Abstract
A boom in respiratory tract infection cases has inflicted a socio-economic burden on the healthcare system worldwide, especially in developing countries. Limited alternative therapeutic options have posed a major threat to human health. Nanotechnology has brought an immense breakthrough in the pharmaceutical industry in a jiffy. The vast applications of nanotechnology ranging from early diagnosis to treatment strategies are employed for respiratory tract infections. The research avenues explored a multitude of nanosystems for effective drug delivery to the target site and combating the issues laid through multidrug resistance and protective niches of the bacteria. In this review a brief introduction to respiratory diseases and multifaceted barriers imposed by bacterial infections are enlightened. The manuscript reviewed different nanosystems, i.e. liposomes, solid lipid nanoparticles, polymeric nanoparticles, dendrimers, nanogels, and metallic (gold and silver) which enhanced bactericidal effects, prevented biofilm formation, improved mucus penetration, and site-specific delivery. Moreover, most of the nanotechnology-based recent research is in a preclinical and clinical experimental stage and safety assessment is still challenging.
1. Introduction
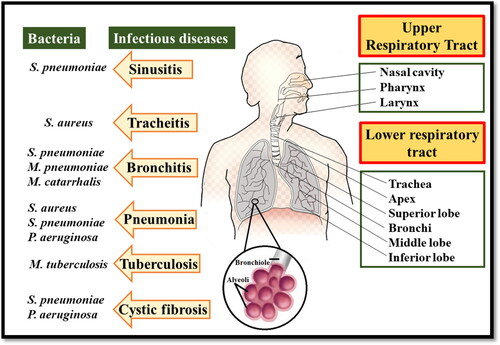
Respiratory infections are the leading cause of mortality and it is estimated that by 2030 the death rate due to respiratory-related diseases would reach 20% of all deaths (Mizgerd, Citation2006; Troy & Bosco, Citation2016). The respiratory tract is continuously exposed to microorganisms (bacteria, viruses, and fungi). If pathogenic microorganisms are not cleared from the lungs upon inhalation, it leads to respiratory tract infections. These infections are categorized into upper and lower respiratory tract infections. Lower respiratory tract infections are mainly caused due to bacterial infections which have more deleterious effects and represent the highest death rates in developing countries. Thus, putting a gruesome financial burden on the health care system (Z. Huang et al., Citation2021). S. pneumoniae, H. influenzae, Moraxella catarrhalis, S. aureus, P. aeruginosa, M. tuberculosis, S. pyogenes are predominant pathogens in respiratory tract infections (Shima et al., Citation2016). Respiratory tract infections are difficult to treat owing to the localization of microbes that resides deep inside the tract and are embedded with thick protective mucus and biofilms layer (Tucker et al., Citation2021). Therapeutics can be administered to the target site either systematically or directly through the respiratory tract. A higher dosage of the drug is required in case of intravenous or oral delivery (systemic delivery) to reach the target site (Baranyai et al., Citation2021). While in the case of pulmonary delivery, the drugs in solution form either have low retention time in the lungs or metabolic enzymes inactivate them, therefore, making the therapeutics ineffective. Though antibiotics like macrolides and quinolone can enter the host cell through diffusion, they are removed from the host cell via an efflux pump (X. Z. Li & Nikaido, Citation2009; López et al., Citation2021). Antimicrobial resistance is another major concern in public health which is escalating because of the misuse of drugs. The emergence of ‘superbugs’ has rendered multifaceted challenges for the pharmaceutical industry. Respiratory infection-causing pathogens (P. aeruginosa, M. tuberculosis, S. pneumonia, and H. influenza) exhibit lower susceptibility to the many classes of antibiotics. Thus, the higher demand for antimicrobials further contributes to the development of antimicrobial resistance and aggravates the situation (Guitor & Wright, Citation2018).
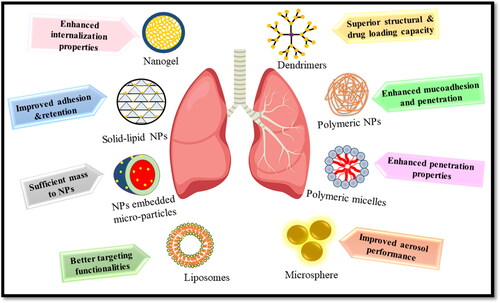
The huge cost and strenuous efforts required in the discovery of new antimicrobials have limited their discovery and the focus has been shifted toward the development of effective formulations to deliver the drug at the targeted site. Nanotechnology is one of the most promising alternative approaches to evade the limitations of conventional formulations. Nano-encapsulation of drugs provides various advantages, i.e. protection of antimicrobial drugs in the biological environment and providing sustained drug release with higher retention time (Falciani et al., Citation2020), enhancement of drug encapsulation and solubility (Ingle et al., Citation2016), reducing the risk of side effects by specific targeting (Y. Yu et al., Citation2022), simultaneous delivery of multiple drugs (Lababidi et al., Citation2020), crossing the barriers, and combating the resistance mechanisms developed by the bacteria (Mullis et al., Citation2021). Nanoparticles enter the mammalian cells either via phagocytosis or pinocytosis. So the employment of nanosystems can help in targeting the intracellular bacteria (Kamaruzzaman et al., Citation2017). Another benefit offered by nanotechnology is the uniform distribution of the drug in the lungs. The homogenous size of nano-suspensions ensures the encapsulation of the drug in each droplet in comparison with the microparticulate form of free antibiotics (Ingle et al., Citation2016) ().
2. Respiratory infectious diseases
2.1. Tuberculosis
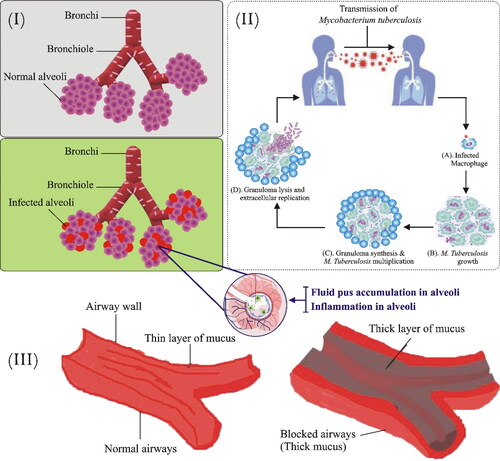
Tuberculosis (TB) is caused by M. tuberculosis and remains a fatal disease across the globe. M. tuberculosis is a facultative intracellular parasite that replicates inside the host after being carried through aerosol droplets. M. tuberculosis may affect other organs, i.e. liver, kidney, bones, and the central nervous system, but predominantly it invades the pulmonary tract. When TB extravagates outside the lungs it is known as extra-pulmonary TB (Da Silva et al., Citation2016; Nasiruddin et al., Citation2017). M. tuberculosis is the most critical pathogen in the global antimicrobial resistance crisis. The first-line drugs used in the treatment of standard TB include four oral antibiotics regimens, i.e. pyrazinamide, isoniazid, rifampin, and ethambutol (Pinheiro et al., Citation2011). Patients find it difficult to stick to this regime due to prolonged and frequent dosing; unable to penetrate the alveolar macrophages where bacilli reside; instability in gastric acid which leads to the development of drug-resistant strains and increases the risk of treatment failure (Ginsberg & Spigelman, Citation2007). Bedaquiline was the first drug approved in more than 40 years (Deoghare, Citation2013). However, reformulation of existing drugs in nanosystems is a possible way for the effective treatment of TB.
2.2. Cystic fibrosis
Cystic fibrosis (CF) is a fatal chronic pulmonary infection that occurs due to a defect in transmembrane protein which is known as cystic fibrosis transmembrane conductance regulator (CFTR) which regulates chloride ion secretion (Winstanley et al., Citation2016). The chloride ion imbalance decreased the volume of surface liquid in the airways, mucus dehydration, and lessens mucus clearance which eventually leads to the secretion and accumulation of viscous mucus in the airways and causes bronchial obstruction (Ong et al., Citation2019). In the early stages of the disease, S. aureus and H. influenzae are found in abundance whereas, in the advance stages P. aeruginosa, Burkholderia cenocepacia, Stenotrophomonas maltophilia, and Achromobacter xylosoxidans are found profusely (Klinger-Strobel et al., Citation2015). P. aeruginosa infection is mostly accountable for most of the premature deceases of CF patients (De Boeck, Citation2020). Thus, inhibiting chronic colonization caused by P. aeruginosa is the key aim for the early treatment of CF. The lungs of CF patients even after receiving antibiotic regimens and aerosolization of tobramycin are persistently colonized by P. aeruginosa (Moreau-Marquis et al., Citation2008). To improve the chloride ion transport functions, CFTR modulators, i.e. ivacaftor, lumacaftor, and tezecaftor have been used. However, refinement in toxicity, specificity, and adverse effects is required to expand the use and effectiveness of these medicines (Robinson et al., Citation2018).
2.3. Asthma
A chronic inflammatory lung disease that is characterized by reversible airway obstruction and bronchial hyper-responsiveness. The Global Initiative for Asthma (GINA) proposed some guidelines for the management of asthma: alleviate and control symptoms; halt the loss of pulmonary function; reduce asthma exacerbations and minimize adverse effects (Cheng & Chen, Citation2019). It is found that S. pneumoniae, H. influenzae, and M. catarrhalis are associated with asthma exaggerations (Kraft, Citation2000). Some uncommon microbial pathogens includes; M. pneumoniae and Chlamydia pneumoniae which induce and exacerbate asthma (Darveaux & Lemanske, Citation2014). The conventional inhaled anti-asthma drugs have improved bioavailability and efficacy. Glucocorticoid, an inhaled corticosteroid (ICS) is the most effective drug to control airway inflammation caused by asthma. However, ICS might not provide immediate relief and cause long-term side effects such as decreased rate of treatment adherence (Adouni Lawani et al., Citation2018) which can accelerate the progression of the disease as well as lung remodeling, and leads to pulmonary deterioration (Pascual & Peters, Citation2005). Moreover, the long-term high dose hormone inhalation will bring harmful effects, i.e. inhibition of the adrenal axis; oral fungal infections; and osteoporosis (L. Wang et al., Citation2019).
2.4. Pneumonia
Pneumonia is an inflammatory condition of the lungs which affect primarily lung air sacs (alveoli). Alveolar spaces are occupied by pus and fluid which distress breathing and confine oxygen uptake. The lesions appear inside alveoli, often associated with buds of granulation tissue that inhibit the bronchiolar lumen resulting in lung abrasions (Al-Tubaikh, Citation2010). Many etiological agents are accountable for pneumonia including pathogenic bacteria; S. pneumoniae, H. influenzae, S. aureus, gram-negative bacilli, M. pneumoniae, Acinetobacter baumanni, Stenotrophomonas maltophilia, and opportunistic fungi that reach alveoli by micro aspiration of oropharynges secretion (Sanivarapu & Gibson, Citation2021). Certain viruses, i.e. coronavirus, adenoviruses, influenza virus, and respiratory syncytial viruses are also responsible for the spread of viral pneumonia (Muhammad et al., Citation2022). The therapy is initiated after the conformation of an etiological agent and the severity of the disease then the treatment starts in a rational way to treat the development of these resistant strains. However, the current treatment of pneumonia is ineffective due to adverse toxic effects related to antibiotics (vancomycin) and inefficient effects against multidrug resistance (B. Kim et al., Citation2018). Therefore, nanotechnology is an emerging technique to overcome hurdles in this regulatory fatal infection ().
Figure 2. Bacterial respiratory infectious diseases. i. Pathophysiology of pneumonia: Pathogens enter via inhalation and reach lower airways. Alveoli releases cytokines and inflammatory mediators which lead to alveolar fluid accumulation. ii. Pathogenesis of TB: M. tuberculosis enters the respiratory tract through inhalation and infects alveoli. In the first step, alveolar macrophages recognize, engulf, and try to destroy bacilli. In the second step, bacilli start to grow within the infected alveolar macrophages which ultimately transform into granuloma. Most human with infected TB don’t exhibit a progression of the disease and remains in a latent state. However, some infected persons progress to the final stage where cavities are filled with free-floating bacteria and spread in the lungs causing pulmonary TB. iii. Comparison of normal and Cystic fibrosis patient airways- Illustration showing normal and highly viscous mucus airways in lungs and respiratory tubes.
3 Bio-barriers: Hindrance in the effective delivery of drugs
Many biological barriers in the respiratory tract impede the delivery of drugs at the targeted site. To formulate an effective drug for respiratory tract infections, it is necessary to consider these biological barriers and the obstacles that are imposed by them. The characteristics of these barriers are different in the case of normal and pathophysiological conditions. Thus, emphasizing the need for rational designing of the therapeutics for effective treatment of respiratory tract infections.
3.1. Micro-environment of the respiratory tract
A heterogeneous lung lining fluid is distributed continuously throughout the respiratory tract. The trachea, bronchi, and bronchioles (conducting parts) are lined with a mucus gel, while the pulmonary surfactants and alveolar sub-phase fluid line the alveoli (A. W. Ng et al., Citation2004). Mucus is composed of water, globular proteins, lipids, DNA, mucins, salts, and cellular debris. It acts as a protective layer and helps in lubrication Mucus blocks the passage of pathogens and foreign substances to the underlying epithelium. Mucins are glycoproteins that contribute to the viscoelasticity of the mucus membrane (Zanin et al., Citation2016). In pathological conditions, the microenvironment of the respiratory tract is affected. The chronic bacterial infections in Cystic fibrosis change the pH of the respiratory tract from almost neutral to acidic. This altered pH induces conformational changes in the structure of mucin protein which can impact the interaction of nanoparticles and mucus (Poschet et al., Citation2002; F. Wan et al., Citation2020). Furthermore, respiratory tract diseases lead to excessive production and dehydration of mucus that also disrupt the interactivity of mucus and therapeutics. The production of extremely viscous mucus in a certain pathological environment may lead to embolism in the trachea, bronchi, and bronchioles, thus further obstructing the passage of drugs from the respiratory tract. The low clearance rate and higher accumulation of mucus create room for microbial growth and thus cause infection.
Pulmonary surfactants are lipid-protein complexes that help in the stabilization of the alveoli and also contribute to innate immune defense. The interaction between the nanoparticles and the surfactant can influence the normal functioning of the surfactants. Certain lung diseases are associated with the abnormal composition and functioning of pulmonary surfactants. These studies reflect the need for novel designing of the nanoparticles for respiratory tract infections, considering the altered microenvironment in case of pathological conditions (Veldhuizen & Haagsman, Citation2000).
3.2. Bacterial biofilms
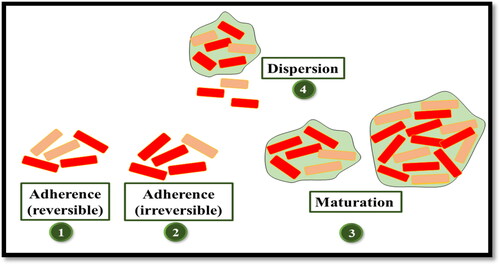
Biofilms refer to the closely associated community of microbes inside a network of carbohydrates, proteins, nucleic acids, and other substances known as extracellular polymeric substances (EPS) (Flemming & Wingender, Citation2010). There are five stages involved in the development of biofilms (). The first stage comprises of reversible adherence of microbes to a surface which is followed by the irreversible attachment of microbes and the release of EPS by microbes. In the next stage which is the early stage of biofilm, small colonies of microbes are immersed in the polymeric matrix. Upon maturation of the biofilms, the microcolonies of bacteria are separated through the open water channels. In the last dispersion stage, the biofilm starts the release of planktonic cells and the aggregates of bacteria (Blasi et al., Citation2016).
Biofilms render antibiotic resistance in bacteria either through the extra-polymeric matrix that acts as a barrier to the entry of antibacterial agents or through the sharing of resistant genes between resistant and susceptible microbes via horizontal gene transfer. Owing to these resistance films it is very difficult to treat bacterial infections. A bacterial biofilm requires 1000 times more concentration of antibiotic than the minimum inhibitory concentration (MIC) of antibiotic needed to kill bacteria (Gnanadhas et al., Citation2015). Biofilms are primarily involved in lower respiratory tract infections which are one of the major causes of death in developing countries (Sharma et al., Citation2021). Kolpen et al. (Citation2022) observed the presence of biofilms in the sputum of both acute and chronic lung infection patients, thus indicating the predominance of biofilms formation in respiratory tract infections. P. aeruginosa which is most prevalent in the respiratory tract infections in cystic fibrosis patients survives the antibiotic treatment due to its ability to form biofilms (Cipolla et al., Citation2014; Koch & Hoiby, Citation1993)
3.3. Intracellular bacteria
Various bacteria create a niche inside the host cells from where they can dodge the host immune system and spread to other sites in the body. These bacteria which localize inside a host cell are known as intracellular bacteria. M. tuberculosis, Salmonella enterica, Chlamydi trachomatis, and Listeria monocytogenes are well-known intracellular bacteria. In addition, some extracellular bacteria can also persist inside the host cell. S. aureus, Escherichia coli, and P. aeruginosa are the few extracellular bacteria that can be hosted inside the cell (Kamaruzzaman et al., Citation2017). Despite having various antibacterial therapeutics almost two-thirds of them are considered ineffective to treat the infection caused by intracellular pathogens. It is also expected that microorganisms may still be present inside the host cells even though the in-vitro susceptibility tests reveal the opposite (Tucker et al., Citation2021). Microorganisms may persist in the respiratory tract for a long time even when prescribed antimicrobials have been expected to be active based on conventional in-vitro susceptibility testing.
M. tuberculosis is an intracellular bacteria that reside inside the macrophages. Bacteria enter the macrophage through complement and mannose receptors. In order to ensure its survival, M. tuberculosis alters the lysosome trafficking and phagolysosome fusion. The host cells defend themselves via apoptosis of macrophages that are resisted by bacteria. However, bacteria can block the apoptosis process of macrophages by utilizing TNF-receptor-dependent mechanisms (Gordon & Read, Citation2002). It is also found that the commensal microorganism which protects against the microbial pathogens at the mucosal surface can also develop infections. These opportunistic bacteria become involved in pathogenicity in case of a compromised immunity of patients, a change in microbiota, or in case of broken skin or mucosal membranes. These microorganisms not only persist but their transmission also went undetected which poses another challenge in the effective treatment and detection of infections (Thakur et al., Citation2019).
4. Nanosystems employed for respiratory tract bacterial infections
4.1. Smart nano-systems
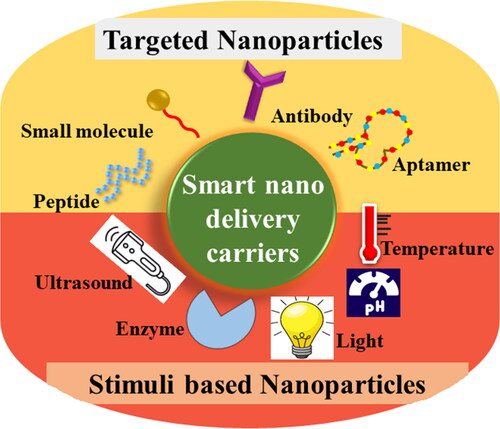
Nanotechnology offers myriad benefits in the medical field. These benefits can be cashed mainly through the engineering of nanoparticles. The focus is now shifted to the engineering of smart nanoparticles. As the name implies, these nanoparticles are fabricated to ensure the drug delivery to specified target sites while minimizing the side effects. These nanoparticles are designed to be target-specific either through targeted delivery (utilizing the receptor-ligand bonding) or through stimuli-responsive delivery of therapeutics .
Baranyai et al., formulated multifunctional nano-systems in which he utilized both targeted delivery of drug and the stimuli-responsive release of drug at the target site. The reactive oxygen species (ROS) responsive, Moxifloxcin encapsulated nano-system of 4-(hydroxymethyl) phenylboronic acid pinacol ester (HPAP)-modified cyclodextrin was prepared for the pulmonary bacterial infections. The phenylboronic acid-modified nano-systems are ROS sensitive and they release the drug when the H2O2 level reaches an optimum range of (0.5–1.0 mM). Thus, the higher levels of the ROS at the site of infection were availed for the stimulus-responsive release of moxifloxacin. For the targeted delivery of drug to the macrophages and mucus, the surface of nano-systems was functionalized with Polyethylene glycol (PEG) and folic acid (FA). These smart nanosystems exhibited more promising results than the free drug (Moxifloxcin) or the non-targeted nanoparticles on the P. aeruginosa infected mouse models. A novel pH-sensitive, surface-modified, and imipenem-loaded silver nanocomposite (IPM@AgNPs-PEG-NOTA) was constructed to evaluate the antibacterial activity in MLE-12 (mouse lung epithelial) cell lines and mice against carbapenem-resistant A. baumannii. The results demonstrated efficient antibacterial activity, increased ROS production, and membrane damage which induced oxidative stress, halted cell wall formation, and interfered with metabolic pathways. Additionally, the nanosystem also inhibited the formation of biofilm. In-vivo studies showed the secretion of proinflammatory cytokines was restrained and repair of infected tissues in mice was promoted as compared to imipenem. This novel drug delivery system also represented effective bactericidal properties in clinical isolates of A. baumannii which paved way for clinical studies (X. Li et al., Citation2021) ().
4.2. Liposomes
Liposomes are spherical lipid vesicles mainly composed of phospholipids and cholesterol. This colloidal form is a self-assembled lipid bilayer with an amphiphilic domain, inner hydrophobic core, and outer layer of the lipid bilayer (Bulbake et al., Citation2017). Liposomes can encapsulate drugs with different solubility either in water core or phospholipid bilayer and enhance the solubility of loaded drugs (Ngan & Asmawi, Citation2018). Due to the similar composition of the lipid bilayer and cell membrane, the liposome carriers could cross various biological barriers and enhance the absorption and therapeutic effects of encapsulated drugs (Rudokas et al., Citation2016). Liposomal formulations that contain fusogenic lipid bilayers can interfere with bacterial membrane, thereby allowing greater drug retention and intracellular delivery of entrapped drugs (Sachetelli et al., Citation2000). Additionally, liposomes attained close proximity to bacteria because of their penetration through the mucus layer (T. Yu et al., Citation2015). This had led to the approval of liposomal antibiotic formulations, i.e. Doxil and AmBisome by FDA (Barenholz, Citation2012; Boswell et al., Citation1998). In particular, ARIKAYCE, a liposomal antibiotic formulation was approved by FDA for the treatment of Mycobacterium avium complex lung disease (O. Khan & Chaudary, Citation2020). Recently, the amikacin encapsulated liposomes demonstrated greater penetration in biofilms and significantly higher cellular uptake in macrophages than non-formulated amikacin (J. Zhang et al., Citation2018). Liposomal formulations are a suitable candidate for pulmonary drug delivery because they provide superior safety profiles, decreased macrophage clearance, and sustained release (El-Sherbiny et al., Citation2011; Cipolla et al., Citation2014; Zhou et al., Citation2015). Wang and his coworkers successfully designed ciprofloxacin and colistin co-loaded liposomal formulations to evaluate the synergistic effects of antibiotics. Liposomal formulation with the size of 100 nm gave a sustained release. The in-vitro cytotoxicity studies showed efficient and safe antibacterial activity against clinical strains of P. aeruginosa and A459 (human epithelial cell lines) as compared to monotherapies (S. Wang et al., Citation2018). In another study, fucoidan-coated liposomes loaded with usnic acid were designed to target M. tuberculosis-infected macrophages (H37Ra). The liposomal vesicle size was 168 nm −1.18 µm in diameter and −5.41 mV zeta potential. The in-vitro cytotoxicity analysis revealed higher cellular uptake and lower IC50 values against infected macrophages as compared to uncoated liposomes (Lima Salviano et al., Citation2021). A. baumannii is a severe pathogen with a higher mortality rate and has clinical challenges due to limited therapeutic options. Liposomal thymoquinone was prepared, characterized and toxicity was evaluated by analyzing hematological, liver and kidney functional parameters against resistant A. baumannii. The in-vivo results revealed higher survival rate and reduced the bacterial load in lung tissues, inflammation markers, leukocytes, and neutrophils in blood of infected mice in compasrion with controls (Allemailem et al., Citation2021). Some recent liposomal formulations are described herein in .
Table 1. Liposomes as a drug delivery system for the infectious respiratory tract.
4.3. Solid lipid nanoparticles (SLN)
SLN is another physiological lipid-based nanoscale aqueous suspension comprised of triglycerides and phospholipids which are structurally different from liposomes. SLN offers a variety of advantages over traditional drug delivery systems such as increased drug loading, high drug stability, sustained and prolonged release, encapsulation of both hydrophilic and hydrophobic drugs, avoidance of organic solvent in formulation, low carrier toxicity, large-scale production, and site-specific drug delivery (Bi et al., Citation2009; Üner & Yener, Citation2007; Weber et al., Citation2014). Due to the physiological components and less toxicity, SLN formulations are more acceptable for pulmonary delivery (). Phospholipids are omnipresent in deep areas of the lungs and are important for the functioning of breathing. At the alveolar surface, phospholipid-based surfactant protein is available which maintains the optimal surface tension and reduces friction in the lung tissues (Beck-Broichsitter et al., Citation2011). The toxicity of SLN in in-vitro and in-vivo lung models showed SLN20 (20% phospholipids in lipid matrix) could be a safe pulmonary drug delivery carrier (M. Nassimi et al., Citation2010; Nassimi et al., Citation2009). Recently, the chitosan-coated SLN loaded with rifampicin was formulated which showed higher loading capacity, enhanced mucoadhesive property, and efficient permeability in alveolar epithelial cell A549 (Vieira et al., Citation2018). In another study, Pastro and coworkers developed sodium colistimethate-loaded SLN to evaluate the antibacterial activity against P. aeruginosa and to assess the in-vivo pulmonary distribution. The results revealed higher antimicrobial activity against clinically isolated P. aeruginosa. The in-vivo distribution showed the homogeneous spread of nanoparticles through the lung with no migration of SLN to other organs (Pastor et al., Citation2014). The targeting efficacy of SLN can be improved via different modifications which thereby increase the drug concentration at a specific site and decrease toxicity. Mannose modified SLN increased the absorption capacity of macrophages of encapsulated drug and enhanced mycobacterial activity against murine macrophage cell lines (Maretti et al., Citation2019).
Table 2. SLN as drug delivery systems for the infectious respiratory tract.
4.4. Polymeric nanoparticles (PNPs)
A polymer is a group of large molecules comprising various small homogenous molecules. Polymers are classified into natural (chitosan, albumin, cyclodextrin, gelatin, collagen, and alginate) and synthetic [poly-lactic-co-glycolic acid (PLGA); polyethylene glycol (PEG), polyacrylates, poly-anhydrides, and poly-ethyleneimine (Rytting et al., Citation2008). Polymers offer feasible encapsulation strategies for the drug in different forms, i.e. microparticles, nanoparticles, and nano-embedded microparticles (Sung et al., Citation2007). The small size of PNPs allows the capillary penetration and uptake by cells which ultimately increases concentration at a specific site (L. Singh et al., Citation2017). PNPs have numerous advantages such as excellent biocompatibility, efficient targeting ability, stability, surface fabrication ability, protection of the drug from degradation, high encapsulation of drug, long shelf life, and sustained drug release (Menon et al., Citation2014). PNPs can deliver different agents which are incorporated into the surface of the polymer or dispersed in a polymeric matrix (Marasini et al., Citation2017). Polymers are considered a suitable candidate for pulmonary delivery. A variety of polymers are used to treat different bacterial respiratory infections, some of them are summarized in . HPOX (hydroxy benzyl alcohol incorporated polyoxalate) is a biodegradable polymeric prodrug of hydroxy benzyl (HBA) and a drug carrier that incorporates HBA and peroxalate through an ester linkage. HPOX suppresses the generation of ROS and has antioxidant and anti-inflammatory properties (Park et al., Citation2010). Recently, Yoo et al., prepared HPOX nanoparticles to evaluate their potency as a therapeutic agent for airway inflammatory diseases in-vitro and in-vivo. The nanoparticles were administered through the intratracheal route. The in-vitro analysis showed a decrease in the level of pro-inflammatory cytokines and ROS. The HPOX nanoparticles significantly reduced recruitment of pro-inflammatory cells in asthma allergic mice. Thus, these polymeric nanoparticles exhibit tremendous ability as therapeutics to treat airway inflammation and asthma (Yoo et al., Citation2013). PLGA is one of FDA approved co-polymer for therapeutic use in humans in numerous drug delivery systems because of its superior biocompatibility, biodegradability profile, and safety (Zhao et al., Citation2014). PLGA nanoparticle fabricated with tufstin (a natural immunostimulatory tetra-peptide with macrophage targeting and stimulatory property) derivative demonstrated enhanced internalization rate and intracellular activity of encapsulated drug against M. tuberculosis (Horváti et al., Citation2018). Günday Türeli et al., formulated ciprofloxacin-loaded PLGA nanoparticles to test the therapeutic effects on bacterial infection-induced CF in Calu-3 cell lines. The nanoparticles gave effective drug loading and permeability. Additionally, the low drug dose decreased the side effects in both in-vitro and in-vivo studies. The hydrophobic nature and nonspecific interaction of PLGA hinder their diffusion in mucus and bacterial biofilm. The surface modification of PNPs with PEG showed a high degree of free movement in biofilm in comparison with drug and lipophilic molecules (Forier et al., Citation2013; Sigurdsson et al., Citation2013). PEGylated nanoparticles can transport antimicrobial agents in biofilms of P. aueroginosa, Burkolderia cepecia, and Burkholderia multivorans (Forier et al., Citation2013; Messiaen et al., Citation2013). Another polymer that is in demand for its antimicrobial and mucoadhesive properties is chitosan. Chitosan is a natural, biocompatible polysaccharide with low immunogenicity and nontoxic nature. Recently, in-vitro studies of clofazimine-loaded chitosan nanoparticles demonstrated enhanced anti-mycobacterial activity, internalization, and bio-adhesion against C2C12 and H37Rv (a standard strain of M. tuberculosis) (Pawde et al., Citation2020).
Table 3. PNP as drug delivery systems for the infectious respiratory tract.
4.5. Dendrimers
Dendrimers are highly branched three-dimensional polymeric nanostructures that are different from traditional polymers. Dendrimers have been used as suitable nanosystems due to internal cavity and surface modification. The functional groups are decorated on the surface of dendrimers through electrostatic interactions to enhance biocompatibility and versatility (D. Huang & Wu, Citation2018; Mehta et al., Citation2019). The drug can be encapsulated in interval cavities or conjugated to the surface of the dendrimer. Additionally, the dendrimers with amphiphilic characteristics can deliver drugs with different solubilities (Ahmad et al., Citation2015). Several types of dendrimers have been described based on dendrimers’ synthesis, shape, physicochemical properties, and structures (Filipczak et al., Citation2021). Polyamidoamine (PAMAM) and polypropylene amine are the most common and oldest dendrimers which are used in drug delivery to their efficient solubilizing ability, reduce toxicity, and enhanced biocompatibility (Idris et al., Citation2020; Kretzmann et al., Citation2017). Recently, PAMAM dendrimers were used for the delivery of siRNA to treat chronic lung inflammation. The siRNA delivery was evaluated in-vitro using RAW264.7 macrophage cell lines and in-vivo through the murine model of lipopolysaccharide-induced lung inflammation. The PAMAM/siRNA nanosystem showed increased cellular uptake in macrophages while in-vivo studies revealed tumor necrosis factor α inhibition upon administration (Bohr et al., Citation2020). Another study reported the inhalable nano drug-using rifampicin-loaded PAMAM dendrimers for the treatment of tuberculosis. The PAMAM dendrimers gave controlled release, improved drug absorption, and bioavailability in comparison with intravenous administration (Rajabnezhad et al., Citation2016). Some recent advancements in dendrimers are explained in .
Table 4. Applications of dendrimers as drug delivery systems for the infectious respiratory tract.
PNPs have gained considerable progress so far due to the ease of their penetration through the mucus layer and gave efficient therapeutic effects even at a very low dose. However, some polymers, i.e. chitosan have shown biodegradability concerns in in-vivo administration. Polymer-based nanosystems may also demonstrate a sustained release, although the drug release mechanism is still unclear and under scrutiny. Thus, in-vivo optimization is required to evaluate the safety of nanoparticulate.
4.6. Nanogels
Hydrogels are the meshed network of polymers that can absorb high water content. The term nanogel is used for hydrogels that lie in the nano scale range. Thus the nanogels possess dual properties of nanoparticles and hydrogels. Due to their small size, the nanogels not only interact with the cell but also penetrate inside the cell. The network of nanogels provides space for the integration of drugs inside them (Keskin et al., Citation2021). Nanogels possess a higher loading capacity of the drug (more than 30% of their weight) and are one of the best candidates for the controlled, stimuli-responsive release of the drug. By changing the chemical composition of the nanogels the desired characteristics (size, charge, porosity, softness) can be achieved (Soni et al., Citation2016). The surface of nanogels can be engineered for ligand attachment and their higher mechanical strength contributes to their higher stability (Hamzah et al., Citation2017). The properties like high loading capacity, improved stability, larger surface area for bioconjugation, tunable size, and responsiveness to environmental stimuli make the nanogels promising carriers for drug delivery. Kabanov & Vinogradov (Citation2009) formulated a nanogel for S. pneumonia which is involved in respiratory tract infections. The cholesteryl-group-containing pullulan (CHP) was assembled into a spherical shape nanogel through hydrophobic interactions. Pneumococcal surface proteins A (PspA) antigen is encapsulated inside this pullulan nanogel and the pneumococcal surface proteins A (PspA) antigen incorporated CHP nanogel (CHP-PspA) was developed as a vaccine against S. pneumoniae. The administration of PspA-loaded nanogel to mice through the pulmonary route exhibited more promising results than the administration of PspA antigen alone. The nanogel inhibited the growth of S. pneumoniae in the nasal cavity and lungs of mice. The clinical trials of this nanogel on non-human primates are delivering encouraging results, thus opening new avenues for the better treatment of respiratory tract infections (Aderibigbe & Naki, Citation2018; Fukuyama et al., Citation2015; Gyu Kong et al., Citation2013; Nakahashi-Ouchida et al., Citation2018).
4.7. Metal nanoparticles
Metal nanoparticles have gained attention due to their surface energy, greater surface-to-volume ratio, spatial confinement, and negligible imperfections. Metal nanoparticles have distinctive physicochemical, thermal, electrical, optical, mechanical, thermal, and biological properties. Biosythesized noble nanoparticles (gold and silver nanoparticles) have emerged as potential weapons in the antibacterial arsenal because of their antimicrobial efficacy Noble nanoparticles have also shown tremendous biomedical applications for treating a wide range of diseases (Ahangarpour et al., Citation2018; Ameen et al., Citation2018; Sathishkumar et al., Citation2016).
4.7.1. Silver nanoparticles (AgNPs)
AgNPs are defined as nanomaterials with all dimensions ranging between 1 and 100 nm. AgNPs exhibit unique electrical, catalytic, and optical properties which have led to the investigation and modification of products for imaging (Kumar et al., Citation2018), diagnosis (Hasanzadeh et al., Citation2019), targeted drug delivery (Kooti et al., Citation2018), detection and antimicrobial activity (Roh et al., Citation2019). Silver is an excellent antibacterial agent, nontoxic inorganic metal, and nontoxic to human cells with a concentration limit of 350 µg/day. The AgNPs have been proved to be a promising antimicrobial agent to combat in-vitro and in-vivo bacterial infections (Bruna et al., Citation2021). AgNPs showed efficient antibacterial activity to a wide range of microorganisms including E.coli (W. R. Li et al., Citation2010), Candida albicans (K. J. Kim et al., Citation2009), P. aeruginosa (Kora & Arunachalam, Citation2011), and S. aureus (W. R. Li et al., Citation2011). and The combined action of antibiotics and AgNPs has proved capable of killing microorganisms via different mechanisms. These mechanisms include; direct contact with bacterial components, i.e. biofilms and cell wall; generation of reactive oxygen species (ROS); inhibition of bacterial DNA replication; release of bioactive ions (Ag+); alteration of cell wall and cytoplasm; disruption of metabolic pathways. The bactericidal effect of AgNPs depends on nanoparticle size, shape, surface area, surface modification, and rate of Ag+ generation, structural differences in gram-positive and gram-negative bacteria (Tăbăran et al., Citation2020). Recently, AgNPs gave remarkable antibacterial activity in comparison with tobramycin (antibiotic) against bacterial strains. Pompilio et al., have formulated novel AgNPs against P. aeruginosa, S. maltophilia, S. aureus, and B. cepacia recovered from patients with CF. The AgNPs in-vitro assayed showed a rapid bactericidal effect against B. cepacia and P. aeruginosa. The viability reduction, destruction of extracellular matrix, and cell death were also observed against S. aureus and P. aeruginosa biofilms (Pompilio et al., Citation2018). Biogenic AgNPs also exhibited promising in-vitro and in-vivo antibacterial activity against S. aureus. Biogenic AgNPs were synthesized from Gardenia thailandica leaf extract followed by their characterization. The characterized nanoparticles were spherical with 11.02 − 17.92 nm diameter. The antibacterial activity of AgNPs was evaluated in-vitro and in-vivo against P. aeruginosa clinical isolates. The in-vitro flow cytometry demonstrated that AgNPs reduced the membrane potential and also decreased the membrane integrity. The in-vivo examination was done on S. aureus infected wounds in rats. The AgNPs treated rats resulted in epidermis regeneration and reduced infiltration of inflammatory cells (Attallah et al., Citation2022). Respiratory tract microbes infections can also be treated through biogenic AgNPs in combination with antibiotics. Aremu et al., developed AgNPs from Hypoxis hemerocallidea (Southern African plant) to evulate antibacterial activity against S. pneumonia, P. aeruginosa, Bacillus cereus, E. coli, and M. catarrhalis. Broad spectrum of antibacterial activity was observed against all respiratory pathobionts. However, AgNPs also synergistically increased the antibacterial effect of streptomycin when they were used in combination (Aremu et al., Citation2021). Similarly, AgNPs were combined with antibiotics (polymixinB, rifampicin and tigecycline) against resistant A. baumannii clinically isolated from patients. The AgNPs showed significant antibacterial activity with no cytotoxicity against A549 and HL-7702 cells. In-vivo studies increased the survival rate and decreased the level of proinflammatory cytokines, interlukins and tumor nacrosis factor alpha (TNF-α) in mice (G. Wan et al., Citation2016). Therefore, AgNPs have dual function which can synergistically enhance activity and also act as a carrier for small molecules.
4.7.2. Gold nanoparticles (AuNPs)
AuNPs are widely used in biochemical and pharmacological research owing to their chemically inert character, ease of penetration, the possibility of being functionalized with different molecules, biocompatibility, controlled release, low toxicity, and higher stability among metallic nanoparticles (de Menezes et al., Citation2021; Dykman & Khlebtsov, Citation2012; Y. Zhang et al., Citation2015). Targeted molecular imaging (Lan et al., Citation2013), biosensing (Kao et al., Citation2013), and targeted drug delivery (Hema et al., Citation2018) can also be achieved through AuNPs. AuNPs are highly explored and used as therapeutic agents against microbial infections (F. Khan et al., Citation2019; Rajkumari et al., Citation2017; Rice et al., Citation2019). Recently, Alsamhary et al., used tricetin to synthesize AuNPs to evaluate the in-vitro antibacterial efficacy against bacterial pathogens which were isolated from immunocompromised patients suffering from respiratory infections. The antibacterial studies confirmed the broad-spectrum antimicrobial activity of AuNPs against S. aureus, Acinetobacter pittii, P. aeruginosa, Enterobacter xiangfangensis, Proteus mirabilis, Bacillus licheniformis, Aeromonas enteropelogenes, and Escherichia fergusonii. The in-vitro cytotoxicity results revealed that biosynthesized AuNPs were biocompatible on primary normal human dermal fibroblast cells up to 50 µg/mL (Alsamhary et al., Citation2020). In another study, folic acid-decorated docetaxel-loaded AuNPs were synthesized to evaluate anti-cancer activities by in-vitro studies against lung cancer cell lines (H520). The cytotoxicity analysis revealed a 50% reduction in cell viability in comparison with control due to the combined effect of AuNPs-based nanoconjugates (Thambiraj et al., Citation2019). Water-soluble and highly stable chitosan oligosaccharide capped gold nanoparticles formulation (COS-AuNPs) was designed to inhibit the phenotypic traits (biofilm formation, virulence factors production), and motility of P. aeruginosa. COS-AuNPs inhibited the formation of biofilm and eradicated the preexisted mature biofilm. COS-AuNPs also hindered the bacterial hemolysis and reduced the production of some virulence factors from P. aeruginosa. Attenuation of bacterial swimming and twitching motilities were observed during the treatment of COS-AuNPs. However, an efficacy test using an animal model is required that will confirm that COS-AuNPs can be used as a potential agent to control the infections associated with P. aeruginosa (F. Khan et al., Citation2019). Thus, AuNPs-based nanoconjugates can be considered an alternative and promising carrier for the treatment of respiratory infections.
Most of the metallic nanoparticles were effective in in-vitro antibacterial activity such as bacterial toxic cations release or ROS but these mechanisms were diminished by numerous in-vivo considerations. Metal cations might be attracted by host molecules and can deviate nanoparticles from antibacterial activity while ROS produced by nanoparticles could be decreased or neutralized in in-vivo from biomolecules (uric acid, albumin, ascorbic acid, glutathione). Furthermore, biodistribution and excretion kinetics have to be studied in detail for different animal models because metallic nanoparticles are not biodegradable. The excretion of metallic nanoparticles from the liver and spleen can take up to 3–4 months that possibly can induce inflammation. Aggregated nanoparticles can exhibit catalytic activity and interfere with numerous diagnostic techniques. Above mentioned concerns, along with toxicity are the challenges of metallic nanoparticles in clinical applications.
5 Mitochondrial STAT 3 and the role of nanocarriers
Signal transducer and activator of transcription 3 (STAT 3) is a transcription factor that functions either through the classical pathway or non-classical pathway. In the classical pathway, it is translocated to the nucleus where it mediates gene transcription while in the non-classical pathway it is translocated to the mitochondria (Yang & Rincon, Citation2016). STAT 3 causes effective regeneration of NAD + in respiration by interacting with the complex I in mitochondria which leads to an increased dehydrogenase activity of Nicotinamide Adenine Dinucleotide (NADH). The NAD + results in the induction of antioxidant genes as it acts as a retrograde signal (Lahiri et al., Citation2021). Fu et al. (Citation2022) demonstrated that mitochondrial STAT 3 plays a crucial role in the functioning of innate lymphoid cells (ILC2) that are involved in type 2 immune response. STAT 3 enhances the ILC2-led allergic inflammation in the lungs. However, decreasing the STAT3 levels and inhibiting the translocation of STAT3 to mitochondria has reduced the inflammatory allergic responses of ILC2. Das et al. (Citation2014) formulated Polyethylenimine (PEI) and PLGA nanoparticles for the encapsulation of siRNA to inhibit the STAT 3. The nano-encapsulated siRNA exhibited effective silencing of STAT 3 in A549 cells while the unencapsulated siRNA administration did not deliver promising results. This indicates the effectiveness of nanocarriers in lung cancer therapy.
6. Detection through nanodiagnostics
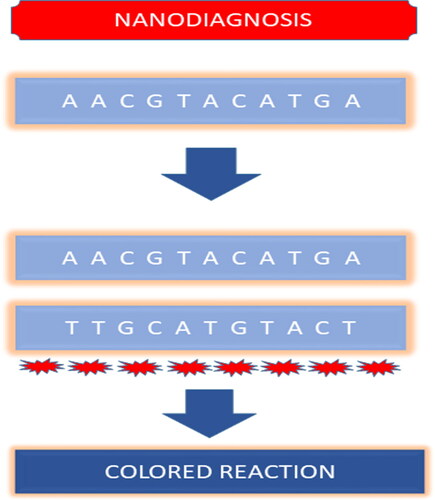
Infectious diseases are the primary cause of death in developing countries with more than 95% of deaths related to infectious diseases being caused because of the failure of effective diagnosis and treatment strategies. Approaches like culture and microscopy, immunology, and PCR are conventionally utilized for the detection of pathogens. However, these conventional detection methods are inefficient for the early and rapid detection of pathogens as these are time-consuming (e.g. culture and microscopy method), expensive, sophisticated, and also carry the risk of cross-contamination. Thus, to overcome the challenges of the conventional diagnostic techniques, nanoparticles and nanodevices-based diagnosis could be carried out for infectious pathogens. Nano diagnostics are robust, cost-effective, portable, and user-friendly (Noah & Ndangili, Citation2019). The high surface area of nanoparticles makes the nanodiagnostics more sensitive and robust(Prasad, Citation2014). Currently, nanomaterials like gold nanoparticles, silver nanoparticles, magnetic nanoparticles, carbon nanotubes, and quantum dots have been explored for their potential as diagnostic tools. Gold nanoparticles are more feasible for clinical diagnosis owing to their properties like biocompatibility, inertness, and unique optical features. Magnetic nanoparticles like iron oxide are extensively studied for their applications in biomedicine. They are employed to detect pathogens by functionalizing their surface with recognition molecules (antibodies, carbohydrates) (Homayoonnia et al., Citation2021; J. Kim et al., Citation2017). Magnetic nanomaterials like gold and silver nanoparticles exhibit Surface Plasmon Resonance and are used in place of labels to detect the pathogens upon hybridization of complementary bases the nanomaterial will change color (Lambe et al., Citation2016). Simple nanoparticle-based diagnosis could not deliver the anticipated results in the diagnosis of pathogens in complicated infections (e.g. where many strains of pathogens are involved) and thus calls for the need for more advanced diagnostic tools. So, nano-diagnostic devices are engineered through the integration of nanotechnology in many techniques (Y. Wang et al., Citation2017). These nanotechnology-based diagnostic assays are also known as pen side tests and lab on chip diagnostic tests as they surpass the need for sophisticated labs and skilled personnel (Lambe et al., Citation2016). DNA-based technologies for the analytical process like Microarray, rolling circle amplification, etc. utilize nanoparticles to aid the process of diagnosis (Shi et al., Citation2014). Shi et al. investigated the respiratory tract infection-causing bacteria S. pneumonia by using the rolling cycle amplification of DNA and utilizing gold nanoparticles as sensors (Salieb-Beugelaar & Hunziker, Citation2015; Shi et al., Citation2014).
7 Pre-clinical and clinical strategies
Various nanotechnology-based oral delivery systems have been formulated for the delivery of antibiotics and tested on animal models. The anti TB drugs such as rifampicin, pyrazinamide, and isoniazid-loaded SLN have been tested in rodents to improve their bioavailability (H. Singh et al., Citation2015). In infected mice, 46 daily doses of the free drug were administered orally to achieve the therapeutic effect. However, only five doses of SLN on every 10th day didn’t detect any tubercle bacilli (Pandey et al., Citation2005). Pulmonary administration of rifampicin-loaded SLN in rodents enabled delivery to alveolar macrophages (Chuan et al., Citation2013). Bacillus Calmette-Geurin (BCG), a TB vaccine that was approved in 1921 showed limited protection as compared to aerosolized formulation of BCG nano-microparticles. When this formulation was administered to guinea pigs, they produced elevated immune responses and resistance to TB infections than standard vaccines (Garcia-Contreras et al., Citation2008; Hawn et al., Citation2014; Kaufmann et al., Citation2017). Additionally, nano chitosan-based recombinant DNA vaccine enhanced immunologic and therapeutic effects in mice model against TB (Feng et al., Citation2013). Despite promising results in preclinical studies, only a few clinical trials have been conducted to test the nano-therapy in respiratory infections. A clinical trial has been completed to check the efficacy of Pitavastatin-loaded PLGA nanoparticles (Nakamura et al., Citation2017). Two liposomal formulations, i.e. amikacin and ciprofloxacin have reached the clinical stages for the treatment of CF-associated lung infection (Paranjpe & Müller-Goymann, Citation2014). However, no groundbreaking studies have been put forward in humans till now, and studies concerning treatment are still at the beginning that demands in-depth investigation.
8 Conclusion and future perspectives
Respiratory tract infections represent a myriad of challenges to the pharmaceutical industries and thus signal alternative approaches to combat the highly resistant and unaccessible pathogens lying deep inside the respiratory tract. Nanotechnology exhibits pronounced potential to overcome these challenges and nanomedicines have provided promising therapeutic effects (). Although preclinical trials have shown broad development progress, clinical effects need to be verified. The toxicity and ultimate fate of nanoparticles in the body called for the rational design of nanoparticles. This manuscript focuses on the advantages and limitations of different nanosystems. Lipid-based colloidal systems (SLN and liposomes) have advantages over others due to the presence of physiological components in their formulations. Many liposomal formulations have been clinically approved by FDA, ARIKAYCE is one of the antibiotic formulations to treat infectious agent M. avium. A. baumannii is one of the challenging clinical microbes, thymoquinone liposomal formulation also gave promising in-vivo results against clinical isolates of this pathogen and the time is not far when we see a bunch of nanomedicines being approved after the clinical trials. Some other freeze-dried liposomal formulations have also reached clinical phase III studies. Future implications may involve targeted multi-functional drugs that will not only effectively target the bacterial cell but also suppress the resistance mechanism of bacteria. Such advanced multifunctional therapy will provide targeted delivery of drugs with a different mode of action; increase the efficacy of therapeutics to treat infected diseases and limit adverse effects on healthy tissues. Nanoantioxidant therapy is another approach that overcomes oxidative stress caused by free radicals and could display more promising results by limiting the side effects of antioxidants. However, for successful applications in therapeutics, more research needs to be conducted in the field of nanotoxicity and the ultimate fate of nanoantioxidant in the body. Valuable insight in this regard will be to evaluate the antioxidant potential of biopolymers as delivering these materials at nanoscale would result in enhanced bioavailability and less toxicity. The optimum dosage of nanoantioxidants is another avenue to be worked on. Furthermore, through nanotechnology new vistas of bacterial vaccines will open that can prevent the onset of bacterial respiratory infections.
Data availability statement
Not applicable.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Aderibigbe BA, Naki T. (2018). Design and efficacy of nanogels formulations for intranasal administration. Molecules 23:1241.
- Adouni Lawani M, Zongo F, Breton MC, et al. (2018). Factors associated with adherence to asthma treatment with inhaled corticosteroids: a cross-sectional exploratory study. J Asthma 55:318–29.
- Ahangarpour A, Oroojan AA, Khorsandi L, et al. (2018). Solid Lipid nanoparticles of myricitrin have antioxidant and antidiabetic effects on streptozotocin-nicotinamide-induced diabetic model and myotube cell of male mouse. Oxid Med Cell Longev 2018:7496936.
- Ahmad J, Akhter S, Rizwanullah M, et al. (2015). Nanotechnology-based inhalation treatments for lung cancer: state of the art. Nanotechnol Sci Appl 8:55–66.
- Ahmed R, Aucamp M, Ebrahim N, Samsodien H. (2021). Supramolecular assembly of rifampicin and PEGylated PAMAM dendrimer as a novel conjugate for tuberculosis. J Drug Delivery Sci Technol 66:102773.
- Al-Nemrawi NK, Alshraiedeh NH, Zayed AL, Altaani BM. (2018). Low molecular weight chitosan-coated PLGA nanoparticles for pulmonary delivery of tobramycin for cystic fibrosis. Pharmaceuticals 11:28.
- Al-Tubaikh JA. (2010). Alveolar lung diseases. Internal Med 113:113–118. https://doi.org/10.1007/978-3-642-03709-2_19
- Alhariri M, Omri A. (2013). Efficacy of liposomal bismuth-ethanedithiol-loaded tobramycin after intratracheal administration in rats with pulmonary Pseudomonas aeruginosa infection. Antimicrob Agents Chemother 57:569–78. https://doi.org/10.1128/AAC.01634-12/ASSET/AE4AB036-23BF-4C0C-B64E-23D180FF5BC6/ASSETS/GRAPHIC/ZAC0021315380009.JPEG
- Allemailem KS, Alnuqaydan AM, Almatroudi A, et al. (2021). Safety and therapeutic efficacy of thymoquinone-loaded liposomes against drug-sensitive and drug-resistant Acinetobacter baumannii. Pharmaceutics 13:677.
- Alsamhary K, Al-Enazi N, Alshehri WA, Ameen F. (2020). Gold nanoparticles synthesised by flavonoid tricetin as a potential antibacterial nanomedicine to treat respiratory infections causing opportunistic bacterial pathogens. Microb Pathog 139:103928.
- Ameen F, AlYahya SA, Bakhrebah MA, et al. (2018). Flavonoid dihydromyricetin-mediated silver nanoparticles as potential nanomedicine for biomedical treatment of infections caused by opportunistic fungal pathogens. Res Chem Intermed 44:5063–73. https://doi.org/10.1007/S11164-018-3409-X/FIGURES/6
- Aremu OS, Qwebani-Ogunleye T, Katata-Seru L, et al. (2021). Synergistic broad-spectrum antibacterial activity of Hypoxis hemerocallidea-derived silver nanoparticles and streptomycin against respiratory pathobionts. Sci Rep 11:1–11.
- Attallah NGM, Elekhnawy E, Negm WA, et al. (2022). In vivo and in vitro antimicrobial activity of biogenic silver nanoparticles against Staphylococcus aureus clinical isolates. Pharmaceuticals 15:194.
- Banoub NG, Saleh SE, Helal HS, Aboshanab KM. (2021). Antibiotics combinations and chitosan nanoparticles for combating multidrug resistance Acinetobacter baumannii. Infect Drug Resist 14:3327–39. https://doi.org/10.2147/IDR.S328788
- Baranyai Z, Soria-Carrera H, Alleva M, et al. (2021). Nanotechnology-based targeted drug delivery: an emerging tool to overcome tuberculosis. Adv Therap 4:2000113.
- Barenholz Y. (2012). Doxil®-the first FDA-approved nano-drug: lessons learned. J Control Release 160:117–34.
- Beck-Broichsitter M, Ruppert C, Schmehl T, et al. (2011). Biophysical investigation of pulmonary surfactant surface properties upon contact with polymeric nanoparticles in vitro. Nanomedicine 7:341–50.
- Bellini RG, Guimarães AP, Pacheco MAC, et al. (2015). Association of the anti-tuberculosis drug rifampicin with a PAMAM dendrimer. J Mol Graph Model 60:34–42.
- Bi R, Shao W, Wang Q, Zhang N. (2009). Solid lipid nanoparticles as insulin inhalation carriers for enhanced pulmonary delivery. J Biomed Nanotechnol 5:84–92.
- Blasi F, Page C, Rossolini GM, et al. (2016). The effect of N-acetylcysteine on biofilms: implications for the treatment of respiratory tract infections. Respir Med 117:190–7.
- Bohr A, Tsapis N, Foged C, et al. (2020). Treatment of acute lung inflammation by pulmonary delivery of anti-TNF-α siRNA with PAMAM dendrimers in a murine model. Eur J Pharm Biopharm 156:114–20.
- Boswell GW, Buell D, Bekersky I. (1998). AmBisome (Liposomal Amphotericin B): a comparative review. J Clin Pharmacol 38:583–92.
- Bruna T, Maldonado-Bravo F, Jara P, Caro N. (2021). Silver nanoparticles and their antibacterial applications. IJMS 22:7202.
- Bulbake U, Doppalapudi S, Kommineni N, Khan W. (2017). Liposomal formulations in clinical use: an updated review. Pharmaceutics 9:12.
- Castellani S, Trapani A, Spagnoletta A, et al. (2018). Nanoparticle delivery of grape seed-derived proanthocyanidins to airway epithelial cells dampens oxidative stress and inflammation. J Transl Med 16:1–15. https://doi.org/10.1186/S12967-018-1509-4/FIGURES/6
- Chai G, Park H, Yu S, et al. (2019). Evaluation of co-delivery of colistin and ciprofloxacin in liposomes using an in vitro human lung epithelial cell model. Int J Pharm 569:118616.
- Cheng W-C, Chen C-H. (2019). Nanotechnology bring a new hope for asthmatics. Ann Transl Med 7:516.
- Chuan J, Li Y, Yang L, et al. (2013). Enhanced rifampicin delivery to alveolar macrophages by solid lipid nanoparticles. J Nanopart Res 15:1–9. https://doi.org/10.1007/S11051-013-1634-1/FIGURES/5
- Cipolla D, Shekunov B, Blanchard J, Hickey A. (2014). Lipid-based carriers for pulmonary products: preclinical development and case studies in humans. Adv Drug Deliv Rev 75:53–80.
- Craparo EF, Porsio B, Sardo C, et al. (2016). Pegylated polyaspartamide-polylactide-based nanoparticles penetrating cystic fibrosis artificial mucus. Biomacromolecules 17:767–777. https://doi.org/10.1021/ACS.BIOMAC.5B01480/SUPPL_FILE/BM5B01480_SI_001.PDF
- Da Silva PB, De Freitas ES, Bernegossi J, et al. (2016). Nanotechnology-based drug delivery systems for treatment of tuberculosis-a review. J Biomed Nanotechnol 12:241–260.
- Darveaux JI, Lemanske RF. (2014). Infection-related asthma. J Allergy Clin Immunol Pract 2:658–663.
- Das J, Das S, Paul A, et al. (2014). Assessment of drug delivery and anticancer potentials of nanoparticles-loaded siRNA targeting STAT3 in lung cancer, in vitro and in vivo. Toxicol Lett 225:454–466.
- De Boeck K. (2020). Cystic fibrosis in the year 2020: a disease with a new face. Acta Paediatr 109:893–899.
- de Menezes BRC, Rodrigues KF, Schatkoski VM, et al. (2021). Current advances in drug delivery of nanoparticles for respiratory disease treatment. J Mater Chem B 9:1745–1761.
- Deoghare S. (2013). Bedaquiline: a new drug approved for treatment of multidrug-resistant tuberculosis. Indian J Pharmacol 45:536–537.
- Derbali RM, Aoun V, Moussa G, et al. (2019). Tailored nanocarriers for the pulmonary delivery of levofloxacin against pseudomonas aeruginosa: a comparative study. Mol Pharm 16:1906–1916. https://doi.org/10.1021/ACS.MOLPHARMACEUT.8B01256/SUPPL_FILE/MP8B01256_SI_001.PDF
- Dykman L, Khlebtsov N. (2012). Gold nanoparticles in biomedical applications: recent advances and perspectives. Chem Soc Rev 41:2256–2282.
- El-Sherbiny IM, Villanueva DG, Herrera D, Smyth HDC. (2011). Overcoming lung clearance mechanisms for controlled release drug delivery. Control Pulmon Drug Delivery 101–126. https://doi.org/10.1007/978-1-4419-9745-6_5
- Falciani C, Zevolini F, Brunetti J, et al. (2020). Antimicrobial peptide-loaded nanoparticles as inhalation therapy for Pseudomonas aeruginosa infections. Int J Nanomed 15:1117–1128.
- Feng G, Jiang Q, Xia M, et al. (2013). Enhanced immune response and protective effects of nano-chitosan-based dna vaccine encoding T cell epitopes of Esat-6 and FL against mycobacterium tuberculosis infection. PLoS ONE 8:e61135.
- Filipczak N, Yalamarty SSK, Li X, et al. (2021). Developments in treatment methodologies using dendrimers for infectious diseases. Molecules 26:3304.
- Flemming HC, Wingender J. (2010). The biofilm matrix. Nat Rev Microbiol 8:623–633.
- Forier K, Messiaen AS, Raemdonck K, et al. (2013). Transport of nanoparticles in cystic fibrosis sputum and bacterial biofilms by single-particle tracking microscopy. Nanomedicine (Lond) 8:935–949.
- Fu L, Zhao J, Huang J, et al. (2022). A mitochondrial STAT3-methionine metabolism axis promotes ILC2-driven allergic lung inflammation. J Allergy Clin Immunol 149:2091–2104.
- Fukuyama Y, Yuki Y, Katakai Y, et al. (2015). Nanogel-based pneumococcal surface protein A nasal vaccine induces microRNA-associated Th17 cell responses with neutralizing antibodies against Streptococcus pneumoniae in macaques. Mucosal Immunol 8:1144–1153.
- Garcia-Contreras L, Wong YL, Muttil P, et al. (2008). Immunization by a bacterial aerosol. Proc Natl Acad Sci U S A 105:4656–4660. https://doi.org/10.1073/PNAS.0800043105/SUPPL_FILE/00043SUPPAPPENDIX.PDF
- Ghodake V, Vishwakarma J, Vavilala SL, Patravale V. (2020). Cefoperazone sodium liposomal formulation to mitigate P. aeruginosa biofilm in Cystic fibrosis infection: a QbD approach. Int J Pharm 587:119696
- Ginsberg AM, Spigelman M. (2007). Challenges in tuberculosis drug research and development. Nat Med 13:290–294.
- Gnanadhas DP, Elango M, Datey A, Chakravortty D. (2015). Chronic lung infection by Pseudomonas aeruginosa biofilm is cured by L-methionine in combination with antibiotic therapy. Sci Rep 5:16043–14.
- Gordon SB, Read RC. (2002). Macrophage defences against respiratory tract infections the immunology of childhood respiratory infections. Br Med Bull 61:45–61.
- Guitor AK, Wright GD. (2018). Antimicrobial resistance and respiratory infections. Chest 154:1202–1212.
- Günday Türeli N, Torge A, Juntke J, et al. (2017). Ciprofloxacin-loaded PLGA nanoparticles against cystic fibrosis P. aeruginosa lung infections. Eur J Pharm Biopharm 117:363–371.
- Gupta PV, Nirwane AM, Nagarsenker MS. (2018). Inhalable levofloxacin liposomes complemented with lysozyme for treatment of pulmonary infection in rats: effective antimicrobial and antibiofilm strategy. AAPS PharmSciTech 19:1454–1467. https://doi.org/10.1208/S12249-017-0945-4/FIGURES/13
- Gyu Kong I, Sato A, Yuki Y, et al. (2013). Nanogel-based PspA intranasal vaccine prevents invasive disease and nasal colonization by Streptococcus pneumoniae. Infect Immun 81:1625–1634.
- Hamzah Y, Bin Hashim S, Rahman WAWA. (2017). Synthesis of polymeric nano/microgels: a review. J Polym Res 24:1–19.
- Hasanzadeh M, Feyziazar M, Solhi E, et al. (2019). Ultrasensitive immunoassay of breast cancer type 1 susceptibility protein (BRCA1) using poly (dopamine-beta cyclodextrine-Cetyl trimethylammonium bromide) doped with silver nanoparticles: a new platform in early stage diagnosis of breast cancer and efficient management. Microchem J 145:778–783.
- Hawn TR, Day TA, Scriba TJ, et al. (2014). Tuberculosis vaccines and prevention of infection. Microbiol Mol Biol Rev 78:650–671. https://doi.org/10.1128/MMBR.00021-14/ASSET/0B1E08E5-B499-450E-AE31-CFE7575C5171/ASSETS/GRAPHIC/ZMR9990923760002.JPEG
- Hema S, Thambiraj S, Shankaran DR. (2018). Nanoformulations for targeted drug delivery to prostate cancer: an overview. J Nanosci Nanotechnol 18:5171–5191.
- Hill M, Twigg M, Sheridan EA, et al. (2019). Alginate/chitosan particle-based drug delivery systems for pulmonary applications. Pharmaceutics 11:379.
- Homayoonnia S, Lee Y, Andalib D, et al. (2021). Micro/nanotechnology-inspired rapid diagnosis of respiratory infectious diseases. Biomed Eng Lett 11:335–365.
- Horváti K, Gyulai G, Csámpai A, et al. (2018). Surface layer modification of poly(d, l-lactic-co-glycolic acid) nanoparticles with targeting peptide: a convenient synthetic route for pluronic F127-tuftsin conjugate. Bioconjug Chem 29:1495–1499. https://doi.org/10.1021/ACS.BIOCONJCHEM.8B00156/SUPPL_FILE/BC8B00156_SI_001.PDF
- Huang D, Wu D. (2018). Biodegradable dendrimers for drug delivery. Mater Sci Eng C Mater Biol Appl 90:713–727.
- Huang Z, Kłodzińska SN, Wan F, Nielsen HM. (2021). Nanoparticle-mediated pulmonary drug delivery: state of the art towards efficient treatment of recalcitrant respiratory tract bacterial infections. Drug Deliv Transl Res 11:1634–1654.
- Idris AO, Mamba B, Feleni U. (2020). Poly(propylene imine) dendrimer: a potential nanomaterial for electrochemical application. Mater Chem Phys 244:122641.
- Ingle AP, Shende S, Pandit R, et al. (2016). Nanotechnological applications for the control of pulmonary infections. Microbiol Respirator Syst Infect 223–235. https://doi.org/10.1016/B978-0-12-804543-5.00015-4
- Juntke J, Murgia X, Günday Türeli N, et al. (2021). Testing of aerosolized ciprofloxacin nanocarriers on cystic fibrosis airway cells infected with P. aeruginosa biofilms. Drug Deliv and Transl Res 11:1752–1765. https://doi.org/10.1007/S13346-021-01002-8/FIGURES/8
- Kabanov AV, Vinogradov SV. (2009). Nanogels as pharmaceutical carriers: finite networks of infinite capabilities. Angew Chem Int Ed Engl 48:5418–5429.
- Kamaruzzaman NF, Kendall S, Good L. (2017). Targeting the hard to reach: challenges and novel strategies in the treatment of intracellular bacterial infections. Br J Pharmacol 174:2225–2236.
- Kao HW, Lin YY, Chen CC, et al. (2013). Evaluation of EGFR-targeted radioimmuno-gold-nanoparticles as a theranostic agent in a tumor animal model. Bioorg Med Chem Lett 23:3180–3185.
- Kaufmann SHE, Weiner J, von Reyn CF. (2017). Novel approaches to tuberculosis vaccine development. Int J Infect Dis 56:263–267.
- Keskin D, Zu G, Forson AM, et al. (2021). Nanogels: a novel approach in antimicrobial delivery systems and antimicrobial coatings. Bioact Mater 6:3634–3657.
- Khan F, Lee JW, Manivasagan P, et al. (2019). Synthesis and characterization of chitosan oligosaccharide-capped gold nanoparticles as an effective antibiofilm drug against the Pseudomonas aeruginosa PAO1. Microb Pathog 135:103623
- Khan F, Manivasagan P, Lee JW, et al. (2019). Fucoidan-stabilized gold nanoparticle-mediated biofilm inhibition, attenuation of virulence and motility properties in Pseudomonas aeruginosa PAO1. Mar Drugs 17:208.
- Khan O, Chaudary N. (2020). The use of amikacin liposome inhalation suspension (Arikayce) in the treatment of refractory nontuberculous mycobacterial lung disease in adults. Drug Des Devel Ther 14:2287–2294.
- Khatak S, Mehta M, Awasthi R, et al. (2020). Solid lipid nanoparticles containing anti-tubercular drugs attenuate the Mycobacterium marinum infection. Tuberculosis 125:102008.
- Kim B, Pang HB, Kang J, et al. (2018). Immunogene therapy with fusogenic nanoparticles modulates macrophage response to Staphylococcus aureus. Nat Commun 9:1–13.
- Kim J, Mohamed MAA, Zagorovsky K, Chan WCW. (2017). State of diagnosing infectious pathogens using colloidal nanomaterials. Biomaterials 146:97–114.
- Kim KJ, Sung WS, Suh BK, et al. (2009). Antifungal activity and mode of action of silver nano-particles on Candida albicans. Biometals 22:235–242. https://doi.org/10.1007/S10534-008-9159-2/FIGURES/5
- Klinger-Strobel M, Lautenschläger C, Fischer D, et al. (2015). Aspects of pulmonary drug delivery strategies for infections in cystic fibrosis-where do we stand? Expert Opin Drug Deliv 12:1351–1374.
- Koch C, & Hoiby N. (1993). Pathogenesis of cystic fibrosis. Lancet 341:1065–1069.
- Kolpen M, Kragh KN, Barraza J, et al. (2022). Bacterial biofilms predominate in both acute and chronic human lung infections. Thorax thoraxjnl-2021-217576. thoraxjnl-2021-217576. https://doi.org/10.1136/THORAXJNL-2021-217576
- Komalla V, Allam VSRR, Kwok PCL, et al. (2020). A phospholipid-based formulation for the treatment of airway inflammation in chronic respiratory diseases. Eur J Pharm Biopharm 157:47–58.
- Kooti M, Sedeh AN, Motamedi H, Rezatofighi SE. (2018). Magnetic graphene oxide inlaid with silver nanoparticles as antibacterial and drug delivery composite. Appl Microbiol Biotechnol 102:3607–3621. https://doi.org/10.1007/S00253-018-8880-1/FIGURES/10
- Kora AJ, Arunachalam J. (2011). Assessment of antibacterial activity of silver nanoparticles on Pseudomonas aeruginosa and its mechanism of action. World J Microbiol Biotechnol 27:1209–1216. https://doi.org/10.1007/S11274-010-0569-2/FIGURES/9
- Kraft M. (2000). The role of bacterial infections in asthma. Clin Chest Med 21:301–313.
- Kretzmann JA, Ho D, Evans CW, et al. (2017). Synthetically controlling dendrimer flexibility improves delivery of large plasmid DNA. Chem Sci 8:2923–2930.
- Kumar SSD, Houreld NN, Kroukamp EM, Abrahamse H. (2018). Cellular imaging and bactericidal mechanism of green-synthesized silver nanoparticles against human pathogenic bacteria. J Photochem Photobiol B 178:259–269.
- Lababidi N, Montefusco-Pereira CV, de Souza Carvalho-Wodarz C, et al. (2020). Spray-dried multidrug particles for pulmonary co-delivery of antibiotics with N-acetylcysteine and curcumin-loaded PLGA-nanoparticles. Eur J Pharm Biopharm 157:200–210.
- Lahiri T, Brambilla L, Andrade J, et al. (2021). Mitochondrial STAT3 regulates antioxidant gene expression through complex I-derived NAD in triple negative breast cancer. Mol Oncol 15:1432–1449. https://doi.org/10.1002/1878-0261.12928
- Lambe U, Brar B, Guray M, et al. (2016). Nanodiagnostics: a new frontier for veterinary and medical sciences. JEBAS 4:307–320.
- Lan MY, Hsu Y, Bin Hsu CH, et al. (2013). Induction of apoptosis by high-dose gold nanoparticles in nasopharyngeal carcinoma cells. Auris Nasus Larynx 40:563–568.
- Li WR, Xie XB, Shi QS, et al. (2011). Antibacterial effect of silver nanoparticles on Staphylococcus aureus. Biometals 24:135–141. https://doi.org/10.1007/S10534-010-9381-6/TABLES/1
- Li WR, Xie XB, Shi QS, et al. (2010). Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Appl Microbiol Biotechnol 85:1115–1122. https://doi.org/10.1007/S00253-009-2159-5/FIGURES/6
- Li X, Gui R, Li J, et al. (2021). Novel multifunctional silver nanocomposite serves as a resistance-reversal agent to synergistically combat carbapenem-resistant Acinetobacter baumannii. ACS Appl Mater Interfaces 13:30434–30457. https://doi.org/10.1021/ACSAMI.1C10309/ASSET/IMAGES/LARGE/AM1C10309_0016.JPEG
- Li XZ, Nikaido H. (2009). Efflux-mediated drug resistance in bacteria: an update. Drugs 69:1555–1623. https://doi.org/10.2165/11317030-000000000-00000
- Li Y, Tang C, Zhang E, Yang L. (2017). Electrostatically entrapped colistin liposomes for the treatment of Pseudomonas aeruginosa infection. Pharm Dev Technol 22:436–444.
- Lima Salviano T, dos Santos Macedo DC, de Siqueira Ferraz Carvalho R, et al. (2021). Fucoidan-coated liposomes: a target system to deliver the antimicrobial drug usnic acid to macrophages infected with Mycobacterium tuberculosis. J Biomed Nanotechnol 17:1699–1710.
- López Y, Muñoz L, Gargallo-Viola D, et al. (2021). Uptake of ozenoxacin and other quinolones in gram-positive bacteria. IJMS 22:13363.
- Ma C, Wu M, Ye W, et al. (2021). Inhalable solid lipid nanoparticles for intracellular tuberculosis infection therapy: macrophage-targeting and pH-sensitive properties. Drug Deliv Transl Res 11:1218–1235. https://doi.org/10.1007/S13346-020-00849-7/FIGURES/6
- Marasini N, Haque S, Kaminskas LM. (2017). Polymer-drug conjugates as inhalable drug delivery systems: a review. Curr Opin Colloid Interf Sci 31:18–29.
- Maretti E, Costantino L, Buttini F, et al. (2019). Newly synthesized surfactants for surface mannosylation of respirable SLN assemblies to target macrophages in tuberculosis therapy. Drug Deliv Transl Res 9:298–310. https://doi.org/10.1007/S13346-018-00607-W/FIGURES/11
- Mehta M, Deeksha Tewari D, Gupta G, et al. (2019). Oligonucleotide therapy: an emerging focus area for drug delivery in chronic inflammatory respiratory diseases. Chem Biol Interact 308:206–215.
- Menon JU, Ravikumar P, Pise A, et al. (2014). Polymeric nanoparticles for pulmonary protein and DNA delivery. Acta Biomater 10:2643–2652.
- Messiaen AS, Forier K, Nelis H, et al. (2013). Transport of nanoparticles and tobramycin-loaded liposomes in burkholderia cepacia complex biofilms. PLoS ONE 8:e79220
- Mignani S, Tripathi VD, Soam D, et al. (2021). Safe polycationic dendrimers as potent oral in vivo inhibitors of mycobacterium tuberculosis: a new therapy to take down tuberculosis. Biomacromolecules 22:2659–2675.
- Mizgerd JP. (2006). Lung infection-a public health priority. PLoS Med 3:e76
- Moreau-Marquis S, Stanton BA, O’Toole GA. (2008). Pseudomonas aeruginosa biofilm formation in the cystic fibrosis airway. Pulm Pharmacol Ther 21:595–599.
- Muhammad W, Zhai Z, Wang S, Gao C. (2022). Inflammation-modulating nanoparticles for pneumonia therapy. Wiley Interdiscip Rev Nanomed Nanobiotechnol 14:e1763. https://doi.org/10.1002/WNAN.1763
- Mullis AS, Peroutka-Bigus N, Phadke KS, et al. (2021). Nanomedicines to counter microbial barriers and antimicrobial resistance. Curr Opin Chem Eng 31:100672.
- Nakahashi-Ouchida R, Yuki Y, Kiyono H. (2018). Cationic pullulan nanogel as a safe and effective nasal vaccine delivery system for respiratory infectious diseases. Hum Vaccin Immunother 14:2189–2193.
- Nakamura K, Matsubara H, Akagi S, et al. (2017). Nanoparticle-mediated drug delivery system for pulmonary arterial hypertension. JCM 6:48.
- Nasiruddin M, Neyaz MK, Das S. (2017). Nanotechnology-based approach in tuberculosis treatment. Tuberc Res Treat 2017:4920209–12.
- Nassimi M, Schleh C, Lauenstein HD, et al. (2010). A toxicological evaluation of inhaled solid lipid nanoparticles used as a potential drug delivery system for the lung. Eur J Pharm Biopharm 75:107–116.
- Nassimi M, Schleh C, Lauenstein HD, et al. (2009). Low cytotoxicity of solid lipid nanoparticles in in vitro and ex vivo lung models. Inhalation Toxicol 21:104–109.
- Ng AW, Bidani A, Heming TA. (2004). Innate host defense of the lung: effects of lung-lining fluid pH. Lung 182:297–317.
- Ng ZY, Wong JY, Panneerselvam J, et al. (2018). Assessing the potential of liposomes loaded with curcumin as a therapeutic intervention in asthma. Colloids Surf B Biointerfaces 172:51–59.
- Ngan CL, Asmawi AA. (2018). Lipid-based pulmonary delivery system: a review and future considerations of formulation strategies and limitations. Drug Deliv Transl Res 8:1527–1544.
- Noah NM, Ndangili PM. (2019). Current trends of nanobiosensors for point-of-care diagnostics. J Anal Methods Chem 2019:2179718
- Ong V, Mei V, Cao L, et al. (2019). Nanomedicine for cystic fibrosis. SLAS Technol 24:169–180.
- Pandey R, Sharma S, Khuller GK. (2005). Oral solid lipid nanoparticle-based antitubercular chemotherapy. Tuberculosis (Edinb) 85:415–420.
- Paranjpe M, Müller-Goymann CC. (2014). Nanoparticle-mediated pulmonary drug delivery: a review. Int J Mol Sci 15:5852–5873.
- Park H, Kim S, Kim S, et al. (2010). Antioxidant and anti-inflammatory activities of hydroxybenzyl alcohol releasing biodegradable polyoxalate nanoparticles. Biomacromolecules 11:2103–2108. https://doi.org/10.1021/BM100474W/ASSET/IMAGES/LARGE/BM-2010-00474W_0009.JPEG
- Pascual RM, Peters SP. (2005). Airway remodeling contributes to the progressive loss of lung function in asthma: an overview. J Allergy Clin Immunol 116:477–486.
- Pastor M, Moreno-Sastre M, Esquisabel A, et al. (2014). Sodium colistimethate loaded lipid nanocarriers for the treatment of Pseudomonas aeruginosa infections associated with cystic fibrosis. Int J Pharm 477:485–494.
- Patel KK, Agrawal AK, Anjum MM, et al. (2020). DNase-I functionalization of ciprofloxacin-loaded chitosan nanoparticles overcomes the biofilm-mediated resistance of Pseudomonas aeruginosa. Appl Nanosci 10:563–575. https://doi.org/10.1007/S13204-019-01129-8/FIGURES/7
- Pawde DM, Viswanadh MK, Mehata AK, et al. (2020). Mannose receptor targeted bioadhesive chitosan nanoparticles of clofazimine for effective therapy of tuberculosis. Saudi Pharmaceut J 28:1616–1625.
- Pignatello R, Leonardi A, Fuochi V, et al. (2018). A Method for efficient loading of ciprofloxacin hydrochloride in cationic solid lipid nanoparticles: formulation and microbiological evaluation. Nanomaterials 8:304.
- Pinheiro M, Lúcio M, Lima JLFC, Reis S. (2011). Liposomes as drug delivery systems for the treatment of TB. Nanomedicine (Lond) 6:1413–1428.
- Pompilio A, Geminiani C, Bosco D, et al. (2018). Electrochemically synthesized silver nanoparticles are active against planktonic and biofilm cells of Pseudomonas aeruginosa and other cystic fibrosis-associated bacterial pathogens. Front Microbiol 9:1349. https://doi.org/10.3389/FMICB.2018.01349/BIBTEX
- Pompilio A, Geminiani C, Mantini P, et al. (2018). Peptide dendrimers as “lead compounds” for the treatment of chronic lung infections by Pseudomonas aeruginosa in cystic fibrosis patients: in vitro and in vivo studies. Infect Drug Resist 11:1767–1782.
- Poschet J, Perkett E, Deretic V. (2002). Hyperacidification in cystic fibrosis: links with lung disease and new prospects for treatment. Trends Mol Med 8:512–519.
- Prabhu P, Fernandes T, Chaubey P, et al. (2021). Mannose-conjugated chitosan nanoparticles for delivery of Rifampicin to Osteoarticular tuberculosis. Drug Deliv Transl Res 11:1509–1519. https://doi.org/10.1007/S13346-021-01003-7/TABLES/4
- Prasad S. (2014). Nanobiosensors: the future for diagnosis of disease? NDD 3:1–10.
- Rajabnezhad S, Casettari L, Lam JKW, et al. (2016). Pulmonary delivery of rifampicin microspheres using lower generation polyamidoamine dendrimers as a carrier. Powder Technol 291:366–374.
- Rajkumari J, Busi S, Vasu AC, Reddy P. (2017). Facile green synthesis of baicalein fabricated gold nanoparticles and their antibiofilm activity against Pseudomonas aeruginosa PAO1. Microb Pathog 107:261–269.
- Rice KM, Ginjupalli GK, Manne NDPK, et al. (2019). A review of the antimicrobial potential of precious metal derived nanoparticle constructs. Nanotechnology 30:372001. https://doi.org/10.1088/1361-6528/AB0D38
- Robinson E, MacDonald KD, Slaughter K, et al. (2018). Lipid nanoparticle-delivered chemically modified mRNA restores chloride secretion in cystic fibrosis. Mol Ther 26:2034–2046.
- Rodrigues B, Shende P. (2020). Monodispersed metal-based dendrimeric nanoclusters for potentiation of anti-tuberculosis action. J Mol Liq 304:112731.
- Roh SG, Robby AI, Phuong PTM, et al. (2019). Photoluminescence-tunable fluorescent carbon dots-deposited silver nanoparticle for detection and killing of bacteria. Mater Sci Eng C Mater Biol Appl 97:613–623.
- Rudokas M, Najlah M, Alhnan MA, Elhissi A. (2016). Liposome delivery systems for inhalation: a critical review highlighting formulation issues and anticancer applications. Med Princ Pract 25:60–72.
- Rytting E, Nguyen J, Wang X, Kissel T. (2008). Biodegradable polymeric nanocarriers for pulmonary drug delivery. Expert Opin Drug Deliv 5:629–639.
- Sachetelli S, Khalil H, Chen T, et al. (2000). Demonstration of a fusion mechanism between a fluid bactericidal liposomal formulation and bacterial cells. Biochim Biophys Acta BBA Biomembr 1463:254–266.
- Salieb-Beugelaar GB, Hunziker PR. (2015). Towards nano-diagnostics for bacterial infections. Eur J Nanomed 7:37–50. https://doi.org/10.1515/EJNM-2015-0010/PDF
- Sanivarapu, R. R., & Gibson, J. (2021). Aspiration pneumonia. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK470459/
- Sathishkumar P, Vennila K, Jayakumar R, et al. (2016). Phyto-synthesis of silver nanoparticles using Alternanthera tenella leaf extract: an effective inhibitor for the migration of human breast adenocarcinoma (MCF-7) cells. Bioprocess Biosyst Eng 39:651–659. https://doi.org/10.1007/S00449-016-1546-4/FIGURES/5
- Sharma A, Kumar D, Dahiya K, et al. (2021). Advances in pulmonary drug delivery targeting microbial biofilms in respiratory diseases. Nanomedicine (Lond) 16:1905–1923. https://doi.org/10.2217/NNM-2021-0057/ASSET/IMAGES/LARGE/FIGURE2.JPEG
- Shi D, Huang J, Chuai Z, et al. (2014). Isothermal and rapid detection of pathogenic microorganisms using a nano-rolling circle amplification-surface plasmon resonance biosensor. Biosens Bioelectron 62:280–287.
- Shima K, Coopmeiners J, Graspeuntner S, et al. (2016). Impact of micro-environmental changes on respiratory tract infections with intracellular bacteria. FEBS Lett 590:3887–3904.
- Sigurdsson HH, Kirch J, Lehr CM. (2013). Mucus as a barrier to lipophilic drugs. Int J Pharm 453:56–64.
- Singh H, Jindal S, Singh M, et al. (2015). Nano-formulation of rifampicin with enhanced bioavailability: development, characterization and in-vivo safety. Int J Pharm 485:138–151.
- Singh L, Kruger HG, Maguire GEM, et al. (2017). The role of nanotechnology in the treatment of viral infections. Ther Adv Infect Dis 4:105–131.
- Soni KS, Desale SS, Bronich TK. (2016). Nanogels: an overview of properties, biomedical applications and obstacles to clinical translation. J Control Release 240:109–126.
- Sung JC, Pulliam BL, Edwards DA. (2007). Nanoparticles for drug delivery to the lungs. Trends Biotechnol 25:563–570.
- Tăbăran AF, Matea CT, Mocan T, et al. (2020). Silver Nanoparticles for the Therapy of Tuberculosis. Int J Nanomed 15:2231–2258.
- Thakur A, Mikkelsen H, Jungersen G. (2019). Intracellular pathogens: host immunity and microbial persistence strategies. J Immunol Res 2019:1356540.
- Thambiraj S, Shruthi S, Vijayalakshmi R, Ravi Shankaran D. (2019). Evaluation of cytotoxic activity of docetaxel loaded gold nanoparticles for lung cancer drug delivery. Cancer Treat Res Commun 21:100157.
- Troy NM, Bosco A. (2016). Respiratory viral infections and host responses; insights from genomics. Respir Res 17:1–12.
- Truzzi E, Nascimento TL, Iannuccelli V, et al. (2020). In vivo biodistribution of respirable solid lipid nanoparticles surface-decorated with a mannose-based surfactant: a promising tool for pulmonary tuberculosis treatment? Nanomaterials 10:568.
- Tucker AN, Carlson TJ, Sarkar A. (2021). Challenges in drug discovery for intracellular bacteria. Pathogens 10:1172. https://doi.org/10.3390/PATHOGENS10091172/S1
- Üner M, Yener G. (2007). Importance of solid lipid nanoparticles (SLN) in various administration routes and future perspectives. Int J Nanomed 2:289. /pmc/articles/PMC2676658/
- Veldhuizen EJA, Haagsman HP. (2000). Role of pulmonary surfactant components in surface film formation and dynamics. Biochim Biophys Acta BBA Biomembr 1467:255–270.
- Vieira ACC, Chaves LL, Pinheiro S, et al. (2018). Mucoadhesive chitosan-coated solid lipid nanoparticles for better management of tuberculosis. Int J Pharm 536:478–485.
- Viswanathan V, Pharande R, Bannalikar A, et al. (2019). Inhalable liposomes of Glycyrrhiza glabra extract for use in tuberculosis: formulation, in vitro characterization, in vivo lung deposition, and in vivo pharmacodynamic studies. Drug Dev Ind Pharm 45:11–20.
- Wan F, Herzberg M, Huang Z, et al. (2020). A free-floating mucin layer to investigate the effect of the local microenvironment in lungs on mucin-nanoparticle interactions. Acta Biomater 104:115–123.
- Wan F, S-R Bohr S, Natalie Kłodzin S, et al. (2019). Ultrasmall TPGS − PLGA hybrid nanoparticles for site-specific delivery of antibiotics into Pseudomonas aeruginosa biofilms in lungs. ACS applied materials & interfaces 12(1):380–389. https://doi.org/10.1021/acsami.9b19644
- Wan G, Ruan L, Yin Y, et al. (2016). Effects of silver nanoparticles in combination with antibiotics on the resistant bacteria Acinetobacter baumannii. Int J Nanomed 11:3789–3800.
- Wang L, Feng M, Li Q, et al. (2019). Advances in nanotechnology and asthma. Ann Transl Med 7:180–180.
- Wang S, Yu S, Lin Y, et al. (2018). Co-delivery of ciprofloxacin and colistin in liposomal formulations with enhanced in vitro antimicrobial activities against multidrug resistant Pseudomonas aeruginosa. Pharm Res 35:1–13.
- Wang Y, Yu L, Kong X, Sun L. (2017). Application of nanodiagnostics in point-of-care tests for infectious diseases. Int J Nanomed 12:4789–4803.
- Weber S, Zimmer A, Pardeike J. (2014). Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for pulmonary application: a review of the state of the art. Eur J Pharm Biopharm 86:7–22.
- Winstanley C, O’Brien S, Brockhurst MA. (2016). Pseudomonas aeruginosa evolutionary adaptation and diversification in cystic fibrosis chronic lung infections. Trends Microbiol 24:327–337.
- Yang R, Rincon M. (2016). Mitochondrial Stat3, the need for design thinking. Int J Biol Sci 12:532–544.
- Yoo D, Guk K, Kim H, et al. (2013). Antioxidant polymeric nanoparticles as novel therapeutics for airway inflammatory diseases. Int J Pharm 450:87–94.
- Yu T, Chan KWY, Anonuevo A, et al. (2015). Liposome-based mucus-penetrating particles (MPP) for mucosal theranostics: demonstration of diamagnetic chemical exchange saturation transfer (diaCEST) magnetic resonance imaging (MRI). Nanomedicine 11:401–405.
- Yu X, Zou L, Ling T, et al. (2021). Preparation and evaluation of bergenin-loaded cationic liposome for anti-asthma treatment. Mat Express 11:601–617.
- Yu Y, Zhang Y, Cheng Y, et al. (2022). NIR-activated nanosystems with self-modulated bacteria targeting for enhanced biofilm eradication and caries prevention. Bioact Mater 13:269–285.
- Zanin M, Baviskar P, Webster R, Webby R. (2016). The interaction between respiratory pathogens and mucus. Cell Host Microbe 19:159–168.
- Zhang J, Leifer F, Rose S, et al. (2018). Amikacin liposome inhalation suspension (ALIS) penetrates non-tuberculous mycobacterial biofilms and enhances amikacin uptake into macrophages. Front Microbiol 9:915. https://doi.org/10.3389/FMICB.2018.00915/BIBTEX
- Zhang Y, Shareena Dasari TP, Deng H, Yu H. (2015). Antimicrobial activity of gold nanoparticles and ionic gold. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 33:286–327.
- Zhao L, Seth A, Wibowo N, et al. (2014). Nanoparticle vaccines. Vaccine 32:327–337.
- Zhong Q, Bielski ER, Rodrigues LS, et al. (2016). Conjugation to poly(amidoamine) dendrimers and pulmonary delivery reduce cardiac accumulation and enhance antitumor activity of doxorubicin in lung metastasis. Mol Pharm 13:2363–2375. https://doi.org/10.1021/ACS.MOLPHARMACEUT.6B00126/SUPPL_FILE/MP6B00126_SI_001.PDF
- Zhou QT, Leung SSY, Tang P, et al. (2015). Inhaled formulations and pulmonary drug delivery systems for respiratory infections. Adv Drug Deliv Rev 85:83–99.