Abstract
The intrinsic viscosity and flow behaviour of aqueous solutions of different concentrations of Arabic gum were studied. The molecular weight of this polymer was calculated using the value of the intrinsic viscosity, employing both Huggins and Kramer and Mark-Houwink equations. The value of the apparent viscosity and the influence of the shear rate on the rheological behaviour of different polymer concentrations in aqueous solution were determined. The effect of temperature over the flow behaviour was also studied. The Ostwald model has been employed to fit the experimental data and model parameters have been obtained.
INTRODUCTION
Non-Newtonian polymers are present in industries such as food, textile, pharmaceutical or cosmetics ones,[Citation1, Citation2] due to their important properties in terms of gelling and shear-thinning. For example, polymers are used as additives to stabilize some industrial products or to contribute their texture. Industrially manufactured products contain additives to optimize the flow behaviour. This is also valid for the food industry, where the selection of the best additive must be made employing rheological parameters as a criterion. The consistency index (k) and flow index (n) are typical rheological parameters used in the power law model. Different authors[Citation3] have concluded that index k is a strong function of the solution and the temperature concentration, whereas n does not have a strong dependence on the concentration and temperature of the polymeric solution.
In this work we have studied the change in the behaviour of some aqueous solutions when different quantities of polymers were added. The polymer employed has been Arabic gum, which is widespread used in the soft drinks industry for the stabilization of dispersed flavour oils. The Arabic gum can enhance not only the viscosity of the aqueous medium like most of other polysaccharide gums, but it also forms a thick macromolecular layer around the emulsion droplets resulting in good steric stabilization.[Citation4] Arabic gum is also an important hydrocolloid because it is compatible with a great number of polymers and it is frequently employed to reduce the viscosity of different liquid phases.[Citation5] Due to the importance of this polymer as additive in different food formulations and other industrial processes, it is important to know about the flow behaviour of its aqueous solutions with the aim of predicting the influence and behaviour caused by the presence of this hydrocolloid in a more complex system. Calculations in the processes involving fluid flows, such as pump sizing, extraction or filtration, require our knowledge of rheological data.[Citation6]
The aim of this work was studying the influence of polymer concentration and temperature on the rheological behaviour of aqueous solutions of Arabic gum. The polymer was characterized regarding its average molecular weight by the use of intrinsic viscosity obtained from viscosimetric measurements. The influence of temperature must also be analysed since food products are subject to variations in their temperature during production, transport, storage, etc.[Citation7] To fit the influence of shear rate upon the shear stress and the apparent viscosity experimental data, the power law (Ostwald model) was used. This model was chosen for its simplicity and the good fit to the experimental data.
MATERIALS AND METHODS
Materials
Arabic gum was supplied by Fluka (CAS number 9000-01-5) and the aqueous solutions were prepared by mass using a balance with a precision of ±10−7 kg. For Arabic gum samples preparation, doubly distilled water was employed. The density of Arabic gum aqueous solutions was measured with an Anton Paar DSA 5000 vibrating tube densimeter, with an accuracy of ±10−6 g·cm−3. To carry out the rheological measurements, an Anton Paar DV-1P digital thermostated rotational viscosimeter with two coaxial cylinders was used. The Arabic gum aqueous solutions used were in the range of 0 to 400 g·L−1. In order to study the effect of temperature on rheology, the measurements were carried out at 10–50°C.
The average molecular weight determination implies the calculation of the intrinsic viscosity by means of Huggins and Kramer equations.[Citation8] The experimental set up to carry out these experiments was a capillary viscosimeter (Schott Gerate AVS 350), that was connected to a thermostated bath (Selecta Frigiterm) with a precision of ± 0.1°C. An electronic stopwatch with an accuracy of ± 0.01 s was used for measuring efflux times. The solutions were prepared in NaCl 0.1 M due to the fact that the Mark-Houwink constants[Citation9] were determined in literature using this solvent to prevent the polyelectrolyte expansion in solution during the parameter determination. The intrinsic viscosity was obtained by the combined application of Huggins and Kramer equations (Eqs. Equation1 Equation–2). A similar procedure was employed by different authors.[Citation10, Citation11]
EquationEquations 1 and Equation2 employ the specific (η sp ) and relative viscosity (η r ). Both variables are defined in EquationEqs. 3 and Equation4.
RESULTS AND DISCUSSION
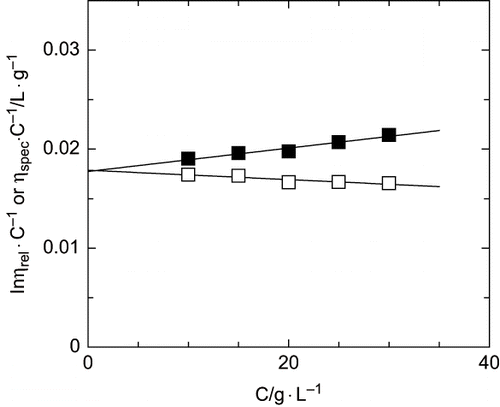
shows the values of kinematic viscosity and density corresponding to low concentration aqueous solutions of Arabic gum employed in intrinsic viscosity determination. shows the representation of Huggins and Kramer equations and allows the calculation of an intrinsic viscosity value of 1.8·10−2 L·g−1. This value is used to calculate the average molecular weight of this polymer, that results in a 845 kDa employing the equations previously shown in the Materials and Methods section. Similar results were obtained in previous studies with different samples of Arabic gum obtained from trees.[Citation9]
Table 1 Densities and kinematic viscosities for aqueous solutions of Arabic gum
Aqueous solutions of Arabic gum employed in the present work present a non-Newtonian flow behaviour due to the influence of the shear rate upon the apparent viscosity, shown in . An increase in the shear rate produces a decrease in the apparent viscosity value, with a higher decrease at low values of shear rate applied to the liquid phase. This behaviour indicates that the non-Newtonian behaviour is a pseudoplastic type. The same behaviour was shown for all the samples analysed. Few researchers have studied the rheological behaviour of different kinds of polymer solutions,[Citation12, Citation13] and these studies have concluded that this kind of aqueous solutions frequently show a non-Newtonian and pseudoplastic behaviour in almost all cases. In the present study, the solutions also exhibit a non-Newtonian flow behaviour. These results agree with previous studies that have used this kind of polymer.[Citation14] As regards the influence of temperature, the viscosity was expected to decrease when temperature increased, and this was the behaviour observed. shows an example of the experimental results obtained for apparent viscosity when varying both experimental variables (shear rate and temperature).
Figure 2 Effect of temperature and shear rate upon the apparent viscosity value. [AG] = 150 g·L−1. 10°C (○), 20°C (•), 30°C (□), 40°C (▪]), and 50°C (Δ).
![Figure 2 Effect of temperature and shear rate upon the apparent viscosity value. [AG] = 150 g·L−1. 10°C (○), 20°C (•), 30°C (□), 40°C (▪]), and 50°C (Δ).](/cms/asset/51c8858e-e09f-4fab-9edf-6b96aadad4d7/ljfp_a_259543_o_f0002g.gif)
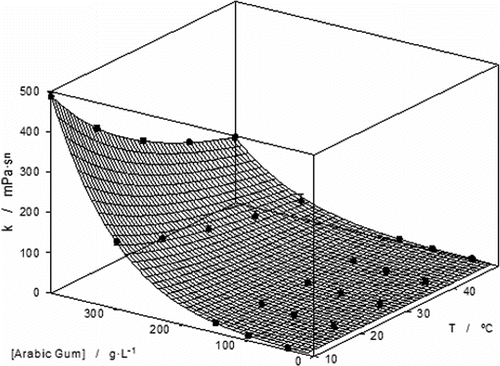
The experimental data for rheological characterization was fitted with the Ostwald's equation (Eq. 7). The rheological parameters have been calculated (see and ) using linearized equation 7. shows an example, where the Ostwlad model is pointed as suitable to fit the rheological behaviour of Arabic gum aqueous solutions under the experimental conditions employed in the present work.
Figure 3 Experimental data fit using Ostwald model. [AG] = 150 g·L−1: (○) T = 10°C; (•) T = 50°C; [AG] = 40 g·L−1: (□) T = 10°C.
![Figure 3 Experimental data fit using Ostwald model. [AG] = 150 g·L−1: (○) T = 10°C; (•) T = 50°C; [AG] = 40 g·L−1: (□) T = 10°C.](/cms/asset/2608c456-1ffb-43c4-bb81-06ae4b465e75/ljfp_a_259543_o_f0003g.gif)
Figure 4 Influence of temperature upon the consistence index. (○) 0 g·L−1; (•) 40 g·L−1; (□) 100 g·L−1; (▪) 150 g·L−1; (Δ) 300 g·L−1; (▴) 400 g·L−1.
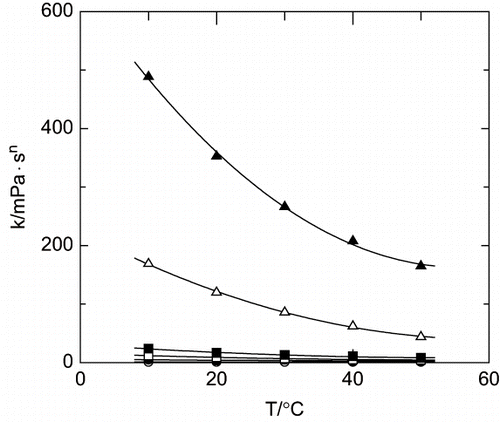
Table 2 Flow behaviour index corresponding to Ostwald model
Table 3 Consistency index corresponding to Ostwald model
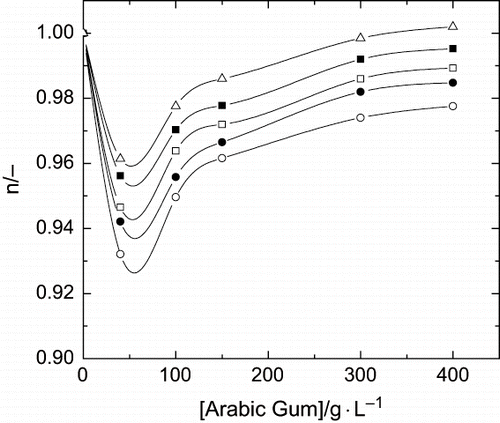
Another characteristic of the system formed by the aqueous solutions of Arabic gum in relation to the value of the flow index, is the existence of a minimum in the value of this parameter when the influence of polymer concentration was analysed (see ). When the hydrocolloid concentration increases in the liquid phase, the behaviour index decreases. This occurs with different polymers aqueous solutions, but it reaches a minimum and then, a higher increase in Arabic gum concentration produces an increase in the value of this rheological parameter up to values near to 1 (Newtonian behaviour). This behaviour is similar to the previous ones observed by our research studies and others, for systems formed by different polymers.[Citation11, Citation14] With regard to the values calculated for the flow behaviour index, a previous study has shown values close to 1,[Citation5] with a clear difference as regards other hydrocolloids aqueous solutions, that show very low values for behaviour index.[Citation15]
Figure 6 Influence of polymer concentration and temperature upon flow index. (○) T = 10°C; (•) T = 20°C; (□) T = 30°C; (▪) T = 40°C; (Δ) T = 50°C.
The effect of temperature on the rheological behaviour of the polymers solutions and power law parameters has also been studied. An increase in temperature produces an increase in Newtonian behaviour of the polymer aqueous solutions, with values of behaviour index near to the Newtonian behaviour (n = 1). A linear trend between behaviour index and temperature was found, and a liquid phase polymer concentration has no influence in the slope of the plot.
CONCLUSIONS
Arabic gum aqueous solutions showed a non-Newtonian and pseudoplastic behaviour. The polymer employed has been characterized in relation to intrinsic viscosity and average molecular weight. The power law model has been employed to describe this behaviour under shear in rotational concentric cylinder system. The rheological parameters have been determined and the influence of polymer concentration and temperature has been evaluated.
REFERENCES
- Sanford , P.A. and Baird , J. 1983 . “ Industrial utilization of polysaccharides ” . In The polysaccharides , Edited by: Aspinall , G.O. and Aspinall , G.O. New York, NY : Academic Press, Inc .
- Feddersen , R.L. and Thorp , S.N. 1993 . “ Starch-based gums ” . In Industrial Gums , Edited by: BeMiller , J. and Whistler , R. New York, NY : Academic Press .
- Wanchoo , R.K. , Sharma , S.K. and Bansal , R. 1996 . Rheological parameters of some water-soluble polymers . J. Polym. Mater. , 13 : 49 – 55 .
- İbanoğlu , E. 2002 . Rheological behaviour of whey protein stabilized emulsions in the presence of gum Arabic . J. Food Eng. , 52 : 273 – 277 .
- Ahmed , J. , Ramaswamy , H.S. and Ngadi , M.O. 2005 . Rheological characteristics of Arabic gum in combination with guar and xanthan gum using response surface methodology: Effect of temperature and concentration . Int. J. Food. Prop. , 8 : 179 – 192 .
- Bueno , S.M. and García-Cruz , C.H.. 2001 . The influence of fermentation time and the presence of salts in the rheology of the fermentation broth of a polysaccharide-producing bacteria free of soil . J. Food Eng. , 50 : 41 – 46 .
- Álvarez , E. , Cancela , M.A. and Maceiras , R. 2006 . Effect of temperature on rheological properties of different jams . Int. J. Food Prop. , 9 : 135 – 146 .
- Rao , M.A. 1999 . Rheology of fluids and semisolid foods: principles and applications , 3 – 15 . Gaithersburg, MD : Aspen .
- Idris , O.H.M. , Williams , P.A. and Phillips , G.O. 1998 . Characterization of gum from Acacia Senegal trees of different ages and location using multidetection gel permeation chromatography . Food Hydrocoll. , 12 : 379 – 388 .
- Chuah , H.H. , Lin-Vien , D. and Soni , U. 2001 . Poly(trimethylene terephthalate) molecular weight and Marl-Houwink equation . Polymer , 42 : 7137 – 7139 .
- Gómez-Díaz , D. and Navaza , J.M. 2003 . Rheology of aqueous solutions of food additives. Effect of concentration, temperature and blending . J. Food Eng. , 56 : 387 – 392 .
- Lindberg , J.J. , Sirviö , H. and Martinmaa , J. 1987 . Rheological studies on CMC . Cellulose Chem. Technol. , 21 : 379 – 385 .
- King , K. 1994 . Changes in the functional properties and molecular weight of sodium alginate following γ irradiation . Food Hydrocoll. , 8 : 83 – 96 .
- Mothé , C.G. and Rao , M.A. 1999 . Rheological behavior of aqueous dispersions of cashew gum and gum arabic: effect of concentration and blending . Food Hydrocoll. , 13 : 501 – 506 .
- Gómez-Díaz , D. , Navaza , J.M. and Quintáns-Riveiro , L.C. 2006 . Influence of concentration and temperature upon rheology of κ-carrageenan aqueous solutions . EJEAFChE. , 5 : 1213 – 1220 .