine residues that are positioned near the cleavage site of enzymes; 3) glycation induced cross-linking that decreases accessibility of cleavage sites for proteases. When initial trypsin digestion is inhibited, this may hinder digestion by other intestinal proteases including brush border membrane proteases as one of the final steps before amino acid absorption, overall thus affecting the release amino acids during digestion. The decreased enzyme accessibility as a result of a different structure is also termed steric hindrance. In contrast, three studies show that glycation is able to increase digestibility. This was seen for WPI and α-lactalbumin (Cattaneo et al. Citation2017; Hiller and Lorenzen Citation2010; Joubran, Moscovici, and Lesmes Citation2015). Unfolding of the globular whey proteins as a result of heating and carbohydrate binding may have made hidden cleavage sites better accessible. Thus, for whey proteins, a balance between protein unfolding and steric hindrance, depending on the exact processing conditions, will determine whether an increase or decrease in digestibility is found after glycation. Most studies show that this balance leans more towards inaccessible cleavage sites, resulting in an overall lower digestibility.
The extent of the Maillard reaction differs between and within studies, but the number of studies that distinguish between early and advanced Maillard reaction products or possible Maillard reaction-induced cross-linking is limited. An exception is the study by Rérat et al. (Citation2002) who used a heated skimmed milk powder (SMP) representative of the early Maillard reaction. After ingestion of the early Maillard SMP, plasma appearance of lysine and total amino acids was decreased compared to a SMP low in MRPs, suggesting that already early Maillard reaction events have an effect on protein digestion and absorption. In another study, Hellwig and Henle (Citation2013) focused on the specific conversion of lysine to the AGE pyrraline. A higher pyrraline content resulted in a decreased formation of small peptides which could be explained by the modification of lysine to pyrraline that is not recognizable for trypsin. Moreover, Corzo-Martínez et al. (Citation2010) used pyridoxamine (PM) to partially inhibit aggregation and cross-linking in the advanced stages of the Maillard reaction. Addition of PM resulted in an increased peptide formation and disappearance of intact β-lactoglobulin after 15 minutes intestinal hydrolysis of β-lactoglobulin conjugated with galactose or tagatose, suggesting an increased hydrolysis when aggregation and cross-linking is inhibited. Lastly, Sarriá, López-Fandiño, and Vaquero (Citation2000) compared consumption of different forms of infant formula in rats and discussed that advanced MRPs are mostly excreted through feces, indicating reduced protein digestibility. Early MRPs were suggested to be partially absorbed, but metabolic utilization was minimal because most of it was excreted via urine. Altogether, it appears that early MRPs decrease amino acid bioavailability, and when absorbed, metabolic utilization is minimal, while advanced MRPs and Maillard-induced cross-linking further diminish overall protein digestibility.
3.1.2. Glycation in combination with other protein modifications
While the aforementioned studies focus on glycation in particular, various studies include heating processes where both glycation and denaturation or aggregation of the proteins occurred. For example, protein digestibility of powdered infant formula was significantly higher than liquid concentrates of the same manufacturer in a rat balance study (Sarwar, Peace, and Botting Citation1989). Likewise, when several infant formulas were digested using an infant in vitro model, it was concluded that digestibility of protein was generally highest for powdered formula, followed by liquid concentrate and ready-to-feed formula suggesting that processing does have an effect (Liusuwan and Lönnerdal Citation1996). Wada and Lönnerdal (Citation2014, Citation2015) conducted two studies where differently heated milk products were digested both in vitro and in rats. In the first, raw milk was either pasteurized, plate UHT treated, steam infused, steam injected, or in-can sterilized, while in the second, enteral formulas were steam injected or in-can sterilized. This resulted in products that differed in glycation, denaturation and aggregation. Overall, they suggested that denaturation can improve protein digestibility, while formation of aggregates or formation of lactulosyllysine (early MRP) can reduce digestibility (Wada and Lönnerdal Citation2014, Citation2015). Differences in glycation and aggregation may also result in different peptides formed during digestion of WPC powders heated with lactose (Liu et al. Citation2016). This is in line with the results of Pinto et al. (Citation2014), who showed that native β-lactoglobulin remained intact during gastric digestion, while heated β-lactoglobulin was digested and addition of glucose limited this heat-induced increase in hydrolysis. This illustrates that the balance of denaturation and glycation affects the digestion of β-lactoglobulin. For this reason, it is important to distinguish between different protein modifications when evaluating digestion of a heated product. For example, Nooshkam and Madadlou (Citation2016) suggested that Maillard conjugation could improve gastric digestibility of whey proteins, while this effect is likely more related to denaturation of the whey proteins. Besides an effect via the Maillard reaction, other protein modifications after heating a more complex matrix could affect protein hydrolysis and digestibility by other means. These include protein-lipid interactions or different formation of coagulates in the stomach (Rudloff and Lönnerdal Citation1992; Wada and Lönnerdal Citation2014). The various modifications that occur in parallel make it hard to distinguish the effect of a single modification specifically which may be an interesting topic for further research.
3.2. Denaturation and aggregation
An overview of studies that investigated the impact of denaturation and aggregation on dairy protein digestion is listed in . Studies were divided in four categories: a human study, animal studies, static in vitro studies and (semi)-dynamic in vitro digestion experiments. These different methods enable to distinguish between the effect of heating on protein digestion and protein digestion kinetics, although direct and quantitative comparability of different studies becomes more complex and the full complexity of in vivo digestion is not simulated in these in vitro models. Furthermore, it is important to note that most studies use heating as a way to induce denaturation and aggregation, but do not actually measure the level of these modifications or other modifications that may occur in the protein samples used prior to digestion.
Table 3. Literature overview of studies investigating the impact of denaturation and aggregation on dairy protein digestibility.
3.2.1. The impact of denaturation and aggregation on gastric protein hydrolysis and gastric behavior
3.2.1.1. Whey proteins and caseins
Zooming into the digestion of the major milk proteins, casein is almost completely digested in the stomach, while native whey proteins are more resistant to hydrolysis and are still intact after gastric digestion (Tunick et al. Citation2016). The resistance of whey proteins to gastric proteolysis is thought to relate to their conformation, which is required to exert specific physiological functions, whereas the open casein structure is mainly thought to relate to nutritional purposes, i.e. supply of amino acids (Guo et al. Citation1995). Most in vitro studies focused on hydrolysis of β-lactoglobulin. Generally, and in different matrices, it is described that heated (>75 °C), denatured β-lactoglobulin becomes more digestible by pepsin in vitro, due to unfolding and the consequential increased accessibility of protein cleavage sites (Guo et al. Citation1995; Kitabatake and Kinekawa Citation1998; Sánchez-Rivera et al. Citation2015; Wang et al. Citation2018). Rat studies confirm in vitro results by showing intact β-lactoglobulin in the rat stomach for native β-lactoglobulin, while heated β-lactoglobulin did not show intact β-lactoglobulin in the stomach (Kitabatake and Kinekawa Citation1998). When using a dynamic gastric digestion model, where gastric emptying is included, only intact β-lactoglobulin was emptied into the intestinal compartment from non-heated whey protein isolate, whereas peptides were emptied during digestion of heated whey protein isolate (Wang et al. Citation2018). On the other hand, gastric digestion of heated serum protein concentrate was also found to be delayed compared to its non-heated comparator, most likely by formation of whey protein aggregates and increased particle size in this particular setting (Stender et al. Citation2018) although it is likely that other reactions may have occurred simultaneously (see section 4.3).
Three different forms of β-lactoglobulin can be formed depending on the exact heating conditions. Dimers are formed with shorter heating times (i.e. 5 minutes, 90 °C) that are mostly still undigestible by pepsin. To a certain extent, the native confirmation may still be present in those dimers, thereby preventing accessibility by pepsin. With longer heating times (i.e. 60 to 120 minutes, 90 °C), other aggregates are formed, including non-native aggregates linked by disulfide-bonds that are rapidly digested by pepsin. Lastly, intermediates may be formed by disulfide-bonding of β-lactoglobulin monomers with peptides, giving a structure that is digested slower by pepsin than its unbound form (Loveday et al. Citation2014; Peram et al. Citation2013). Overall, this suggests that β-lactoglobulin may not be affected in low-temperature pasteurization processes, but high pasteurization and sterilization will increase its gastric hydrolysis.
Impact of heating on digestion of casein is less well studied compared to whey proteins. In general, hydrolysis of casein by pepsin in the stomach is high because of its open structure. However, a single study found that heating increased the resistance of casein to gastric hydrolysis in heated vs unheated skimmed milk, which was attributed to the formation of aggregates between caseins and whey proteins (Sánchez-Rivera et al. Citation2015). The resulting peptides formed from caseins differed between unheated and heated skimmed milk (Sánchez-Rivera et al. Citation2015). On the other hand, it has been argued that complexes of whey proteins with kappa-casein are better digestible by pepsin, trypsin and chymotrypsin than when they do not form complexes (Singh and Creamer Citation1993).
Overall, the available evidence indicates that induction of milk protein denaturation by heating seems to enhance gastric protein hydrolysis (mainly for whey proteins). However, it cannot be ruled out that aggregate formation between whey proteins themselves, between whey and casein, and potential interactions with lipids (Rudloff and Lönnerdal Citation1992) can alter gastric hydrolysis. For casein, its coagulation behavior at low pH in the stomach may alter digestive kinetics, as discussed in the next paragraph.
3.2.1.2. Milk matrix
Static in vitro experiments show that heating milk at higher temperatures increased the formation of peptides within the first 30 minutes of gastric digestion (Lamothe et al. Citation2017). This may be explained by the observation that heat treatment of milk results in a more fragmented and crumbled coagulum under gastric conditions, leading to a greater rate of gastric protein hydrolysis (Mulet-Cabero et al. Citation2019; Ye et al. Citation2017). The less consistent coagulum as a result of heating is suggested to be caused by: 1) binding of denatured whey proteins to the casein micelles, thereby blocking micelle aggregation; 2) denatured whey proteins and κ-casein forming soluble complexes, incorporating whey proteins in the coagulum, and 3) calcium concentration being reduced in the serum, changing the ion equilibrium, and thereby the coagulum structure (Mulet-Cabero et al. Citation2019). The increased hydrolysis is confirmed in commercial milk samples where intact whey proteins, and β-lactoglobulin in particular, in (in-can) sterilized or UHT-treated milk are better digested by pepsin than in pasteurized milk (Rinaldi, Gauthier, et al. Citation2014; Tunick et al. Citation2016; Wada and Lönnerdal Citation2014). Caseins and α-lactalbumin were hydrolyzed independent of heat treatments in all samples (Tunick et al. Citation2016). In contrast, heating skimmed milk for 10 minutes at 90 °C resulted in intact caseins that were still present after up to 50 minutes of gastric digestion. This could be due to the formation of aggregates between caseins and whey proteins (Sánchez-Rivera et al. Citation2015), as also discussed in section 1. The widely differing heat treatments amongst the studies discussed may, at least to a certain extent, explain the variation in results.
Although the full complexity of human digestion cannot be simulated, dynamic in vitro models and animal models enable to give a more reliable picture of protein digestion kinetics over time. The different kinetics are mainly caused in the stomach, because heating may result in different gastric behavior and emptying. While this may not influence overall digestibility, differences in kinetics of digestion may be relevant for their physiological consequences (see section 7).
The lower consistency of the coagulum as a result of heating influences gastric emptying of protein by increasing protein release in the beginning of digestion (Mulet-Cabero et al. Citation2019). Whey proteins that were incorporated in the coagulum of heated milk were digested together with the casein. On the other hand, no whey proteins were incorporated in the raw milk coagulum but they were present in the serum phase, resulting in emptying of intact whey proteins (Ye et al. Citation2017). This confirms the findings from static in vitro models that non-heated whey proteins are still intact after gastric digestion, resulting in intact whey proteins emptied in the intestine.
A limited number of animal studies investigated kinetics of digestion and absorption of heated milk. Barbé et al. (Citation2013, Citation2014) used cannulas shortly after the pylorus to sample duodenal effluents in minipigs. They showed that stomach retention time was increased for heated milk compared to raw liquid milk. For raw milk, intact caseins only appeared in the duodenum in the first minutes of digestion, and were completely hydrolyzed in the following duodenal effluents. These pattern was similar for heated milk. In contrast, intact β-lactoglobulin was visible up to 3 h after ingestion of the raw milk, while it disappeared 50 minutes after ingestion of heated milk. This suggests that β-lactoglobulin was susceptible to gastric digestion after heating the milk, resulting in disappearance of the intact form in duodenal effluents of the heated milk from 50 minutes onwards. The differences in raw and heated milk led to different postprandial plasma amino acid concentrations with an increased maximal and average indispensable amino acid concentration for heated milk (Barbé et al. Citation2013, Citation2014). This is in line with another study in pigs, where nitrogen was mainly absorbed in the first 2 h postprandial for milk and heated yogurt, while this was more distributed over 4 h postprandial for non-heated yogurt. It was suggested that the non-heated yogurt retained its viscosity, while this was reduced after heating. In turn, the milk and heated yogurt were emptied more rapidly from the stomach resulting in a faster postprandial absorption, while this was more delayed for the more viscous non-heated yogurt (Mpassi et al. Citation2001).
Altogether, both in vitro and animal studies show that heating milk or yogurt could alter gastric behavior and gastric emptying of the milk, thereby affecting the rate of post prandial plasma amino acid appearance.
3.2.2. The impact of denaturation and aggregation on intestinal protein digestibility
Heating-induced enhanced proteolysis demonstrated in a gastric model does not automatically mean an increased overall digestibility. Therefore, including a gastric and intestinal phase to assess protein digestibility is of importance. Different results are found after intestinal digestion for individual proteins and the total milk matrix.
3.2.2.1. β-lactoglobulin
Heating mainly impacts whey protein (predominantly β-lactoglobulin) hydrolysis in the stomach. While pancreatic enzymes are able to hydrolyze β-lactoglobulin completely anyways, it was shown for β-lactoglobulin that proteolysis by pancreatic enzymes was faster when it had been heated at different time-temperature combinations (Guo et al. Citation1995; Kitabatake and Kinekawa Citation1998; Rahaman, Vasiljevic, and Ramchandran Citation2017). This was shown for whey protein based gels as well (Lorieau et al. Citation2018; Singh et al. Citation2014). In contrast, Carbonaro et al. (Citation1997) and Lindberg et al. (Citation1998) showed that severe heating of whey protein extracts or WPC above a critical temperature (≥75 °C) decreased overall digestibility of its proteins, probably due to formation of more compact whey proteins and aggregates (Carbonaro et al. Citation1997; Lindberg et al. Citation1998; Wada and Lönnerdal Citation2014). However, it cannot be ruled out that confounding factors including protein glycation and glycation-induced aggregation, as discussed in section 4.3, underlie these observed effects. A single study showed that intestinal proteolysis was similar for native and heated serum protein concentrate (Stender et al. Citation2018). Interestingly, the studies that found an increased hydrolysis used isolated β-lactoglobulin, while the studies that found a decreased hydrolysis used whey protein extracts or whey protein concentrates and no effect was seen for serum protein concentrates. This suggests that the production and matrix of the product may influence the denaturation and aggregation state of the proteins, subsequently resulting in a different digestibility for the different matrices.
3.2.2.2. Casein
Intense processing of skimmed milk powders could result in higher resistance of intact caseins to gastrointestinal digestion. This mainly involves αs2-casein and κ-casein that are able to form disulfide bridges with denatured whey proteins. Therefore it was suggested that whey-casein aggregates formed during heating may result in a decreased hydrolysis of caseins, although this is in conflict with the results of a study by Singh and Creamer (Citation1993) (Dupont, Boutrou, et al. Citation2010; Singh and Creamer Citation1993). The interaction of caseins with denatured whey proteins may thus affect casein digestion, but the resulting effects on digestion differ between studies.
3.2.2.3. Milk matrix
In a milk matrix, several in vitro studies show that, comparable to the gastric hydrolysis results, heating of liquid milk results in (small) increases of protein hydrolysis by pancreatic enzymes (Carbonaro et al. Citation1997; Rinaldi et al. Citation2015). On the other hand, other in vitro studies show a similar intestinal proteolysis between raw, pasteurized and sterilized milk (Lamothe et al. Citation2017; Tunick et al. Citation2016). Overall, the effect of heating of liquid milk on intestinal hydrolysis thus seems small or absent.
Also, animal studies show that denaturation after heating of different dairy matrices does not affect overall digestibility. When comparing milk, yoghurt, and heated yoghurt, overall nitrogen absorption determined by portal labeled nitrogen absorption was similar in growing pigs (Mpassi et al. Citation2001). Milk being raw, pasteurized, UHT sterilized, or boiled also did not affect protein digestibility (Efigenia, Povoa, and Moraes-Santos Citation1997) or biological value of milk protein in rats (Lacroix et al. Citation2006). In young pigs, no effect on apparent fecal and ileal digestibility and dietary nitrogen utilization was shown for skimmed milk powders that were differently heated during the liquid processing. This result was found despite a different flocculation behavior found for the powders in vitro that could have influenced gastric emptying and digestion kinetics. This indicates that denaturation does not affect overall digestibility but can affect digestion kinetics (Moughan et al. Citation1989).
Those results are in line with a single human study that used labeled nitrogen to compare postprandial kinetics of UHT milk with microfiltered (MF) and pasteurized milk (Lacroix et al. Citation2008). Overall, no significant differences were shown for different treatments on serum amino acid concentrations. However, a significant increase in plasma amino acid levels from baseline was more sustained for UHT milk than MF milk. Moreover, postprandial dietary protein retention was reduced in the UHT group. Together, this suggests that the more rapid digestive kinetics of UHT milk resulted in a higher loss of dietary N compared to MF and pasteurized milk (Lacroix et al. Citation2008).
Altogether, most studies, including animal studies and a single human study, conclude that heating of milk proteins in a liquid milk matrix does not affect overall protein digestibility and bioavailability, but it can affect digestive kinetics mainly through a different stomach behavior, that could impact further metabolism.
3.3. Other modifications
Glycation, denaturation, and aggregation could affect protein digestion and quality, as described in section 3.1 and 3.2. Moreover, other modifications that have been demonstrated to occur during dairy processing, as evidenced by their presence in actual dairy products, could impact protein digestion and quality (Liardon and Hurrell Citation1983; Meltretter et al. Citation2007). For example, oxidation is demonstrated when isolated whey proteins are heated with lactose, while racemization occurs in heated milk powders. However, those modifications seem to be less abundant than glycation (Liardon and Hurrell Citation1983; Meltretter et al. Citation2007). The exact conditions, and in which stage of dairy processing these other modifications actually occur, is not yet known ().
3.3.1. Oxidation
Although less studied than Maillard-based modifications, oxidation as a result of industrial processing is another chemical amino acid modification that has been demonstrated to affect protein digestion and quality. To date, several studies applying different experimental oxidation strategies have demonstrated effects of oxidation on digestibility and protein quality (Chang and Zhao Citation2012; Feng et al. Citation2015; Rutherfurd, Montoya, and Moughan Citation2014). Oxidation strategies include treatment of the proteins by an oxidative system containing oxidase-enzymes (Chang and Zhao Citation2012), a free radical-generation system with hydrogen peroxide (Feng et al. Citation2015), or incubation with performic acid (Rutherfurd, Montoya, and Moughan Citation2014). Rutherfurd and colleagues applied a chemical strategy to oxidize several protein sources including the milk proteins casein and α-lactalbumin to study true ileal amino acid digestibility (TIAAD) in rats. Oxidation of all protein sources resulted in an almost complete modification of methionine, tyrosine, and tryptophan. Importantly, as these amino acid are considered either essential (methionine and tryptophan) or conditionally essential (tyrosine), care should be taken with excluding these for further ileal digestibility calculations. Nonetheless, the authors excluded these amino acids from further TIAAD calculations because they were destroyed during the oxidation process of the proteins. For the other amino acids, it was demonstrated that TIAAD of oxidized casein was significantly lowered whereas that of oxidized α-lactalbumin was unchanged. The decreased overall TIAAD for casein, excluding the oxidized amino acids, indicates that other amino acids are affected as well, meaning oxidation can affect overall digestibility of casein. Other studies provided more detailed information about chemical and structural changes as a result of oxidation by using either a free radical generating system and WPI (Feng et al. Citation2015) or a peroxidase system and sodium caseinate (Chang and Zhao Citation2012). Despite the different approaches for oxidation of the milk proteins, both studies revealed that oxidation caused physico-chemical and structural changes, such as loss of thiol groups, formation of dityrosine and carbonyls, increased surface hydrophobicity, turbidity and particle size, which coincided with an overall decreased in vitro hydrolysis for both gastric and overall gastrointestinal conditions.
3.3.2. Racemization
Racemization, the conformation of L-amino acids to their enantiomer D-form, may affect protein quality predominately as the physiological response to L-amino acids may be different than to the D-forms (Friedman Citation2010). Specifically for milk proteins (both caseins and β-lactoglobulin), racemization as a result of heat and alkali treatment has been demonstrated to decrease true ileal protein digestibility in minipigs (de Vrese et al. Citation2000). It is suggested that enzymatic breakdown and absorption of peptides containing D-amino acids and free D-amino acids is affected, because they are not recognized by the peptidases. This may affect other amino acids in the peptides as well, resulting in a decreased ileal protein digestibility (de Vrese et al. Citation2000). These results are in line with previous findings where racemization of milk proteins was induced in a more artificial manner and digestibility values were directly related to the degree of racemization and to the amount of lysinoalanine (Swaisgood and Catignani Citation1985). Thus, although studied to a lesser extent than glycation, racemization appears to be another modification that occurs during processing of milk proteins that may decrease overall digestibility and protein quality.
3.3.3. Dephosphorylation
Milk proteins, predominantly caseins, have a high degree of phosphorylation which contributes to their structure and functionality (Huppertz, Fox, and Kelly Citation2018). Phosphorylation has generally been considered to be sensitive to heat (Singh Citation2004) although limited studies determined the impact of milk protein dephosphorylation on digestion. Fox et al. (Citation2004) discuss that partial dephosphorylation of casein reduces the size of protein precipitates under acid conditions such as occurring during gastric digestion. This would suggest a faster gastric emptying, at least for the casein fraction that would normally coagulate and form a protein network. However, as demonstrated by scintigraphy analyses in this study, gastric emptying of the dephosphorylated casein appeared to be faster than the unmodified casein. With respect to direct measurements of digestion, Wada and Lönnerdal (Citation2014) determined that, out of several tested industrial heating procedures for raw milk, in-can sterilization resulted in the most prominent casein dephosphorylation in addition to increases in other protein modifications including denaturation, aggregation and lactulosyllysine and carboxymethyllysine formation. Although not directly linked to dephosphorylation, and likely predominantly an effect of denaturation, these heating-induced modifications increased the degree of digestion as assessed by hydrolysis of major milk proteins by in vitro and in vivo digestion experiments.
Similar results were obtained with model enteral formulas by the same group where in-can sterilization resulted in higher protein modifications including carboxymethyllysine formation and dephosphorylation of serine residues in the major milk proteins (Wada and Lönnerdal Citation2015). In this more complex matrix, in vitro and in vivo experiments in rats show that digestive differences of model enteral formulas were larger.
In conclusion, although mostly coinciding with other modifications, available literature suggests that dephosphorylation of milk proteins may impact digestibility. More studies are however required to obtain a more complete understanding of dephosphorylation, preferably adopting modification strategies that are able to separate dephosphorylation from other processing-induced modifications.
3.3.4. Cross-linking
Heating of dairy proteins could also result in the formation of cross-links between different amino acids within a protein. A well-known example is lysinoalanine (LAL) that can be formed during heating of proteins. Cross-linking could affect digestibility of a protein by changing enzyme accessibility. However, an in vitro infant digestion of infant formula model systems suggested that different protein cross-linking as assessed by LAL content did not change protein hydrolysis (Cattaneo et al. Citation2017). In another study, in vitro digestion of different infant formulas showed no direct correlation between digestibility and LAL content, lysine blockage, or HMF content (Pompei, Rossi, and Mare Citation1988). Moreover, an animal study in minipigs showed that true digestibility of casein was not related to LAL content, but it was still suggested that small amounts of LAL could impair digestion by interfering with enzymatic cleavage (de Vrese et al. Citation2000). In line with that, LAL formation in α-lactalbumin resulted in a reduced protein digestibility and protein quality in rats (Gilani and Sepehr Citation2003). Thus, available studies indicate that cross-linking may or may not affect digestibility of proteins, thereby showing a need for further research.
3.4. Physiological consequences
The effects of dairy processing, as described in section 1 of this review, can affect milk proteins in many different ways (). This can cause changes in their digestibility or digestion kinetics as detailed in sections 3.1–3.3. In this section, the possible physiological consequences of these differences in digestion are discussed.
3.4.1. Protein digestion, bioavailability and metabolic utilization
To a certain extent, heating of milk proteins may increase the speed of digestion as a result of heat induced protein denaturation and better protease accessibility (predominantly demonstrated for β-lactoglobulin). However, the discussed non-enzymatic modifications that occur simultaneously will modify specific amino acids and decrease bioavailability and functionality of these amino acids. Furthermore, blocking of specific amino acids may hinder further proteolytic activity thereby impairing overall protein digestibility. In addition, cross-linking can occur with formation of novel structures that may further inhibit enzymatic hydrolysis and overall digestibility. As a consequence, non-enzymatic protein modification may be considered indicative for a reduced protein quality as demonstrated by direct comparisons of their non-modified forms as discussed in sections 3.1 and 3.3.
Intestinal epithelial cells contribute to degradation of dietary proteins in different ways. Brush border membrane proteases represent the last step of luminal protein digestion, hydrolyzing proteins/peptides in an absorbable form. Furthermore, during transport across the intestinal epithelium, enterocytes contribute to further hydrolysis of absorbed di- and tri-peptides intracellularly. Epithelial degradation and transport of heating induced modified β-lactoglobulin may be affected, as demonstrated by in vitro studies using different intestinal epithelial cell-models (Bernasconi, Fritsché, and Corthésy Citation2006; Rytkönen et al. Citation2006).
The fate of modified amino acids remains largely unexplored to date as most studies investigating digestion of modified and non-modified proteins only discuss decreased bioavailability. Decreased digestibility and absorption of dietary proteins may increase protein fermentation in the lower gastrointestinal tract with increased production of microbial protein fermentation products, including branched-chain fatty acids, ammonia, phenol, p-cresol, indole and hydrogen sulfide (Nyangale, Mottram, and Gibson Citation2012), which may be unfavorable from several perspectives. In contrast to fecal carbohydrate fermentation, microbiome protein fermentation is less extensively researched and the toxic potential of these compounds is predominantly derived from in vitro cell-toxicity and animal studies determining effects of purified individual protein fermentation by-products (Verbeke et al. Citation2015). Minimally irritating concentrations were assessed as a marker of local effects and exceeded 1% for all compounds, which is well above the concentrations occurring in vivo in the colon. Moreover, in human studies there is little evidence for adverse effects of protein fermentation metabolites. Further studies are therefore required and especially more chronic exposure of combinations of modified amino acids/digestion products and effects on the longer term remain to be elucidated.
To a certain extent, the microbiome also appears to be equipped to metabolize modified lysine, which may be one of the explanations why fecal extraction of glycated lysine was rather low in rat, pig, and human studies, typically around 4% of the ingested amount (Lee and Erbersdobler Citation2005; Rérat et al. Citation2002). Interestingly, one particular microbial specie, Intestinimonas, is able to convert lysine and Amadori products thereof into butyrate and acetate, which are considered important microbiome derived signaling molecules in the gut (Bui et al. Citation2015).
Post absorption, nitrogen metabolism may be affected by processing-induced protein modifications and result in a larger splanchnic extraction, as demonstrated in rats fed differentially processed milk protein preparations (Lacroix et al. Citation2006). Interestingly, in this study lactosylation was likely the most important driver for increased splanchnic extraction as spray dried soluble milk proteins did not denature or aggregate (as illustrated by preserved solubility at pH 4.6) but did show the most prominent effect on splanchnic extraction. In a study in humans, Lacroix et al., (Citation2008) showed that UHT milk gives a more rapid appearance of amino acids in plasma compared to MF milk, ultimately resulting in a lower postprandial nitrogen retention. This suggests that protein digestive kinetics can influence metabolic utilization of the ingested protein. Additional work by Rérat et al. (Citation2002) in pigs reveals that loss of nutritional quality as a result of additional heating of milk powders is mainly driven by loss of lysine bioavailability and to a lesser extent by the decrease in digestibility of other essential amino acids. Nonetheless, fecal excretion of almost all amino acids was higher in milk with 50% blocked lysine. Interestingly, in this study fructoselysine was detected postprandially suggesting that, at least to a certain extent, the galactose residue of lactuloselysine is released in the gut lumen or brush border. Moreover, urine levels of fructoselysine recorded over 72 h revealed that urinary excretion of glycated lysine is around 20% of the ingested amount. In humans, urinary excretion of protein-bound fructoselysine ranged from 1.4 to 3.5% of the amount ingested as determined using test meals enhanced with fructoselysine (Lee and Erbersdobler Citation2005).
For advanced glycation end-products (AGE), most available data reflect the health impact of endogenously formed AGE, whereas data on the impact of dietary AGE is limited. Basically, AGE are known to induce effects in the body by two distinct mechanisms, including deformation or cross-linking of AGE with endogenous proteins and interactions with AGE receptors (Poulsen et al. Citation2013). For AGE receptors, studies related to milk proteins are available (discussed in section 3.4.3), whereas deformation and cross-linking of AGE with endogenous proteins has almost exclusively been related to endogenous formation of AGE, while effects of dietary AGE are still largely unknown.
During the digestion of milk protein, bioactive peptides are formed that may induce physiological responses predominantly along the GI tract (Nongonierma and FitzGerald Citation2015). Well known examples in this area are casein-derived sequences termed casomorphins. Cattaneo et al. (Citation2017) demonstrate that processing may impact the formation of β-casomorphins. Thus, besides differences in amino acid bioavailability, these result highlight potential additional mechanisms of action how intense processing of milk proteins may affect their overall physiological activity.
Taken together it thus appears that processing-induced protein and amino acid modifications may affect digestion, absorption, and metabolism of protein at all levels, although particularly about the fate of modified amino acids post-digestion more data is needed.
3.4.2. Gastrointestinal physiology
Beyond an altered digestion and reduced bioavailability, protein modifications may have additional physiological consequences. Proliferation and differentiation responses to food intake may be changed. Amongst others, this is evident from in vivo studies on the effect of differently treated milk protein preparations in preterm pigs. Immaturity of the gut predisposes these animals to gut complications and milk protein preparations (particular sow milk or minimally processed bovine milk protein preparations and colostrum) have been demonstrated to reduce the risk for such complications (Li, Ostergaard, et al. Citation2013; Li et al. Citation2018; Støy et al. Citation2016). Preterm pigs fed formula containing differentially processed whey protein concentrates (WPCs) displayed differences in intestinal structure, function, and integrity as determined by studying gut maturation events including villus height, disaccharide digestion/absorption, gut permeability, and intestinal inflammatory responses (Li et al. Citation2018). It was concluded that optimization of processing technology may be important to preserve the bioactivity and nutritional value of formula for newborns. Particular for colostrum (generally containing high levels of bioactive proteins), pasteurization and spray drying decreased the levels of bioactive proteins, including transforming growth factor β1 and β2, and increased protein aggregation (Støy et al. Citation2016). Although differences were observed, all tested preparations still showed trophic and anti-inflammatory activity effects on the intestine in the preterm pig model. Further in vitro work by the same group demonstrates that, at least in part, these observations could be explained by direct effects of the preparations on intestinal epithelial cells (Nguyen et al. Citation2016). Likely, this mostly relates to inactivation of bioactive whey proteins, although it cannot be excluded that processing-induced protein and amino acid modifications contribute to these observed effects.
For casein, similar results were obtained (Wang and Zhao Citation2017). In an in vitro setting, Wang et al. demonstrated that digests of glycated casein, relative to digests of non-modified casein, show altered proliferative, differentiation, and anti-apoptotic effects on epithelial cells. These results suggest that Maillard-based modifications of casein could have a direct effect on intestinal cells through mechanisms likely involving casein peptides.
3.4.3. Immunology
Food processing can alter the allergenic properties of proteins by hiding, destroying, or exposing allergic epitopes through structural changes. Milk proteins are among the best studied proteins in this area and several studies have assessed their antibody reactivity and allergic potential, after processing, in different models for allergy. As most studies in this area apply different antibodies and different modification strategies, direct comparison of individual studies is, however, challenging. Generally, for clinical relevance and conclusions around allergenicity, patient-derived sera (single or pooled) is preferred, whereas animal derived sera should only be employed for characterization of structure-immune reactivity interactions.
Sletten et al. (Citation2008) studied casein and β-lactoglobulin reactivity after processing and simulated digestion using polyclonal antibodies and milk-allergic patient sera. In this study, UHT treatment resulted in increased stability of IgE and IgG epitopes to simulated gastric digestion but had no effect on specific IgE and IgG binding. Specifically, for β-lactoglobulin, digestive stability decreased although immunogenicity was retained after simulated gastric digestion in both treated and non-treated milk.
In a similar experimental design employing polyclonal antibodies, Li et al. revealed that glycation of whey protein isolate with oligoisomaltose reduced β-lactoglobulin and α-lactalbumin reactivity after simulated gastric digestion (Li, Luo, et al. Citation2013). These results are more or less in line with results using human derived basophils and sera from cows milk allergic patients (Morisawa et al. Citation2009). This study demonstrated that heat treatment reduced the allergenicity of β-lactoglobulin by inducing conformational changes which increased its susceptibility to enzymatic digestion which disrupted B-cell epitopes.
Whey protein aggregates can be formed by heating of whey proteins as discussed in detail in section 3.2, which may affect their immunogenicity. Amongst others, this is evident from a study by Delorenzi et al. (Citation2011) who prepared different aggregates through heating and speculated that structural changes may reduce overall allergenicity of β-lactoglobulin, either through changes in proteolytic accessibility and/or shielding of major epitopes within the aggregate structure.
In another study, the impact of typical variations in industrial processing conditions were studied on αs1-, αs2-, β- and κ-casein binding by monoclonal antibodies (Dupont, Boutrou, et al. Citation2010). Heat-treatment of milk prior to spray-drying was shown to increase residual casein immunoreactivity after digestion, likely as a result of decreased digestion of casein during simulated gastrointestinal digestion and, as a consequence, increased survival of antibody epitopes, which is in line with another study from the same group (Dupont, Mandalari, et al. Citation2010). Interestingly, higher heat treatment regimes in this study also led to the formation of aggregates which may be an important factor to consider regarding uptake of milk proteins by Peyer’s patches and immunological responses including allergic sensitization.
This has been studied in detail in vivo in two elaborate studies employing purified milk proteins and a combination of in vitro and in vivo experiments (Roth-Walter et al. Citation2008; Stojadinovic et al. Citation2014). The study by Roth-Walter et al. (Citation2008) aimed at unraveling the mechanisms underlying milk protein sensitization and the effect of processing, which identified that uptake of aggregated milk proteins though Peyer’s patches may be one of the underlying mechanisms of milk protein sensitization. Typical pasteurization processes caused aggregation of β-lactoglobulin and α-lactalbumin, inhibiting their uptake by intestinal epithelial cells both in vitro and in vivo. Interestingly, in mice uptake of these aggregates appeared to be redirected to Peyer’s patches and promoted a higher Th2-associated antibody and cytokine response relative to their non-pasteurized counterparts. In milk protein sensitized mice, only non-aggregated soluble whey proteins elicited an anaphylactic response when administrated orally, where casein and whey protein aggregates required a systemic administration to induce anaphylaxis. Overall, the authors concluded that allergy development may be affected by processing both pre- and post-sensitization: Initial sensitization by aggregated proteins through uptake of modified protein (aggregates) by Peyer’s patches and eventual uptake of soluble proteins/fragments which induce anaphylaxis. These results are in line with more recent findings comparing crosslinked and native β-lactoglobulin where crosslinking of β-lactoglobulin increased its sensitizing capacity through increased sampling of β-lactoglobulin through Peyer’s patches (Stojadinovic et al. Citation2014). Interestingly, in a similar experimental design, non-native β-lactoglobulin was also demonstrated to cause a more intense local immunologic response in the intestinal mucosa than its native form (Rytkönen et al. Citation2002), which could, speculatively, cause additional local inflammation-associated complications.
In conclusion, processing induced milk protein modification may thus alter their allergic potential through different processes with relevance for both sensitization and subsequent allergic response.
Further, modified milk proteins may affect the immune system through advanced-glycation end-products. In an in vitro setting, Deo et al. (Citation2009) demonstrated that digested dietary AGE, as prepared by dry-heating casein and glucose, complexed with serum albumin, may regulate expression of RAGE (receptor for AGE) and downstream inflammatory pathways.
Poulsen et al. (Citation2016) compared the effect of diets containing milk protein of which different proportions were intensively heated (70 °C for 7 days). Increased intake of intensively heated milk protein was associated with an increased expression of the AGE receptors RAGE and AGER1 in whole blood (predominantly when the protein source was in the form of a milk protein hydrolysate). The group where RAGE and AGER1 was increased also had the highest urinary excretion of the AGE methylglyoxal-derived hydroimidazolone, which is associated with age-related diseases (Maessen, Stehouwer, and Schalkwijk Citation2015). In summary, although it is clear that milk protein modification (predominantly glycation) can affect advanced glycation end-product receptor signaling, the overall physiological consequences remain to be identified.
3.4.4. Antioxidant activity
Milk proteins may exert antioxidant activity, either as intact proteins or as digest (Corrochano et al. Citation2018; Nongonierma and FitzGerald Citation2015). Most studies in this area employ in vitro assays and/or direct cell exposure of proteins and peptides. The physiological relevance of these in vitro assays, however, remains to be elucidated. Although some in vivo studies are available that assess the relevance of milk antioxidant activity (Fardet and Rock Citation2018), there is a need for controlled human intervention studies in this area to reveal the overall impact on health. Processing, and in particular the Maillard reaction, may increase the antioxidant and radical scavenging activity of milk protein and its digest, which is evident from several studies that looked at the antioxidant capacity of milk products before and after processing (Gu et al. Citation2010; Şanlidere Aloǧlu Citation2013). Although the increase in antioxidant capacity may possibly be beneficial, other effects of the Maillard reaction as discussed in this review may be less favorable.
3.4.5. Calcium homeostasis
Milk products and proteins are important dietary contributors to human mineral homeostasis by acting as a source of minerals and regulating their absorption (van den Heuvel and Steijns Citation2018). Milk protein Maillard reaction products may reduce mineral solubility and bioavailability during gastrointestinal digestion which has been discussed for several food preparations and model Maillard reaction products, both in vitro and in vivo (Mesías, Seiquer, and Navarro Citation2009; Sarriá, López-Fandiño, and Vaquero Citation2001). Specifically, for milk and milk proteins, several studies are available. In vitro experiments using heated casein-glucose-fructose solutions revealed that calcium solubility was lowered which resulted in a decreased in vitro absorption of calcium (Seiquer et al. Citation2001). However, in vivo absorption appeared to be unaffected, although urinary calcium excretion was increased compared to non-heated controls. In a more recent study from the same authors, UHT milk was compared with overheated milk (3 severe sterilization heatings, 16 minutes at 116 °C) both in vitro and in vivo (Seiquer et al. Citation2010). Relative to UHT treatment, severe heating resulted in a reduced calcium solubility during in vitro digestion, which in turn resulted in a decreased calcium absorption and retention in rats.
Overall, further long-term and human intervention studies are needed to investigate the overall effect of processing-modified milk proteins on mineral homeostasis.
4. Conclusion and future perspectives
This systematic review demonstrates that dairy processing significantly affects protein quality and can be used as a tool to steer gastrointestinal protein digestion. Glycation as a result of heat processing decreases protein digestibility and amino acid availability, leading to a decrease in protein quality. Oxidation, racemization, dephosphorylation, and cross-linking are less well studied chemical modifications that may impact protein quality as well as digestibility. In contrast, protein denaturation does not affect overall digestibility, despite that gastric hydrolysis of whey proteins (predominantly β-lactoglobulin) is increased when heated. Nonetheless, protein denaturation can be used as a tool to alter gastric emptying of the protein, eventually resulting in a different post-prandial plasma amino acid appearance, although the matrix will play a role as well. Physiological effects for a change in digestibility or digestion kinetics mainly point towards amino acid bioavailability and immunological consequences.
Although the effects of processing-induced milk protein modifications have been extensively studied, several aspects remain to be elucidated in future research.
4.1. Human studies
Although many studies highlight the negative impact on protein digestion and overall protein quality, there is a need for more human studies to identify overall physiological relevance of digestive differences as a result of processing-induced protein modifications. In particular, the metabolic fate and longer-term impact of modified amino acids is, to a large extent, still unclear.
For overall relevance to the food industry, it is preferred to adopt commercially relevant processes and products. The latter as interactions between different food components beyond protein and reducing sugars are likely to affect the overall outcome, including level of modification and overall physiological effects.
4.2. Stages of Maillard reaction and other modifications
As highlighted by a number of studies discussed in this review, the impact of early and late stage Maillard reaction products on digestion and overall protein quality may differ. Moreover, it is evident that small changes in heating conditions may have a large impact. From an industrial perspective, this may be an important area of research as the extreme heating conditions tested in several previous studies are typically not applied in dairy processing. Especially of relevance for the more intense processing conditions, Maillard-based reactions may be coinciding with other chemical changes as discussed in this review (). In this respect, there is thus a need to assess effects of the different stages of the Maillard reaction separately, and in conjunction with other modifications, both from an analytical and functional perspective, as in most studies to date this is mostly not clear.
4.3. Glycosylation stability
Glycosylation of milk proteins (predominantly lactoferrin, immunoglobulins, κ-casein, and proteins of the milk fat globule membrane) contributes to overall milk protein structure and functionality (O’Riordan et al. Citation2014). In addition to being a source of glycans, glycosylation of individual milk proteins has been associated with antipathogenic, prebiotic (mainly bifidogenic), immunomodulatory and satiety effects, mostly by studies that study glycosylation-depleted proteins. That milk protein processing can affect protein glycosylation, and thus its digestion and potentially associated functionalities, is evident from several studies which investigated whey and glycomacropeptide glycosylation patterns (Ferron-Baumy et al. Citation1992; Floris et al. Citation2010; Taylor and Woonton Citation2009). Variation in heat treatments of milk demonstrated that this influenced the level of glycosylation of (cheese-derived) glycomacropeptide and whey protein. Thus, in addition to direct chemical and structural modification of amino acids, processing of milk may affect protein glycosylation stability and as a results the overall structure and activity of individual milk proteins. However, effects of milk protein deglycosylation on their digestion and overall functionality remain to be identified, which could thus be another angle for future research in the field of processing-induced protein modifications.
4.4. In vitro digestion
For in vitro digestion analyses, a harmonized approach would be recommended as, to date, in vitro digestion procedures in the area of milk protein research and processing-induced modifications are not identical. Differences include, for example, proteases and protease concentrations, digestive pH, protein/enzyme ratio’s, incubation time, etc. which to a large extent will affect overall outcomes. In this respect, it is recommended to adopt a straightforward harmonized approach, as e.g. recently discussed by the INFOGEST network (Brodkorb et al. Citation2019).
Although progress has been made to establish a more quantitative relation between glycation and digestion (Deng et al. Citation2017), a harmonized in vitro digestion procedure would also allow to assess better the quantitative relation between processing-induced protein modifications and digestion among the different studies and tested processing conditions. Ultimately, quantitative assessments of milk protein modifications and digestion will enable industry to further develop processes that will have a minimum impact on overall milk protein modification.
With respect to translatability to overall physiological relevance of processing-induced modifications, more complex models may be required. Particularly for studies aimed at unraveling the fate of modified amino acids and studies employing epithelial/immune cells, brush border membrane proteases should ideally be included in the in vitro digestion procedure.
4.5. Prebiotic activity
The dairy industry is mainly aimed at preventing processing-induced protein modifications. Nonetheless, several in vitro studies highlight the potential of Maillard based conjugation of milk proteins and carbohydrates as novel food ingredients with a possible beneficial prebiotic character (Corzo-Martínez et al. Citation2012; Hernandez-Hernandez et al. Citation2011; Luz Sanz et al. Citation2007). Corzo-Martínez et al. (Citation2012) specifically focused on the growth of bifidobacterial and lactic acid bacteria and demonstrated that both the carbohydrate and protein source in the conjugates matter, with strain-specific effects. Hernandez-Hernandez et al. (Citation2011) demonstrated that β-lactoglobulin conjugated with GOS showed similar fecal fermentation effects as purified GOS. However, from the work of Sanz and colleagues it is also evident that GOS conjugation to milk proteins coincides with a reduced digestibility. Taken together, the overall relevance of these postulated novel prebiotics thus need further evaluation (Luz Sanz et al. Citation2007).
References
- Alamir, I., C. Niquet-Leridon, P. Jacolot, C. Rodriguez, M. Orosco, P. M. Anton, and F. J. Tessier. 2013. Digestibility of extruded proteins and metabolic transit of N ε-carboxymethyllysine in rats. Amino Acids 44 (6):1441–9. doi: 10.1007/s00726-012-1427-3.
- Barbé, F., S. Le Feunteun, D. Rémond, O. Ménard, J. Jardin, G. Henry, B. Laroche, and D. Dupont. 2014. Tracking the in vivo release of bioactive peptides in the gut during digestion: Mass spectrometry peptidomic characterization of effluents collected in the gut of dairy matrix fed mini-pigs. Food Research International 63:147–56. doi: 10.1016/j.foodres.2014.02.015.
- Barbé, F., O. Ménard, Y. Le Gouar, C. Buffière, M.-H. Famelart, B. Laroche, S. Le Feunteun, D. Dupont, and D. Rémond. 2013. The heat treatment and the gelation are strong determinants of the kinetics of milk proteins digestion and of the peripheral availability of amino acids. Food Chemistry 136 (3–4):1203–12. doi: 10.1016/j.foodchem.2012.09.022.
- Bernasconi, E., R. Fritsché, and B. Corthésy. 2006. Specific effects of denaturation, hydrolysis and exposure to Lactococcus lactis on bovine β-lactoglobulin transepithelial transport, antigenicity and allergenicity. Clinical & Experimental Allergy 36 (6):803–14. doi: 10.1111/j.1365-2222.2006.02504.x.
- Brodkorb, A., L. Egger, M. Alminger, P. Alvito, R. Assunção, S. Ballance, T. Bohn, C. Bourlieu-Lacanal, R. Boutrou, F. Carrière, et al. 2019. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nature Protocols 14 (4):991–1014. doi: 10.1038/s41596-018-0119-1.
- Bui, T. P. N., J. Ritari, S. Boeren, P. de Waard, C. M. Plugge, and W. M. de Vos. 2015. Production of butyrate from lysine and the amadori product fructoselysine by a human gut commensal. Nature Communications 6 (1):10062. doi: 10.1038/ncomms10062.
- Carbonaro, M., M. Cappelloni, S. Sabbadini, and E. Carnovale. 1997. Disulfide reactivity and in vitro protein digestibility of different Thermal-Treated milk samples and whey proteins. Journal of Agricultural and Food Chemistry 45 (1):95–100. doi: 10.1021/jf950828i.
- Cattaneo, S., M. Stuknytė, F. Masotti, and I. De Noni. 2017. Protein breakdown and release of β-casomorphins during in vitro gastro-intestinal digestion of sterilised model systems of liquid infant formula. Food Chemistry 217:476–82. doi: 10.1016/j.foodchem.2016.08.128.
- Chang, C. H., and X. H. Zhao. 2012. In vitro digestibility and rheological properties of caseinates treated by an oxidative system containing horseradish peroxidase, glucose oxidase and glucose. International Dairy Journal 27 (1-2):47–52. doi: 10.1016/j.idairyj.2012.07.004.
- Corrochano, A. R., V. Buckin, P. M. Kelly, and L. Giblin. 2018. Invited review: Whey proteins as antioxidants and promoters of cellular antioxidant pathways. Journal of Dairy Science 101 (6):4747–61. doi: 10.3168/jds.2017-13618.
- Corzo-Martínez, M., M. Ávila, F. J. Moreno, T. Requena, and M. Villamiel. 2012. Effect of milk protein glycation and gastrointestinal digestion on the growth of bifidobacteria and lactic acid bacteria. International Journal of Food Microbiology 153 (3):420–7. doi: 10.1016/j.ijfoodmicro.2011.12.006.
- Corzo-Martínez, M., A. C. Soria, J. Belloque, M. Villamiel, and F. J. Moreno. 2010. Effect of glycation on the gastrointestinal digestibility and immunoreactivity of bovine β-lactoglobulin. International Dairy Journal 20 (11):742–52. doi: 10.1016/j.idairyj.2010.04.002.
- Culver, C. A., and H. E. Swaisgood. 1989. Changes in the digestibility of dried casein and glucose mixtures occurring during storage at different temperatures and water activities. Journal of Dairy Science 72 (11):2916–20. doi: 10.3168/jds.S0022-0302(89)79442-X.
- de Vrese, M., R. Frik, N. Roos, and H. Hagemeister. 2000. Protein-bound D-amino acids, and to a lesser extent lysinoalanine, decrease true ileal protein digestibility in minipigs as determined with (15)N-labeling. The Journal of Nutrition 130 (8):2026–31. doi: 10.1093/jn/130.8.2026.
- Delorenzi, N. J., A. Moro, P. A. Busti, G. D. Báez, and G. A. Ballerinia. 2011. Effects of structural changes in β-lactoglobulin on its allergenicity. In Whey: Types, composition and health implications, eds. G. M. Ortero and R. M. Benitez, 169–82. Hauppauge, NY: Nova Science Publishers.
- Deng, Y., P. A. Wierenga, H. A. Schols, S. Sforza, and H. Gruppen. 2017. Effect of Maillard induced glycation on protein hydrolysis by lysine/arginine and non-lysine/arginine specific proteases. Food Hydrocolloids 69:210–9. doi: 10.1016/j.foodhyd.2017.02.007.
- Deo, P., J. V. Glenn, L. A. Powell, A. W. Stitt, and J. M. Ames. 2009. Upregulation of oxidative stress markers in human microvascular endothelial cells by complexes of serum albumin and digestion products of glycated casein. Journal of Biochemical and Molecular Toxicology 23 (5):364–72. doi: 10.1002/jbt.20301.
- Desrosiers, T., and L. Savoie. 1991. Extent of damage to amino acid availability of whey protein hearted with sugar. Journal of Dairy Research 58 (4):431–41.
- Desrosiers, T., L. Savoie, G. Bergeron, and G. Parent. 1989. Estimation of lysine damage in heated whey proteins by furosine determinations in conjunction with the digestion cell technique. Journal of Agricultural and Food Chemistry 37 (5):1385–91. doi: 10.1021/jf00089a039.
- Donato, L., and F. Guyomarc’h. 2009. Formation and properties of the whey protein/-casein complexes in heated skim milk – A review. Dairy Science and Technology 89 (1):3–29. doi: 10.1051/dst:2008033.
- Dupont, D., R. Boutrou, O. Menard, J. Jardin, G. Tanguy, P. Schuck, B. B. Haab, and J. Leonil. 2010. Heat treatment of milk during powder manufacture increases casein resistance to simulated infant digestion. Food Digestion 1 (1–2):28–39. doi: 10.1007/s13228-010-0003-0.
- Dupont, D., G. Mandalari, D. Mollé, J. Jardin, O. Rolet-Répécaud, G. Duboz, J. Léonil, C. E. N. Mills, and A. R. Mackie. 2010. Food processing increases casein resistance to simulated infant digestion. Molecular Nutrition & Food Research 54 (11):1677–89. doi: 10.1002/mnfr.200900582.
- Efigenia, M., B. Povoa, and T. Moraes-Santos. 1997. Effect of heat treatment on the nutritional quality of milk proteins. International Dairy Journal 7 (8/9):609–12. doi: 10.1016/S0958-6946(97)00049-6.
- EFSA. 2012. Scientific opinion on dietary reference values for protein. EFSA Journal 10 (2):2557. doi: 10.2903/j.efsa.2012.2557.
- Erbersdobler, H. 1989. Protein reactions during food processing and storage – Their relevance to human nutrition. Bibliotheka Nutrio et Dieta 43:140–55.
- Fardet, A., and E. Rock. 2018. In vitro and in vivo antioxidant potential of milks, yoghurts, fermented milks and cheeses: A narrative review of evidence. Nutrition Research Reviews 31 (1):52–70. doi: 10.1017/S0954422417000191.
- Feng, X., C. Li, N. Ullah, J. Cao, Y. Lan, W. Ge, R. M. Hackman, Z. Li, and L. Chen. 2015. Susceptibility of whey protein isolate to oxidation and changes in physicochemical, structural, and digestibility characteristics. Journal of Dairy Science 98 (11):7602–13. doi: 10.3168/jds.2015-9814.
- Ferrer, E., A. Alegría, R. Farré, P. Abellán, and F. Romero. 1999. Review: Indicators of damage of protein quality and nutritional value of milk. Food Science and Technology International 5 (6):447–61. doi: 10.1177/108201329900500602.
- Ferron-Baumy, C., D. Molle, G. Garric, and J. L. Maubois. 1992. Characterization of caseinomacropeptides released from renneted raw and UHT treated milks. Le Lait 72 (2):165–73. doi: 10.1051/lait:1992211.
- Floris, R., T. Lambers, A. Alting, and J. Kiers. 2010. Trends in infant formulas: A dairy perspective. In Improving the safety and quality of milk, ed. M. Griffiths, 454–74. Amsterdam, the Netherlands: Elsevier. doi: 10.1533/9781845699437.3.454.
- Fox, M., G. Georgi, G. Boehm, D. Menne, M. Fried, and M. Thumshirn. 2004. Dietary protein precipitation properties have effects on gastric emptying in healthy volunteers. Clinical Nutrition 23 (4):641–6. doi: 10.1016/j.clnu.2003.10.013.
- Friedman, M. 1999. Chemistry, biochemistry, nutrition, and microbiology of lysinoalanine, lanthionine, and histidinoalanine in food and other proteins. Journal of Agricultural and Food Chemistry 47 (4):1295–319. doi: 10.1021/jf981000 + .
- Friedman, M. 2010. Origin, microbiology, nutrition, and pharmacology of D-Amino acids. Chemistry & Biodiversity 7 (6):1491–530. doi: 10.1002/cbdv.200900225.
- Gerrard, J. A., M. Lasse, J. Cottam, J. P. Healy, S. E. Fayle, I. Rasiah, P. K. Brown, S. M. BinYasir, K. H. Sutton, and N. G. Larsen. 2012. Aspects of physical and chemical alterations to proteins during food processing – Some implications for nutrition. British Journal of Nutrition 108 (S2):S288–S297. doi: 10.1017/S000711451200236X.
- Gilani, G. S., and E. Sepehr. 2003. Protein digestibility and quality in products containing antinutritional factors are adversely affected by old age in rats. The Journal of Nutrition 133 (1):220–5. doi: 10.1093/jn/133.1.220.
- Gu, F. L., J. M. Kim, S. Abbas, X. M. Zhang, S. Q. Xia, and Z. X. Chen. 2010. Structure and antioxidant activity of high molecular weight Maillard reaction products from casein-glucose. Food Chemistry 120 (2):505–11. doi: 10.1016/j.foodchem.2009.10.044.
- Guo, M. R., P. F. Fox, A. Flynn, and P. S. Kindstedt. 1995. Susceptibility of β-lactoglobulin and sodium caseinate to proteolysis by pepsin and trypsin. Journal of Dairy Science 78 (11):2336–44. doi: 10.3168/jds.S0022-0302(95)76860-6.
- Guyomarc’h, F., F. Warin, D. Donald Muir, and J. Leaver. 2000. Lactosylation of milk proteins during the manufacture and storage of skim milk powders. International Dairy Journal 10 (12):863–72. doi: 10.1016/S0958-6946(01)00020-6.
- Hellwig, M., and T. Henle. 2013. Release of pyrraline in absorbable peptides during simulated digestion of casein glycated by 3-deoxyglucosone. European Food Research and Technology 237 (1):47–55. doi: 10.1007/s00217-013-2027-5.
- Hernandez-Hernandez, O., F. J. Moreno, S. Kolida, R. A. Rastall, and M. L. Sanz. 2011. Effect of glycation of bovine β-lactoglobulin with galactooligosaccharides on the growth of human faecal bacteria. International Dairy Journal 21 (12):949–52. doi: 10.1016/j.idairyj.2011.06.002.
- Hiller, B., and P. C. Lorenzen. 2010. Functional properties of milk proteins as affected by Maillard reaction induced oligomerisation. Food Research International 43 (4):1155–66. doi: 10.1016/j.foodres.2010.02.006.
- Huppertz, T., P. F. Fox, and A. L. Kelly. 2018. The caseins: Structure, stability, and functionality. In Proteins in food processing, ed. R. Y. Yada, 49–92. Amsterdam, the Netherlands: Elsevier. doi: 10.1016/B978-0-08-100722-8.00004-8.
- Joubran, Y., A. Moscovici, and U. Lesmes. 2015. Antioxidant activity of bovine alpha lactalbumin Maillard products and evaluation of their in vitro gastro-duodenal digestive proteolysis. Food & Function 6 (4):1229–40. doi: 10.1039/C4FO01165A.
- Joubran, Y., A. Moscovici, R. Portmann, and U. Lesmes. 2017. Implications of the Maillard reaction on bovine alpha-lactalbumin and its proteolysis during in vitro infant digestion. Food & Function 8 (6):2295–308. doi: 10.1039/C7FO00588A.
- Kitabatake, N., and Y. I. Kinekawa. 1998. Digestibility of bovine milk whey protein and β-lactoglobulin in vitro and in vivo. Journal of Agricultural and Food Chemistry 46 (12):4917–23. doi: 10.1021/jf9710903.
- Lacroix, M., C. Bon, C. Bos, J. Léonil, R. Benamouzig, C. Luengo, J. Fauquant, D. Tomé, and C. Gaudichon. 2008. Ultra high temperature treatment, but not pasteurization, affects the postprandial kinetics of milk proteins in humans. The Journal of Nutrition 138 (12):2342–7. doi: 10.3945/jn.108.096990.
- Lacroix, M., J. Léonil, C. Bos, G. Henry, G. Airinei, J. Fauquant, D. Tomé, and C. Gaudichon. 2006. Heat markers and quality indexes of industrially heat-treated [15N] milk protein measured in rats. Journal of Agricultural and Food Chemistry 54 (4):1508–17. doi: 10.1021/jf051304d.
- Lamothe, S., N. Rémillard, J. Tremblay, and M. Britten. 2017. Influence of dairy matrices on nutrient release in a simulated gastrointestinal environment. Food Research International 92:138–46. doi: 10.1016/j.foodres.2016.12.026.
- Larsen, J. A., A. J. Fascetti, C. C. Calvert, and Q. R. Rogers. 2010. Bioavailability of lysine for kittens in overheated casein is underestimated by the rat growth assay method. Journal of Animal Physiology and Animal Nutrition 94 (5):e102. doi: 10.1111/j.1439-0396.2010.00988.x.
- Lee, K., and H. F. Erbersdobler. 2005. Balance experiments on human volunteers with ε-fructoselysine (FL) and lysinoalanine (LAL). In Maillard reactions in chemistry, food and health, eds. T. P. Labuza, G. A. Reineccius, V. M. Monnier, J. O'Brien, and J.W. Baynes, 358–63. Amsterdam, the Netherlands: Elsevier. doi: 10.1533/9781845698393.4.358.
- Li, Y., M. V. Ostergaard, P. Jiang, D. E. W. Chatterton, T. Thymann, A. S. Kvistgaard, and P. T. Sangild. 2013. Whey protein processing influences Formula-Induced gut maturation in preterm pigs. The Journal of Nutrition 143 (12):1934–42. doi: 10.3945/jn.113.182931.
- Li, Y., D. N. Nguyen, K. Obelitz-Ryom, A. D. Andersen, T. Thymann, D. E. W. Chatterton, S. Purup, A. B. Heckmann, S. B. Bering, and P. T. Sangild. 2018. Bioactive whey protein concentrate and lactose stimulate gut function in formula-fed preterm pigs. Journal of Pediatric Gastroenterology and Nutrition 66 (1):128–34. doi: 10.1097/MPG.0000000000001699.
- Li, Z., Y. Luo, L. Feng, and P. Liao. 2013. Effect of Maillard reaction conditions on antigenicity of β-lactoglobulin and the properties of glycated whey protein during simulated gastric digestion. Food and Agricultural Immunology 24 (4):433–43. doi: 10.1080/09540105.2012.712951.
- Liardon, R., and R. F. Hurrell. 1983. Amino acid racemization in heated and alkali-treated proteins. Journal of Agricultural and Food Chemistry 37:432–7. doi: 10.1021/jf00116a062.
- Lindberg, T., S. Engberg, L. B. Sjöberg, and B. Lönnerdal. 1998. In vitro digestion of proteins in human milk fortifiers and in preterm formula. Journal of Pediatric Gastroenterology & Nutrition 27 (1):30–6. doi: 10.1097/00005176-199807000-00006.
- Liu, F., M. Teodorowicz, H. J. Wichers, M. A. J. S. Van Boekel, and K. A. Hettinga. 2016. Generation of soluble advanced glycation end products receptor (sRAGE)-binding ligands during extensive heat treatment of whey protein/lactose mixtures is dependent on glycation and aggregation. Journal of Agricultural and Food Chemistry 64 (33):6477–86. doi: 10.1021/acs.jafc.6b02674.
- Liusuwan, R. A., and B. Lönnerdal. 1996. Solubility and digestibility of milk proteins in different forms of infant formulas. FASEB Journal 10 (3).
- Lorieau, L., A. Halabi, A. Ligneul, E. Hazart, D. Dupont, and J. Floury. 2018. Impact of the dairy product structure and protein nature on the proteolysis and amino acid bioaccessiblity during in vitro digestion. Food Hydrocolloids 82:399–411. doi: 10.1016/j.foodhyd.2018.04.019.
- Loveday, S. M., M. R. Peram, H. Singh, A. Ye, and G. B. Jameson. 2014. Digestive diversity and kinetic intrigue among heated and unheated β-lactoglobulin species. Food Funct. 5 (11):2783–91. doi: 10.1039/C4FO00362D.
- Luz Sanz, M., M. Corzo-Martínez, R. a Rastall, A. Olano, and F. J. Moreno. 2007. Characterization and in vitro digestibility of bovine beta-lactoglobulin glycated with galactooligosaccharides. Journal of Agricultural and Food Chemistry 55 (19):7916–25. doi: 10.1021/jf071111l.
- Maessen, D. E. M., C. D. A. Stehouwer, and C. G. Schalkwijk. 2015. The role of methylglyoxal and the glyoxalase system in diabetes and other age-related diseases. Clinical Science 128 (12):839–61. doi: 10.1042/CS20140683.
- Mauron, J. 1990. Influence of processing on protein quality. Journal of Nutritional Science and Vitaminology 36 Suppl 1:S57–S69. doi: 10.3177/jnsv.36.4-SupplementI_S57.
- Mehta, B. M., and H. C. Deeth. 2016. Blocked lysine in dairy products: Formation, occurrence, analysis, and nutritional implications. Comprehensive Reviews in Food Science and Food Safety 15 (1):206–18. doi: 10.1111/1541-4337.12178.
- Meltretter, J., S. Seeber, A. Humeny, C.-M. Becker, and M. Pischetsrieder. 2007. Site-Specific formation of Maillard, oxidation, and condensation products from whey proteins during reaction with lactose. Journal of Agricultural and Food Chemistry 55 (15):6096. doi: 10.1021/jf0705567.
- Mesías, M., I. Seiquer, and M. P. Navarro. 2009. Influence of diets rich in Maillard reaction products on calcium bioavailability. Assays in male adolescents and in caco-2 cells. Journal of Agricultural and Food Chemistry 57 (20):9532–8. doi: 10.1021/jf9018646.
- Moher, D., A. Liberati, J. Tetzlaff, D. G. Altman, and P. Grp. 2009. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement (reprinted from Annals of Internal Medicine). Physical Therapy 89 (9):873–80. doi: 10.1371/journal.pmed.1000097.
- Morgan, F., J. Léonil, D. Mollé, and S. Bouhallab. 1999. Modification of bovine β-lactoglobulin by glycation in a powdered state or in an aqueous solution: Effect on association behavior and protein conformation. Journal of Agricultural and Food Chemistry 47 (1):83–91. doi: 10.1021/JF9804387.
- Morisawa, Y., A. Kitamura, T. Ujihara, N. Zushi, K. Kuzume, Y. Shimanouchi, S. Tamura, H. Wakiguchi, H. Saito, and K. Matsumoto. 2009. Effect of heat treatment and enzymatic digestion on the B cell epitopes of cow’s milk proteins. Clinical & Experimental Allergy 39 (6):918–25. doi: 10.1111/j.1365-2222.2009.03203.x.
- Moughan, P. J., M. N. Wilson, C. H. M. Smits, and W. C. Smith. 1989. An evaluation of skim milk powders subjected to different heating conditions during processing as dietary protein sources for the young pig. Animal Feed Science and Technology 22 (3):203–15. doi: 10.1016/0377-8401(89)90062-X.
- Moughan, P. J., M. P. J. Gall, and S. M. Rutherfurd. 1996. Absorption of lysine and deoxyketosyllysine in an early-Maillard browned casein by the growing pig. Journal of Agricultural and Food Chemistry 44 (6):1520–5. doi: 10.1021/jf950428v.
- Moughan, P. J., and S. M. Rutherfurd. 1996. A new method for determining digestible reactive lysine in foods. Journal of Agricultural and Food Chemistry 44 (8):2202–9. doi: 10.1021/jf950032j.
- Mpassi, D., G. Rychen, M. Mertes, and F. Laurent. 2001. Portal absorption of 45Ca from labelled milk, yoghurt or heat treated yoghurt in the growing pig. International Dairy Journal 11 (10):809–15. doi: 10.1016/S0958-6946(01)00107-8.
- Mulet-Cabero, A.-I., A. R. Mackie, P. J. Wilde, M. A. Fenelon, and A. Brodkorb. 2019. Structural mechanism and kinetics of in vitro gastric digestion are affected by process-induced changes in bovine milk. Food Hydrocolloids 86:172–83. doi: 10.1016/j.foodhyd.2018.03.035.
- Nguyen, D. N., P. T. Sangild, Y. Li, S. B. Bering, and D. E. W. Chatterton. 2016. Processing of whey modulates proliferative and immune functions in intestinal epithelial cells. Journal of Dairy Science 99 (2):959–69. doi: 10.3168/jds.2015-9965.
- Nongonierma, A. B., and R. J. FitzGerald. 2015. Bioactive properties of milk proteins in humans: A review. Peptides 73:20–34. doi: 10.1016/j.peptides.2015.08.009.
- Nooshkam, M., and A. Madadlou. 2016. Microwave-assisted isomerisation of lactose to lactulose and Maillard conjugation of lactulose and lactose with whey proteins and peptides. Food Chemistry 200:1–9. doi: 10.1016/j.foodchem.2015.12.094.
- Nowak-Wegrzyn, A., and A. Fiocchi. 2009. Rare, medium, or well done? the effect of heating and food matrix on food protein allergenicity. Current Opinion in Allergy and Clinical Immunology 9 (3):234–7. doi: 10.1097/ACI.0b013e32832b88e7.
- Nyangale, E. P., D. S. Mottram, and G. R. Gibson. 2012. Gut microbial activity, implications for health and disease: The potential role of metabolite analysis. Journal of Proteome Research 11 (12):5573–85. doi: 10.1021/pr300637d.
- O’Riordan, N., M. Kane, L. Joshi, and R. M. Hickey. 2014. Structural and functional characteristics of bovine milk protein glycosylation. Glycobiology 24 (3):220–36. doi: 10.1093/glycob/cwt162.
- Pellegrino, L., S. Cattaneo, and I. De Noni. 2011. Nutrition and health | Effects of processing on protein quality of milk and milk products. In Encyclopedia of dairy sciences, eds. J. W. Fuquay, P. L. H. McSweeney, and P. F. Fox, 1067–74. Cambridge, MA: Academic Press. doi: 10.1016/B978-0-12-374407-4.00383-6.
- Peram, M. R., S. M. Loveday, A. Ye, and H. Singh. 2013. In vitro gastric digestion of heat-induced aggregates of β-lactoglobulin. Journal of Dairy Science 96 (1):63–74. doi: 10.3168/jds.2012-5896.
- Pinto, M. S., J. Léonil, G. Henry, C. Cauty, A. Ô. F. Carvalho, and S. Bouhallab. 2014. Heating and glycation of β-lactoglobulin and β-casein: Aggregation and in vitro digestion. Food Research International 55:70–6. doi: 10.1016/j.foodres.2013.10.030.
- Pompei, C., M. Rossi, and F. Mare. 1988. Protein quality in commercial milk-based infant formulas. Journal of Food Quality 10 (6):375–91. doi: 10.1111/j.1745-4557.1988.tb00298.x.
- Poulsen, M. W., J. M. Andersen, R. V. Hedegaard, A. N. Madsen, B. N. Krath, R. Monošík, M. J. Bak, J. Nielsen, B. Holst, L. H. Skibsted, et al. 2016. Short-term effects of dietary advanced glycation end products in rats. British Journal of Nutrition 115 (4):629–36. doi: 10.1017/S0007114515004833.
- Poulsen, M. W., R. V. Hedegaard, J. M. Andersen, B. de Courten, S. Bügel, J. Nielsen, L. H. Skibsted, and L. O. Dragsted. 2013. Advanced glycation endproducts in food and their effects on health. Food and Chemical Toxicology 60:10–37. doi: 10.1016/j.fct.2013.06.052.
- Rahaman, T., T. Vasiljevic, and L. Ramchandran. 2017. Digestibility and antigenicity of β-lactoglobulin as affected by heat, pH and applied shear. Food Chemistry 217:517–23. doi: 10.1016/j.foodchem.2016.08.129.
- Rérat, A., R. Calmes, P. Vaissade, and P. A. Finot. 2002. Nutritional and metabolic consequences of the early Maillard reaction of heat treated milk in the pig. Significance for man. European Journal of Nutrition 41 (1):1–11. doi: 10.1007/s003940200000.
- Rinaldi, L., S. F. Gauthier, M. Britten, and S. L. Turgeon. 2014. In vitro gastrointestinal digestion of liquid and semi-liquid dairy matrixes. LWT - Food Science and Technology 57 (1):99–105. doi: 10.1016/j.lwt.2014.01.026.
- Rinaldi, L., L. E. Rioux, M. Britten, and S. L. Turgeon. 2015. In vitro bioaccessibility of peptides and amino acids from yogurt made with starch, pectin, or beta-glucan. International Dairy Journal 46:39–45. doi: 10.1016/j.idairyj.2014.09.005.
- Roth-Walter, F., M. C. Berin, P. Arnaboldi, C. R. Escalante, S. Dahan, J. Rauch, E. Jensen-Jarolim, and L. Mayer. 2008. Pasteurization of milk proteins promotes allergic sensitization by enhancing uptake through Peyer’s patches. Allergy 63 (7):882–90. doi: 10.1111/j.1398-9995.2008.01673.x.
- Rudloff, S., and B. Lönnerdal. 1992. Solubility and digestibility of milk proteins in infant formulas exposed to different heat treatments. Journal of Pediatric Gastroenterology and Nutrition 15 (1):25–33. doi: 10.1097/00005176-199207000-00005.
- Rutherfurd, S. M., and P. J. Moughan. 2008. Effect of elevated temperature storage on the digestible reactive lysine content of unhydrolyzed- and hydrolyzed-lactose milk-based products. Journal of Dairy Science 91 (2):477–82. doi: 10.3168/jds.2007-0612.
- Rutherfurd, S. M., C. A. Montoya, and P. J. Moughan. 2014. Effect of oxidation of dietary proteins with performic acid on true ileal amino acid digestibility as determined in the growing rat. Journal of Agricultural and Food Chemistry 62 (3):699–707. doi: 10.1021/jf403146u.
- Rutherfurd, S. M., and P. J. Moughan. 1997. Application of a new method for determining digestible reactive lysine to variably heated protein sources. Journal of Agricultural and Food Chemistry 45 (5):1582–6. doi: 10.1021/jf960788y.
- Rytkönen, J., T. J. Karttunen, R. Karttunen, K. H. Valkonen, M. C. Jenmalm, T. Alatossava, … J. Kokkonen. 2002. Effect of heat denaturation on beta-lactoglobulin-induced gastrointestinal sensitization in rats: Denatured βLG induces a more intensive local immunologic response than native βLG. Pediatric Allergy and Immunology 13 (4):269–77. doi: 10.1034/j.1399-3038.2002.01028.x.
- Rytkönen, J., K. H. Valkonen, V. Virtanen, R. A. Foxwell, J. M. Kyd, A. W. Cripps, and T. J. Karttunen. 2006. Enterocyte and M-cell transport of native and heat-denatured bovine β-lactoglobulin: Significance of heat denaturation. Journal of Agricultural and Food Chemistry 54 (4):1500–7. doi: 10.1021/jf052309d.
- Sánchez-Rivera, L., O. Ménard, I. Recio, and D. Dupont. 2015. Peptide mapping during dynamic gastric digestion of heated and unheated skimmed milk powder. Food Research International 77:132–9. doi: 10.1016/j.foodres.2015.08.001.
- Şanlidere Aloǧlu, H. 2013. The effect of various heat treatments on the antioxidant capacity of milk before and after simulated gastrointestinal digestion. International Journal of Dairy Technology 66 (2):170–4. doi: 10.1111/1471-0307.12021.
- Sarriá, B., R. López-Fandiño, and M. P. Vaquero. 2000. Protein nutritive utilization in rats fed powder and liquid infant formulas.. Food Science and Technology International 9 (1):9–16.
- Sarriá, B., R. López-Fandiño, and M. P. Vaquero. 2001. Does processing of a powder or in-bottle-sterilized liquid infant formula affect calcium bioavailability? Nutrition 17 (4):326–31.
- Sarwar, G., R. W. Peace, and H. G. Botting. 1989. Differences in protein digestibility and quality of liquid concentrate and powder forms of milk-based infant formulas fed to rats. The American Journal of Clinical Nutrition 49 (5):806–13. doi: 10.1093/ajcn/49.5.806.
- Sarwar, G., R. W. Peace, H. G. Botting, and D. Brulé. 1989. Digestibility of protein and amino acids in selected foods as determined by a rat balance method. Plant Foods for Human Nutrition 39 (1):23–32. doi: 10.1007/BF01092398.
- Schaafsma, G. 2012. Advantages and limitations of the protein digestibility-corrected amino acid score (PDCAAS) as a method for evaluating protein quality in human diets. British Journal of Nutrition 108 (S2):S333–S336. doi: 10.1017/S0007114512002541.
- Seiquer, I., C. Delgado-Andrade, A. Haro, and M. P. Navarro. 2010. Assessing the effects of severe heat treatment of milk on calcium bioavailability: In vitro and in vivo studies. Journal of Dairy Science 93 (12):5635–43. doi: 10.3168/jds.2010-3469.
- Seiquer, I., T. Aspe, P. Vaquero, and P. Navarro. 2001. Effects of heat treatment of casein in the presence of reducing sugars on calcium bioavailability: In vitro and in vivo assays. Journal of Agricultural and Food Chemistry 49 (2):1049–55. doi: 10.1021/jf001008v.
- Singh, H. 2004. Heat stability of milk. International Journal of Dairy Technology 57 (2-3):111–9. doi: 10.1111/j.1471-0307.2004.00143.x.
- Singh, H., and L. K. Creamer. 1991. Denaturation, aggregation and heat stability of milk protein during the manufacture of skim milk powder. Journal of Dairy Research 58 (3):269. doi: 10.1017/S002202990002985X.
- Singh, H., and L. K. Creamer. 1993. In vitro digestibility of whey protein/k-casein complexes isolated from heated concentrated milk. Journal of Food Science 58 (2):299–302. doi: 10.1111/j.1365-2621.1993.tb04260.x.
- Singh, T. K., S. K. Øiseth, L. Lundin, and L. Day. 2014. Influence of heat and shear induced protein aggregation on the in vitro digestion rate of whey proteins. Food & Function 5 (11):2686–98. doi: 10.1039/C4FO00454J.
- Sletten, G. B. G., L. Holden, E. Egaas, and C. K. Faeste. 2008. Differential influence of the degree of processing on immunogenicity following proteolysis of casein and β-lactoglobulin. Food and Agricultural Immunology 19 (3):213–28. doi: 10.1080/09540100802350963.
- Stender, E. G. P., G. Koutina, K. Almdal, T. Hassenkam, A. Mackie, R. Ipsen, and B. Svensson. 2018. Isoenergic modification of whey protein structure by denaturation and crosslinking using transglutaminase. Food & Function 9 (2):797–805. doi: 10.1039/C7FO01451A.
- Stojadinovic, M., R. Pieters, J. Smit, and T. C. Velickovic. 2014. Cross-linking of β-lactoglobulin enhances allergic sensitization through changes in cellular uptake and processing. Toxicological Sciences 140 (1):224–35. doi: 10.1093/toxsci/kfu062.
- Støy, A. C. F., P. T. Sangild, K. Skovgaard, T. Thymann, M. Bjerre, D. E. W. Chatterton, … P. M. H. Heegaard. 2016. Spray dried, pasteurised bovine colostrum protects against gut dysfunction and inflammation in preterm pigs. Journal of Pediatric Gastroenterology and Nutrition 63 (2):280–7. doi: 10.1097/MPG.0000000000001056.
- Swaisgood, H. E., and G. L. Catignani. 1985. Digestibility of modified milk proteins: Nutritional implications. Journal of Dairy Science 68 (10):2782–90. doi: 10.3168/jds.S0022-0302(85)81166-8.
- Taylor, C. M., and B. W. Woonton. 2009. Quantity and carbohydrate content of glycomacropeptide fractions isolated from raw and heat-treated milk. International Dairy Journal 19 (12):709–14. doi: 10.1016/j.idairyj.2009.06.010.
- Tunick, M. H., D. X. Ren, D. L. Van Hekken, L. Bonnaillie, M. Paul, R. Kwoczak, and P. M. Tomasula. 2016. Effect of heat and homogenization on in vitro digestion of milk. Journal of Dairy Science 99 (6):4124–39. doi: 10.3168/jds.2015-10474.
- Van Boekel, M. A. J. S. 1998. Effect of heating on Maillard reactions in milk. Food Chemistry 62 (4):403–14. doi: 10.1016/S0308-8146(98)00075-2.
- van den Heuvel, E. G. H. M., and J. M. J. M. Steijns. 2018. Dairy products and bone health: How strong is the scientific evidence? Nutrition Research Reviews 31 (2):164–78. doi: 10.1017/S095442241800001X.
- Vasbinder, A. J., and C. G. de Kruif. 2003. Casein–whey protein interactions in heated milk: The influence of pH. International Dairy Journal 13 (8):669–77. doi: 10.1016/S0958-6946(03)00120-1.
- Verbeke, K. A., A. R. Boobis, A. Chiodini, C. A. Edwards, A. Franck, M. Kleerebezem, A. Nauta, J. Raes, E. A. F. van Tol, and K. M. Tuohy. 2015. Towards microbial fermentation metabolites as markers for health benefits of prebiotics. Nutrition Research Reviews 28 (1):42–66. doi: 10.1017/S0954422415000037.
- Wada, Y., and B. Lönnerdal. 2014. Effects of different industrial heating processes of milk on Site-Specific protein modifications and their relationship to in vitro and in vivo digestibility. Journal of Agricultural and Food Chemistry 62 (18):4175–85. doi: 10.1021/jf501617s.
- Wada, Y., and B. Lönnerdal. 2015. Effects of industrial heating processes of Milk-Based enteral formulas on Site-Specific protein modifications and their relationship to in vitro and in vivo protein digestibility. Journal of Agricultural and Food Chemistry 63 (30):6787–98. doi: 10.1021/acs.jafc.5b02189.
- Walstra, P., J. T. M. Wouters, and T. J. Geurts. 2006. Dairy science and technology. 2nd ed. Boca Raton, FL: CRC Press.
- Wang, X. P., and X. H. Zhao. 2017. Prior lactose glycation of caseinate via the Maillard reaction affects in vitro activities of the pepsin-trypsin digest toward intestinal epithelial cells. Journal of Dairy Science 100 (7):5125–38. doi: 10.3168/jds.2016-12491.
- Wang, X., A. Ye, Q. Lin, J. Han, and H. Singh. 2018. Gastric digestion of milk protein ingredients: Study using an in vitro dynamic model. Journal of Dairy Science 101 (8):6842–52. doi: 10.3168/jds.2017-14284.
- Ye, A., J. Cui, D. Dalgleish, and H. Singh. 2017. Effect of homogenization and heat treatment on the behavior of protein and fat globules during gastric digestion of milk. Journal of Dairy Science 100 (1):36–47. doi: 10.3168/jds.2016-11764.
- Zhao, D., T. T. Le, L. B. Larsen, L. Li, D. Qin, G. Su, and B. Li. 2017. Effect of glycation derived from α-dicarbonyl compounds on the in vitro digestibility of β-casein and β-lactoglobulin: A model study with glyoxal, methylglyoxal and butanedione. Food Research International 102:313–22. doi: 10.1016/j.foodres.2017.10.002.
- Zhao, D., L. Li, T. T. Le, L. B. Larsen, G. Su, Y. Liang, and B. Li. 2017. Digestibility of glyoxal-glycated β-casein and β-lactoglobulin and distribution of peptide-bound advanced glycation end products in gastrointestinal digests. Journal of Agricultural and Food Chemistry 65 (28):5778–88. doi: 10.1021/acs.jafc.7b01951.
