Abstract
Thermal degradation of total carotenoids, lycopene and visual colour of watermelon juice was studied at 50–90°C up to 5 h. Total carotenoids content in fresh watermelon juice was reduced from 4.568 to 0.929 mg/100 g, lycopene from 4.403 to 0.82 mg/100 g and Hunter ‘a × b’ value from 251.66 to 89.59 when heated at 90°C for 5 h. First order model explained the degradation behaviour of total carotenoids, lycopene and Hunter ‘a × b’ value evident from correlation coefficient (R2) higher than 0.93. The dependence of degradation rate constant of total carotenoids, lycopene and Hunter ‘a × b’ value on temperature was adequately explained by Arrhenius equation. The activation energies for total carotenoids, lycopene and Hunter ‘a × b’ value were 24.19, 26.46, and 55.47 kJ/mol, respectively. Total carotenoids and lycopene were correlated with Hunter ‘a × b’ value with R2 > 0.99 indicating that visual colour may be used to predict lycopene and total carotenoids contents in watermelon juice.
INTRODUCTION
The watermelon (Citrullus vulgaris), member of cucurbitaceae family, is a native plant of tropical Africa and cultivated throughout warmer parts of the world. The main pigments in red, orange and yellow-fleshed watermelons are lycopene, β-carotene and both β-carotene and xanthophylls respectively.[Citation1,Citation2] Lycopene is a highly effective antioxidant while lycopene and β-carotene have been positively correlated with reduced cancer incidences.[Citation3] Carotenoid pigments are sensitive to light, heat, oxygen, and acid. Maintenance of natural pigments in thermally processed and stored foods has been a major challenge in food processing.[Citation4] Reports on lycopene content of watermelon juice are available but information on pigments and visual color degradation during thermal processing is scanty. The visual color measurement has been accepted by food processing industry as an on-line quality control technique while measurement of pigment content quantifies the actual degradation during processing.
Degradation of lycopene during heating at 50, 100, or 150°C or illuminated at 25°C for varied lengths of time was found to fit a first order model and degradation rate constant (min−1) of lycopene rose with increasing temperature and the activation energy was calculated to be 61.0 kJ/mol.[Citation5] The degradation kinetics of carotenoids and visual color of papaya puree at different temperatures 70–105°C followed first order model with coefficient of determination > 0.99. The activation energies for carotenoids and visual color in papaya puree were 20.56 and 32.59 kJ/mol respectively.[Citation6] Thermal treatment of tomato pulp at 100°C in the presence of O2, with/without light caused significant loss of lycopene.[Citation7] Concentration of tomato pulp resulted in up to 57% loss of lycopene.[Citation8] Degradation kinetics of lycopene and visual color in tomato peel isolated from pomace at 50–100°C for 10 h resulted in decreased in lycopene content from 1.75–0.92 mg/100 g and visual color values ‘a × b’ from 342.58–239.35, respectively.[Citation9]
Studies on lycopene degradation revealed that heating tomato pulp at 100°C for 120 min decreased lycopene content from 185.5 to 141.5 mg/100 g of total solids. The lycopene loss was highest in the presence of air and light at 25°C, and lowest under vacuum and dark.[Citation10] The degradation rate of lycopene was higher than β-carotene when safflower oil was heated at 75, 85, or 95°C.[Citation11] The lycopene content in tomatoes slightly decreased using different dehydration methods: vacuum-drying (55°C, 4–8 h), conventional air-drying (95°C, 6–10 h), and osmotic-vacuum-drying (first dehydrated with an osmotic treatment at 25°C for 4 h, followed by vacuum-drying at 55°C for 4–8 h. The lycopene content in samples slightly decreased during the dehydration processes. During osmotic treatment, lycopene content remained constant. After osmotic-vacuum-drying, total lycopene retention in tomatoes was greater than that using vacuum-drying. Conventional air-drying decreased lycopene retention greatly in tomato samples, which was attributed to the influence of heat and oxygen.[Citation12]
The degradation kinetics of pigments and visual color in watermelon juice was generally observed to see the effect of processing conditions on the pigments degradation during the watermelon juice. Undesirable degradation of pigments and visual color affects the sensory attributes, health promoting ability and natural appearance of the watermelon juice. This study was conducted to investigate the degradation kinetics of lycopene, total carotenoids and visual color during heat treatment of watermelon juice and their correlation with each other.
MATERIALS AND METHODS
Materials
Ripe watermelons (Citrullus vulgaris) were procured from local market of Amritsar. Fruits were washed, cut into quarters, deseeded, peeled, passed through screw juice extractor (Kalsi Industries Ltd., India) and filtered to get watermelon juice. All the chemicals used for analysis were of analytical grades.
Physicochemical Analysis
Total soluble solids were quantified by using Abbe Mat Refractometer (Milton Roy Co., USA) at 21°C. Titrable acidity (as anhydrous citric acid) was determined using a pH meter (Elico, India). Reducing and total sugars were determined according to Lane and Eyon method.[Citation13]
Thermal Treatment
The watermelon juice was sealed in cultured tubes (19 mm internal diameter × 930 mm length) and was immersed in a water-bath (Brookfield Laboratories, USA) for preset times (0, 1, 2, 3, 4, and 5 h) at 50, 60, 70, 80, and 90°C. The desired temperature was considered to have achieved when the temperature of water-bath reached the set value. The samples were transferred to an ice-water bath immediately after the treatment.
Total Carotenoids
Juice (20 g) was mixed with 100 ml tetrahydrofuran and 50 ml petroleum ether in a separating funnel, the aqueous phase was separated and the organic phase was washed with three aliquots of water (25 ml). The extraction was repeated with petroleum ether until aqueous phase became colorless. Ether extracts was dried on anhydrous sodium sulphate and volume was made to 250 ml. Carotenoids were determined spectrophotometrically (Shimadzu, Japan) by measuring absorbance at 450 nm and were expressed as mg/100 g using extinction coefficient of 13.9 × 104 mol cm−1.[Citation14]
Lycopene
Sample (1 g) was extracted with acetone in a pestle and mortar till residues became colorless. Lycopene was transferred into petroleum ether phase by diluting acetone extract in a separating funnel, passed through sodium sulfate, volume made to 50 ml and absorbance was measured at 503 nm using UV visible spectrophotometer (Shimadzu, Japan).[Citation15] The extinction coefficient (17.2 × 104 mol cm−1) was verified with standard lycopene solution (Sigma Chemical Co, St. Louis, Missouri, U.S.A.), and the lycopene in sample was calculated.
Visual Color
The Hunter Color Lab (Hunter Associates Laboratory, USA) was calibrated with standard white tile (L = 90.55, a = −0.71, b = 0.39). A sample handling dish was charged with samples, placed on the analyzing port and noted the L, a, b values.
Kinetics of Pigments and Visual Color Degradation
The kinetics of degradation of both pigments and visual color has been reported to follow first order reaction adequately.[Citation16,Citation17, Citation18, Citation6, Citation19] The first order kinetic model based on lycopene concentration is:
where, L is the concentration of lycopene at time ‘t’ (mg/100 g), Lo is the initial concentration of lycopene (mg/100 g), k is the degradation rate constant (h−1), and t is heating time (h). Similar model was used for total carotenoids and Hunter ‘a × b’ values by replacing lycopene concentration with total carotenoids concentration and Hunter ‘a × b’ values.
Temperature Dependence of Degradation Rate Constant
The Arrhenius model was applied to describe the temperature dependence of pigments and visual color degradation.
where, ko is Frequency factor (h−1); Ea = Activation energy (J/mol); R is the universal gas constant (8.314 J/mol K); and T is absolute temperature (K).
Relationship Between Pigments Content and Visual Color
The Hunter ‘a × b’ values were correlated with pigments concentration of watermelon juice. The relationship between visual color and pigment concentration has been found to be well described using the linear equation[Citation6]:
where ka and kb are the coefficients, and L is lycopene concentration (mg/100g). To determine relationship between visual color and total carotenoids, ‘C’ replaced ‘L’ where ‘C’ represents total carotenoid concentration (mg/100 g).
Statistical Analysis
The experimental data was analyzed employing given models [Eqs. (Equation1–3)], and adequacy of fit was evaluated by comparing the regression coefficients (R2) and standard error values that were computed using Excel (Microsoft Inc., USA).
RESULTS AND DISCUSSION
Physicochemical Properties
Total soluble solids of watermelon juice varied from 6.8–7.2°B. Similar results for total soluble solids were found for bottled Indo-American hybrid watermelon juice i.e. 6.38–9.0°B[Citation20]. The acidity of watermelon juice varied from 0.06–0.09%. Earlier studies on watermelon juice reported acidity in the range of 0.05-0.8%.[Citation20–22] Reducing sugars and total sugars of watermelon juice varied from 3.47–3.78% and 5.22–5.29%, respectively. Earlier studies reported higher values for reducing sugars and total sugars for Indo-American hybrid watermelon juice i.e. 5.52% and 7.76% which may be due differences in cultivar or processing conditions.[Citation20]
Degradation Kinetics of Total Carotenoids
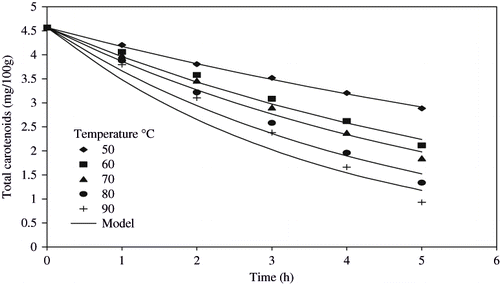
Total carotenoids content of fresh watermelon juice was found to be 4.568 mg/100 g. When the juice was heated at selected temperatures, its total carotenoids content was decreased. Total carotenoids decreased to 2.883 mg/100 g after 5 h of heating at 50°C (). The degradation of total carotenoids increased with increase in temperature and it reduced to 0.929 mg/100 g after 5 h of heating at 90°C. The first order model [EquationEq. (1)] was applied to the data and the degradation parameters were obtained (). The degradation rate constant was 0.09 h−1 at 50°C, which increased progressively to 0.271 h−1 with increase in temperature to 90°C. The predicted values of the total carotenoids were obtained from the model and shown as solid line in . The correlation coefficients (R2) ranged from 0.9309 to 0.9981, whereas standard error varied from 0.0258 to 0.2677 that adequately explained the degradation behavior. Total carotenoids are highly unsaturated compounds and therefore, susceptible to oxidation, isomerisaton, and other chemical changes during processing and storage.[Citation23, Citation24] Present study also gave similar trend in watermelon juice.
Table 1 The kinetic parameters of pigments and visual color degradation with time (EquationEq 1) at selected temperatures (n = 3)
Degradation Kinetics of Lycopene
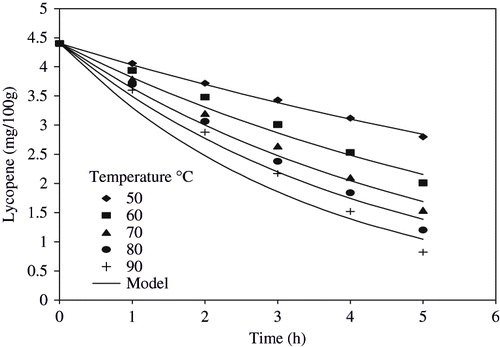
Lycopene content of watermelon juice was found to be 4.403 mg/100 g, which was very near to that of earlier reported value of 4.87 mg/100 g.[Citation25] When the juice was heated, the lycopene content decreased to 2.8 mg/100g after 5 h at 50°C accounting for approximately 36% loss (). Similar trend was observed during heating of the juice at higher temperatures. After 5 h of heating, the lycopene content decreased to 2.01, 1.54, 1.2, and 0.82 mg/100 g at 60, 70, 80 and 90°C, respectively. The lycopene content decreased in tomato pulp as the temperature was increased from 29 to 40°C.[Citation26] Degradation takes place due to various reasons such as high temperature, long processing time, light and oxygen. Cole and Kapur,[Citation7] reported that decline in lycopene was due to destruction by heat and oxidation resulting in fragment products like acetone, methyl heptenone, laevulinic aldehyde and glyoxal.
First order model [EquationEq. (1)] for degradation of lycopene during heating was fitted and the degradation rate constants were found to vary from 0.0874 to 0.2878 h−1 (). It was observed that as the temperature increased, the degradation rate constant increased. The predicted values of the lycopene using first order model are shown by solid line and best fit was found in the plot of lycopene degradation at 50°C (). The R2 value ranged from 0.9367 to 0.9964, while standard error values ranged from 0.0309 to 0.0245, which validated the first order model for explaining degradation changes. Studies on the stability of lycopene during heating and illumination also concluded that the degradation of lycopene fitted the first order model with degradation rate and increased with increasing temperature.[Citation5] Ax et al.[Citation27] reported that in oil-in-water emulsion, the degradation rate constant for total and all trans lycopene increased with increase in temperature. Hackett et al.[Citation28] concluded that lycopene degradation followed first order model and degradation rate constant values followed similar trends. The present findings concluded that degradation of lycopene followed the first order model and degradation rate constant increased with increase in heating temperature and these results were similar with earlier reported observations.
Degradation Kinetics of Hunter Color Value
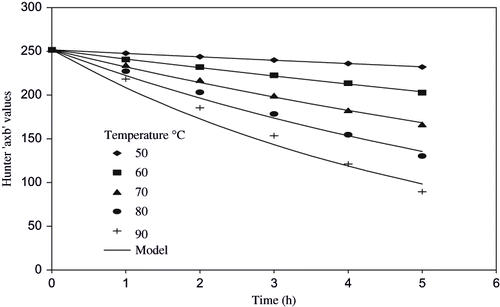
Visual color of thermally processed juice was observed on Hunter color Lab in the form of ‘L’, ‘a’, and ‘b’ values. It was desired to find out the variable that varied in linear fashion and could be adequately used to describe first order degradation kinetics, which can be correlated with pigment degradation of watermelon juice. Thus different combinations of Hunter values ‘L’, ‘a’, ‘b’ were plotted against time and R2 were computed (). The ‘a × b’ values showed maximum R2 value (0.9944) and therefore, selected to describe the visual color change in watermelon juice.
Table 2 Correlation coefficients (R2 ) of change in Hunter values with time (EquationEq. 1) at selected temperatures (n = 3)
In previous studies (a/b)2 values were correlated with lycopene content in tomato juice with R2 of 0.77[Citation29] and the ‘a’ values were correlated with lycopene content of grapefruit juice with R2 of 0.96.[Citation30] Perkins-Veazie et al., [Citation25] found that the ‘a’ and ‘1000a/(b+L)’ were best correlated with lycopene content of melons. The present study has shown different results since ‘a × b’ values gave best correlation but it was closely followed by ‘a’ value. However ‘a × b’ values have been found best suited for broccoli and papaya.[Citation17, Citation6] Davis et al.[Citation31] reported on use of xenon flash colorimeter/spectrophotometer to correlate its absorption with lycopene content of watermelon puree.
Hunter ‘a × b’ value for fresh watermelon juice was observed to be 251.66 and it decreased to 231.17 at 50°C and 89.59 at 90°C after 5 h of heating. The first order model [EquationEq. (1)] was applied and its degradation rate constant was found to vary from 0.016–0.188h−1 at 50–90°C (). The predicted values computed using the models have been given as a solid line (). The R2 values varied from 0.9714 to 0.9995 and standard error values varied from 0.1365 to 8.1225 (). The best relationship was found when watermelon juice was heated at 50°C for 5 h. Ahmed et al.,[Citation6] showed that in case of papaya puree thermal degradation of Hunter ‘a × b’ value followed first order model with R2 values of 0.997− 0.999. Reports on the watermelon were not traceable but present findings are in accordance with earlier studies on papaya puree.
Temperature Dependence of Degradation Rate Constant
Effect of temperature on the degradation rate constants has been shown in . Arrhenius model [EquationEq. (2)] was employed to explain the change in degradation rate constant with temperature. The Ko values for total carotenoids, lycopene and Hunter ‘a × b’ value were 1861.05, 806.9, and 184 × 105h−1 while the activation energies were found to be 55.46, 26.46, and 24.2 kJ/mol, respectively. Higher activation energy for visual color signified greater heat sensitiveness during thermal processing. The R2 values for total carotenoids, lycopene and Hunter ‘a × b’ value were 0.9728, 0.9727, and 0.9803, respectively, indicating that Arrhenius model explained well the dependence of visual color and pigment degradation on temperature.
Figure 4 Dependence of degradation rate constant for total carotenoids, lycopene and Hunter ‘a × b’ values of watermelon juice.
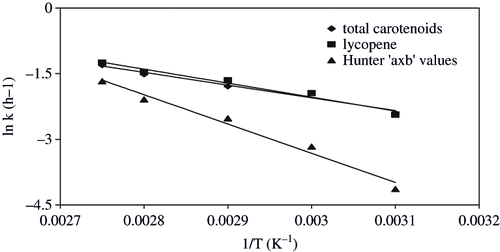
Sharma & Maguer,[Citation10] found the activation energy for lycopene degradation in tomato pulp ranging from 19.9 to 27.7 kJ/mol while Ahmed et al.,[Citation6] reported the activation energies for carotenoids and visual color as 20.56 and 32.59 kJ/mol respectively in papaya puree. Ax et al.,[Citation27] reported that the activation energy of total and all trans lycopene degradation in water emulsion varied from 18–25 kJ/mol. Hackett et al., [Citation28] reported the activation energy of 11.5 to 15 kJ/mol for lycopene degradation in tomato oleoresins. In the present study, the activation energy for pigments and visual color degradation in watermelon juice ranged between 24.2–55.46 kJ/mol.
Relationship Between Visual Color and Pigments Concentration
The Hunter ‘a × b’ values were correlated with total carotenoids and lycopene contents of watermelon juice using the model [EquationEq. (3)] and its coefficients have been given in . The ka value for total carotenoids varied from −16.953 to −1.0578 where as kb value ranged between 0.0223 to 0.0854. It was observed that ka value increased while kb value decreased, with increase in temperature. Total carotenoids were found to be highly related with Hunter ‘a × b’ values with R2 values ranging from 0.9967–0.9999 and standard error varying from 0.0156 to 0.0163.
Table 3 Regression parameters for the correlation of visual color with total carotenoids and lycopene [EquationEq. (3)] content (n = 3)
Similarly, when lycopene was correlated to visual color, ka increased from −16.038 to −1.164 while kb decreased from 0.0219 to 0.0811 with increase in temperature (). The correlation coefficient (R2) ranged between 0.9988–0.9996, and standard error varied from 0.0226–0.04318.
These results suggested that as the total carotenoids and lycopene degradation occurred with processing, the visual color of the product got affected. This might be because lycopene acts as a polyenic chromophore with H-conjugated double bonds, which absorbs and reflects light[Citation32] and thus imparts red color to watermelon. As the pigment degraded due to oxidation and isomerisation, so did the visual color. In previous study absorption on xenon flash colorimeter/spectrophotometer was correlated to lycopene content of watermelon puree and correlation coefficient of 0.9794 was reported.[Citation31] Thus there is a direct relationship between visual color and pigments content in watermelon juice and could be used interchangeably.
CONCLUSION
The thermal degradation kinetics of total carotenoids, lycopene and visual color of watermelon juice were studied at different temperatures 50–90°C for 0–5 h. The total carotenoids content, lycopene content and Hunter ‘a × b’ value of watermelon juice was reduced from 4.568–0.929 mg/100 g, 4.403–0.82 mg/100 g and 251.66–89.59, respectively, when heated at 50–90°C for 5 h. The degradation of the total carotenoids content, lycopene, and Hunter ‘a × b’ values followed first order kinetic model. Total carotenoids degradation, lycopene degradation and color loss followed Arrhenius Model and the activation energies for total carotenoids, lycopene and Hunter ‘a × b’ value were 24.19, 26.46, and 55.47 kJ/mol respectively indicating the greater temperature sensitivity of visual color parameters. The total carotenoids and lycopene were found well correlated to the Hunter ‘a × b’ values, thus could predict the pigment degradation in the watermelon juice.
REFERENCES
- Watanabe , K. , Saito , T. , Hirota , S. and Takahashi , B. 1987 . Carotenoid Pigments in Red, Orange and Yellow Fleshed Fruits of Watermelon (Citrullus vulgaris) . Journal of the Japanese Society for Horticultural Science , 56 : 45 – 50 .
- Collins , J. , Perkins-Veazie , P.M. and Fish , W.W. 2003 . Identification of Carotenoids in Orange and Yellow Fleshed Watermelon . Federation of American Society for Experimental Biology Conference , 17 ( 5 ) : 456.6
- Giovannucci , E.L. , Ascherio , A. , Rimm , E.B. , Stampfer , M.J. , Colditz , G.A. and Willet , W.C. 1995 . Intake of Carotenoids and Retinol in Relationship to Risk of Prostate Cancer . Journal of National Cancer Institute , 87 : 1767 – 1776 .
- Clydesdale , F.M. , Fleischman , D.L. and Francis , F.I. 1970 . Maintenance of Color in Processed Green Vegetables . Journal of Food Product Development , 4 : 127 – 130 .
- Lee , M.T. and Chen , B.H. 2002 . Stability of Lycopene During Heating and Illumination in a Model System . Food Chemistry , 78 : 425 – 432 .
- Ahmed , J. , Shivhare , U.S. and Sandhu , K.S. 2002 . Thermal Degradation Kinetics of Carotenoids and Visual Color of Papaya Puree . Journal of Food Science , 67 ( 7 ) : 2692 – 2695 .
- Cole , E.R. , Kapur , N.S. and The Stability of Lycopene II . 1957 . Oxidation During Heating of Tomato Pulps . Journal of the Science of Food and Agriculture , 8 : 366 – 368 .
- Noble , A.C. 1975 . Investigation of the Color Changes in Heat Concentrated Tomato Pulp . Journal of Agricultural and Food Chemistry , 23 : 48 – 49 .
- Kaur , D. , Sogi , D.S. and Wani , A.A. 2006 . Degradation Kinetics of Lycopene and Visual Color in Tomato Peel Isolated from Pomace . International Journal of Food Properties , 9 ( 4 ) : 781 – 789 .
- Sharma , S.K. and Maguer , M.L. 1996 . Kinetics of Lycopene Degradation in Tomato Pulp Solids Under Different Processing and Storage Conditions . Food Research International , 29 : 309 – 315 .
- Ranganna , S. 1986 . Hand Book of Analysis and Quality Control for Fruit and Vegetable Products , 2nd , New Delhi : Tata McGraw Hill .
- Henry , L.K. , Catignani , G.L. and Schwartz , S.J. 1998 . Oxidative Degradation Kinetics of Lycopene, Lutein, 9-cis and all-trans β-carotene . Journal of the American Oil Chemists Society , 75 : 823 – 829 .
- Shi , J. , Maguer , M.L. , Kakuda , Y. , Liptay , A. and Niekamp , F. 1999 . Lycopene Degradation and Isomerization in Tomato Dehydration . Food Research International , 32 : 15 – 21 .
- Alena , E. , Fallico , B. and Maccahone , E. 2000 . Influence of Carotenoids and Pulps on the Color Modification of Blood Orange Juice . Journal of Food Science , 65 : 458 – 460 .
- Sadler , G. , Davis , J. and Dezman , D. 1990 . Rapid Extraction of Lycopene and β-carotene from Reconstituted Tomato Paste and Pink Grapefruit Homogenates . Journal of Food Science , 55 ( 5 ) : 1460 – 1461 .
- Steet , J.A. and Tong , C.H. 1996 . Degradation Kinetics of Green Color and Chlorophyll in Peas by Colorimetry and HPLC . Journal of Food Science , 61 ( 5 ) : 924 – 927 .
- Weemaes , C.A. , Ooms , V. , Loey , A.M. and Hendrickx , M.E. 1999 . Kinetics of Chlorophyll Degradation and Color Loss in Heated Broccoli Juice . Journal of Agricultural and Food Chemistry , 47 : 2404 – 2409 .
- Gunawan , M.I. and Barringer , S.A. 2000 . Green Color Degradation of Blanched Broccoli (Brassica oleracea) Due to Acid and Microbial Growth . Journal of Food Processing and Preservation , 24 : 253 – 263 .
- Ahmed , J. , Shivhare , U.S. and Singh , G. 2000 . Chlorophyll and Color of Green Chilli Puree as Affected by Mesh Size and Temperature . International Journal of Food Properties , 4 : 305 – 316 .
- Chahal , G.S. and Saini , S.P.S. 1999 . Storability of Juice from New Hybrid Watermelon Variety . Indian Food Packer , : 12 – 18 .
- Hayoglu , A.G.I.A. and Fenercioglu , H.A. 1990 . Research on the Possibility of Using Watermelon in the Fruit Juice Industry . Gida , 15 ( 6 ) : 329 – 332 .
- Gowda , I.N.D. and Jalali . 1995 . Studies on Juice making from Watermelon Fruits . Indian Food Packer , 49 ( 3 ) : 33 – 41 .
- Boskovic , M.A. 1979 . Fate of Lycopene in Dehydrated Tomato Products: Carotenoid Isomerisation in Food System . Journal of Food Science , 44 : 84 – 86 .
- Nguyen , M. , Francis , D. and Schwartz , S. 2001 . Thermal Isomerization Susceptibility of Carotenoids in Different Tomato Varieties . Journal of the Science of Food and Agriculture , 81 : 910 – 917 .
- Perkins-Veazie , P. , Collins , J.K. , Pair , S.D. and Roberts , W. 2001 . Lycopene Content Differs among Red-fleshed Watermelon Cultivars . Journal of the Science of Food and Agriculture , 81 : 983 – 987 .
- Akanbi , C.T. and Oludemi , F.O. 2004 . Effect of Processing and Packaging on Lycopene Content of Tomato Products . International Journal of Food Properties , 7 : 139 – 152 .
- Ax , K. , Miebach , E.M. , Link , B. , Schuchmann , H. and Schubert , H. 2003 . Stability of Lycopene in Oil-in-water Emulsions . Engineering in Life Science , 3 ( 4 ) : 199 – 201 .
- Hackett , M.M. , Lee , J.H. , Francis , D. and Schwartz , S.J. 2004 . Thermal Stability and Isomerization of Lycopene in Tomato Oleoresins from Different Varieties . Journal of Food Science , 69 ( 7 ) : 536 – 541 .
- D'souza , M.C. , Singh , S. and Ingle , M. 1992 . Lycopene Content of Tomato Fruit can be Estimated from Chromaticity Values . Horticulture Science , 27 : 465 – 466 .
- Lee , H.S. 2000 . Objective Measurement of Red Grapefruit Juice Color . Journal of Agricultural and Food Chemistry , 48 : 1507 – 1511 .
- Davis , A.A. , Fish , W.W. and Perkin-Veazie , P. 2003 . A Rapid Hexane-free Method for Analyzing Lycopene Content in Watermelon . Journal of Food Science , 68 : 328 – 332 .
- Davies , B.H. 1976 . “ Carotenoids ” . In Chemistry and Biochemistry of Plant Pigment , 2nd , Edited by: Goodwin , T.W. Vol. 2 , 38 – 165 . New York : Academic Press .