Abstract
Water diffusivity, vitamin C degradation, and color change were assessed in two batches of cactus pear that were dried; one by regular air-drying; and the other one by applying partial osmodehydration followed by air-drying. The drying was done with a convection oven at 40, 50, 60, and 70°C. The pretreatment was performed by immersing the samples for 3 hours in a 40°Brix sucrose solution at 40°C. An analysis of variance (ANOVA) with a confidence level of 95% (p < 0.05) revealed that water diffusivity was mainly affected by the temperature and the pretreatment. Both the vitamin C loss and the color change behaved as a first-order reaction; the set temperature used was found to be the most important factor in the process.
INTRODUCTION
Conventional air-drying is one of the most widely used techniques in food dehydration. This method improves the stability and extends the shelf life of the product.[Citation1] Dehydration also brings about substantial reduction in weight and volume; this reduces packing, storage, and transportation costs. However, the removal of water may considerably reduce the quality of these products. The most common quality defects of dehydrated foods are: slow or incomplete rehydration, loss of typical juiciness of fresh foods, as well as unfavorable changes in color and flavor. The drying time and the temperature applied are the most important factors in the process.[Citation2] The use of low temperatures gives the product better stability, but reduces its quality, since the exposure times have to be longer. Thus, the use of an osmotic treatment before conventional drying as a means of enhancing the texture, taste, nutrient retention and color stability of several products[Citation3,Citation4] has received considerable attention recently. Osmotic treatment consist in partially removing water from the product by immersing it in aqueous solutions of sugar, salt or spices.[Citation5–7] During this treatment, a significant amount of water is removed and simultaneously infusion of desirable solutes takes place.[Citation5–7] However, when the process is concluded, further drying such as convective or freeze-drying, is still necessary to reduce moisture to a minimum. This step improves the stability of the product.
Although Lahsasni et al.[Citation8] have recently proposed convective solar drying and, similarly, Moreno-Castillo et al.[Citation9] have suggested osmotic dehydration for that purpose, there is not published information concerning the use of an osmotic treatment followed by convective drying applied to cactus pear. Therefore, this work was undertaken to investigate this aspect further, and the main objective was to evaluate the effect of the temperature on the drying kinetics, ascorbic acid degradation and color change of fresh and osmotically pretreated cactus pear subjected to air-dehydration. Drying kinetics was described using a diffusional model adapted to slab geometry, whereas first-order kinetics was proposed to describe the rate of vitamin C loss and the rate of color change.
MATERIALS AND METHODS
Experiments with Fresh versus Pretreated Samples
Cactus pear fruits (variety Opuntia ficus indica) with an average weight between 100 and 110 g and similar ripening (considering total soluble solids, °Brix, and peel color) were purchased at a local market. Commercial sucrose was used in experiments. The fruits were carefully washed with water to get rid of the spines, then hand peeled and cut into cylindrical slices 5-mm thick and approximately 49 mm in diameter. Two similar batches were formed with the fruit in order to perform the two different experiments.
For the conventional drying process, an air flow was applied on the two circular faces of the slices. This was performed with a convection oven, using 4 different temperatures (40, 50, 60, and 70°C), and with an air velocity of 1 m/s. The drying process was monitored periodically weighing the samples with an electronic balance (± 0.0001 g), and finally stopped when the weight reached a constant value.
The pre-treatment of the other batch was done by placing the samples into a beaker filled with a 40° Brix sugar solution. Then, the beaker was immersed into a water-bath maintained at a constant temperature of 40°C. Three-hour immersion periods under static conditions and a product/solution ratio of 1/15 were used in these experiments. When the pre-treatment was complete the samples were subjected to the air-drying process described above.
A factorial experimental design was used, all tests were done in triplicate, and a total of 24 runs were carried out (). Samples were taken out of the drier at regular intervals to mesure ascorbic acid content and color change. The former was determined by direct iodine titration (0.1 N) of suitable dilutions of the product in sulphuric acid and starch solutions.[Citation10] In the case of color, the L*, a*, and b* parameters (Hunter values) were measured before and during the drying by means of a colorimeter (AccuProbe HH06TM, Accuracy Microsensors, Inc. Pittsford, New York).
Table 1 Values of the water diffusivity (D W ) and reaction rate constants (k 1 ) and (k 2 ) determined through regression method for each experimental condition
Moisture Diffusivity Calculation
The slices were considered as infinite slabs and the analytical solution of Fick's second law for unsteady state diffusion was fitted to the drying curves by a non-linear regression procedure, taking the first 10 terms of the series[Citation11]:
where: X(t) and Xo represent, respectively, moisture content (g water/g dry matter) at time tand at initial time; Dw is water effective diffusivity (m2/s); Lrepresents the half thickness of the slice (m); and tis the process time (s). In order to apply EquationEq. (1), it was assumed that a) the mass transfer takes place only in one direction; b) the initial moisture content of the product is uniform; c) the slice surface has already reached moisture equilibrium; d) Dw is constant; e) slice shrinkage is negligible; and f) the values of the equilibrium moisture content are relatively small compared to X(t) or Xo .
Kinetics of Ascorbic Acid Degradation
To describe nutrient degradation in thermally processed foods, as well as during food storage periods, a first-order kinetic has been generally used[Citation12]:
where Cis the instantaneous nutrient concentration (mg/100g sample); k 1is the temperature dependent degradation rate constant (min−1); and the exponent mis the reaction order. For isothermal conditions and in the case of a first-order reaction (m = 1), EquationEq. (2)can be analytically integrated to obtain a degradation function:
Co is the initial ascorbic acid concentration and tis the drying time. EquationEq. (3)has been used to assess vitamin C degradation in several processes, such as the storage of orange juice[Citation13] and dehydrated guava,[Citation14] as well as the dehydration of pineapple.[Citation15]
Kinetics of Color Change
The overall color change (ΔE) was measured with Hunter's values L*, a*, and b* using EquationEq. (4):
where L o *, a o *, and b o * denote the color parameters before the drying process; and L*, a*, and b* correspond to the ones obtained afterwards. The experimental data were fitted using a first-order kinetics model[Citation16] to estimate color change as a function of time, as follows:
where ΔE maxis the largest color change reached in the experimental condition; and k 2is a first-order constant (min−1) representing the rate of the food's color change.
Statistical Analyses
The experimental data were fitted using a polynomial model to predict the values of the dependent variable Y(DW, k1and k2 ):
where β i (i= 0, 1 … 5) represents the linear regression coefficients of the model; and T and Tr, are the coded values of the independent variables (temperature, pretreatment). The analysis of variance (ANOVA) was performed with a confidence level of 95% (p < 0.05) using the software Modde 7.0.
RESULTS AND DISCUSSIONS
Drying Rate
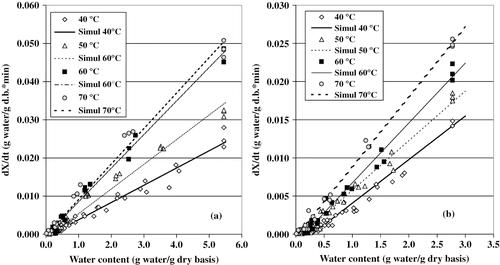
The moisture content versus drying rate are shown in (F samples) and 1b (PT samples), respectively. The drying process took place in the falling rate period, since the drying rate dropped steadily as the moisture content decreased. A similar phenomenon was observed in the dehydration of papaya,[Citation3] pear,[Citation4] and bell pepper[Citation17]; it has been attributed to the colloidal and hydrophilic characteristics of biological materials. In this case, the water molecules are strongly bound and the presence of free-water is insignificant.[Citation17] Actually, diffusivity is the physical mechanism governing the moisture transfer inside foods during the falling rate period. It is a complex mechanism that involves movement of water, in both liquid and vapor phase, and it is often characterized by a coefficient named effective diffusivity (DW ). (a, b) shows that, in general, higher temperatures brought about increments in the drying rate, and that this varied linearly with the moisture content.
Drying Kinetics
(F samples) and 2b (PT samples) presents the variation of the moisture content as a function of time. At the beginning of the process, the moisture content quickly dropped, but this variation slowed down as the drying progressed. The temperature clearly affected the water loss rate, since higher temperatures noticeably reduced the corresponding drying times. The shortest drying times occurred at 60 and 70°C.
Figure 2Effect of the temperature on the drying curves of cactus pear. (a) Fresh samples; and (b) pretreated samples.
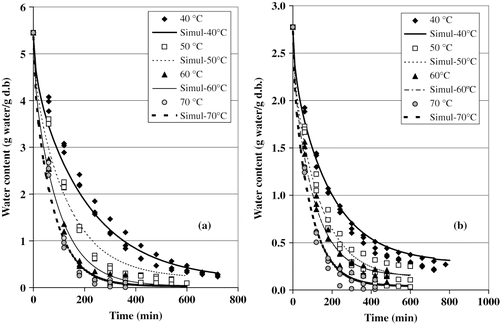
The fresh samples registered an initial moisture content of 5.45 ± 0.39 g of water/g of dry matter, while the pretreated samples had 2.78 ± 0.19 g of water/g of dry matter, which corresponds to 84.5% and 73.5%, respectively; this means that the pretreatment caused an humidity decrease of about 11%.
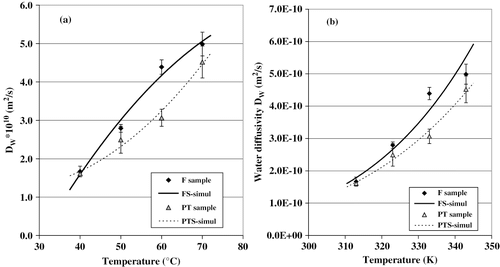
The simulated curves obtained with Fick's equation [EquationEq. (1)] are also shown in . When calculating the values for water diffusivity (Dw ), the corresponding values for the determination coefficients (r2) were all greater than 0.98; they are listed on . The water effective diffusivity varied from 1.51 × 10−10to 5.32 × 10−10m2/s, within the range of temperature (40–70°C) covered in this study. This range of variation is similar to that reported for the dehydration of bell pepper[Citation17]; it is also similar to values reported by Maroulis et al. [Citation18] in their compilation about water diffusivity in biological products.
To evaluate the effects of the experimental conditions variations on the DW -values, a multiple linear regression analysis was performed using EquationEq. (6)and a confidence level of 95%. The regression coefficients of the model are shown in . DW was influenced by the temperature (T), the pretreatment (Tr), and by the T* Trand T 2* Trinteractions. also shows that, according to the lowest p(t)values, the temperature and the pre-treatment were the most important process variables affecting the water diffusivity. The following equations are the transfer mass models used to describe water diffusivity during prickly pear drying (both with R2 = 0.958):
Table 2 Regression coefficients of the polynomial model [EquationEq. (6)] to evaluate the effect of temperature and pretreatment on the D W , k 1 , and k 2 values (p < 0.05). The bold character indicates that the corresponding parameter had a significant effect on the properties evaluated
The graphs in present water diffusivity as a function of temperature. It is clear that Dw increased as the temperature increased; the values recorded for water diffusivity at 70°C were about 2.5 times greater than the ones obtained at 40°C. also shows that the Dw values of the PT samples were slightly smaller than those of the FS samples; a layer of sucrose might have formed on the surface of the pretreated slices, and this in turn might have reduced the amount of water given off by the fruit.
Figure 3Water diffusivity versus air-drying temperature. (a) Polynomial model; and (b) Arrhenius' model.
The relationship between diffusivity and temperature has also been represented with Arrhenius' model by many authors[Citation17,Citation19,Citation20]:
where Do is the pre-exponential factor (m2/s); Eais the activation energy (kJ/mol); Ris the ideal gas constant (kJ/mol K); and T is the absolute temperature of the air (K). Applying EquationEq. (9)to the dehydration of cactus pear, the variation of DW as a function of the temperature would be:
EquationEqs. (10)and Equation(11)allow to determine the activation energy, which was 33.56 kJ/mol for the fresh samples, and 29.45 kJ/mol for the pretreated samples. This was due to initial difference in moisture content (84.5% and 73.5%), which was discussed above. These activation energy values are consistent with the ones obtained for the dehydration of olive cake, [Citation20] and dehydration of yerba mate leaves,[Citation21] but they are about 2 times smaller than the values reported for the dehydration of bell pepper.[Citation17]
By comparing the graphs in , it becomes clear that in both cases, DW varied as a function of the temperature in the same way. This indicates that the mathematical models proposed in this paper (EquationEq. 7and Equation8)are as precise as the Arrhenius model. These graphs also show how the Dw values (predicted and experimental) were influenced by the temperature and pre-treatment.
Ascorbic Acid Degradation
Ascorbic acid is an essential ingredient in people's daily diet, and it is found in a wide variety of foods, mainly in fruits and vegetables. Ascorbic acid is also a natural antioxidant used in foodstuff formulations in order to prevent browning, discoloring and also to enhance shelf-life.[Citation22] It is know to be thermolabile and its degradation depends on several factors such as temperature, water content, pH, storage time and metal ions.[Citation15] It has been indicated by several authors[Citation14] that one of the possible indicators for the effectiveness of the process is ascorbic acid degradation. It is generally observed that, if vitamin C is well retained, the other nutrients are also well retained.[Citation14] Thus, the present work pays especial attention to the degradation undergone by the vitamin C during the processes studied.
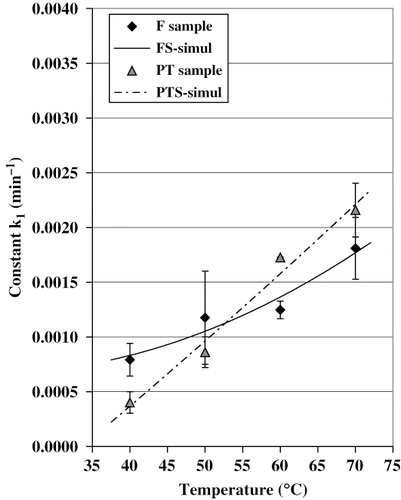
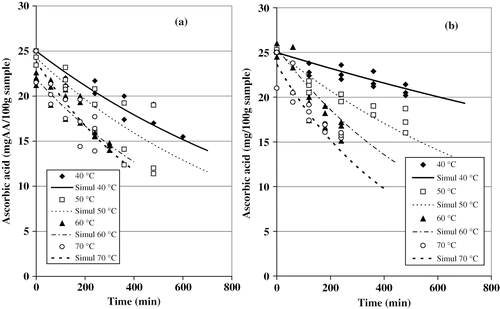
The curves characterizing ascorbic acid loss, for fresh and pretreated samples, can be seen in and b, respectively. The initial ascorbic acid content of the fruits used in the experiences varied between 21 and 26 mg/100 g sample (). also shows the agreement between experimental data and predicted values using EquationEq. (3). The values for k 1and r2obtained are also given in . The proposed model fitted the experimental data with r2values above 0.80. This confirms that the ascorbic acid degradation is a first order reaction. It can be seen that according to reaction rate constant (k 1), vitamin C loss increased as the temperature was raised. Indeed, this nutrient is highly sensitive to heat. Actually, the retention of vitamin C was found to be maximal when the dry temperature was fixed between 40 and 50°C, while a greater degradation of the nutrient was observed during the drying at 70°C. Similar results were reported by Ramallo and Mascheroni[Citation15] in the case of the pineapple dehydration.
Figure 4Effect of the temperature on the ascorbic acid loss in cactus pear. (a) Fresh samples; and (b) pretreated samples.
The coefficients of the polynomial model [EquationEq. (6)] corresponding to the statistical analysis are also shown in . From these results, it is possible to notice that k 1was affected mainly by the temperature (T) and by the T* Trinteraction. The pretreatment (Tr) did not significantly affected the degradation constant (k 1).
Experimental and predicted rate constants [with Eqs. (12, Equation13); R2 = 0.875) for both, fresh and pretreated samples, were plotted versus the drying temperature in .
It can be noted that the magnitude of k 1drastically increased as the temperature was raised. EquationEq. (12)applied to the F samples permits to estimate a degradation level about 2 times grater at 70°C than that obtained at 40°C. Furthermore, this difference became much important in the case of the PT samples: the values of k 1, obtained with EquationEq. (13), were at least 6 times larger for 70°C than those corresponding to 40°C. The pretreatment, evidently, was not favorable to the retention of the vitamin at high drying temperature (60–70°C). However, the lowest k 1-values, corresponding to the best vitamin C retention, were obtained in pretreated samples dried at 40 and 50 °C.
The activation energy necessary for the vitamin C degradation was calculated using EquationEq. (14)and Equation(15):
and the values obtained were 22.57 kJ/mol (F samples) and 51.51 kJ/mol (PT samples). The difference in activation energy is probably due to the sucrose coat formed on the surface of the pretreated slices. This range of variation in activation energy is similar to that reported for high pressurized and thermally pasteurized orange juice[Citation13] and during storage study of citrus juice concentrates.[Citation23]
Color Change
The change in color during thermal processing of foods may occur by any one or more of the following mechanisms: pigments degradation, ascorbic acid oxidation, browning, non-enzymatic reaction, the Maillard reaction, or combinations of the above mechanisms.[Citation24]
The color of natural cactus pear can be described as green-clear. The Lo *, ao *, bo * values were 60.43 ± 2.76, −4.73 ± 1.23, and 19.67 ± 2.63, respectively. In this study, it was observed that all the three parameters were found to determine changes. As the drying progressed, L* decreased, while a* and b* increased (results are not shown). This behavior was also observed by Mayor and Sereno[Citation25] during osmotic dehydration of pumpkin. The mechanisms of these changes are not clear, they can be due to enzymatic and non-enzimatic browning reactions. The decrease in L*and increase in *aand b*values was attributed to occurrence of Maillard reactions during drying of banana.[Citation26] The decrease of L* ties with the browning of foods as it has been reported by several authors.[Citation27] Concerning the increase in a*and b*; this caused the samples to become more yellow-red.
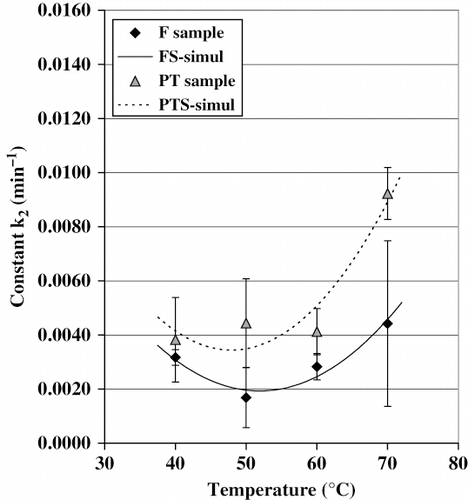
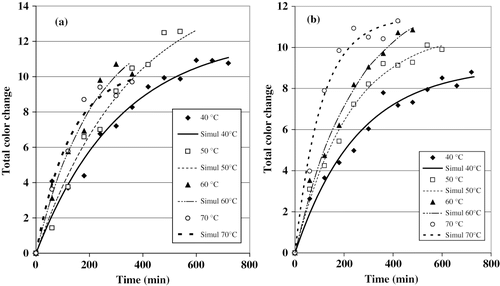
Preliminary results showed that L*, a*, and b* values can be used to monitor the color change in cactus pear, the graphs describing the overall color change are shown in (F samples) and 6b (PT samples). Only the mean values were plotted. The simulated curves obtained with a first-order kinetic model represented by EquationEq. (5)are also given in . The values of the constants k 2and ΔEmax, as well as those obtained for r2 through the experimental data adjustment, are shown in . A statistical analysis based on the total color change (ΔE) was carried out. The procedure and the model [EquationEq. (6)] were the same of those used for the Dw and k 1analysis. In , it can be observed that there was a significant linear and quadratic effect of the temperature on the values of k 2. Minima values of ΔE are observed at 40°C, while the maxima ones occurred at 70°C.
Figure 6Effect of the temperature on the total color change of cactus pear. (a) Fresh samples; (b) pretreated samples.
To predict the k 2variation, the following models were used (both with R2 = 0.709):
The behavior predicted by models (EquationEqs. 16, Equation17)in shown that the magnitude of constant color degradation should increase proportionally to the drying temperature. The pretreatment did not influence k 2 in a significant way, even though the values of this constant tended to be greater in the case of the PT samples. This difference seems to be a result of solids uptake by the samples during the pretreatment.
The activation energy needed to color change in the cactus pear was calculated using EquationEq. (18)and Equation(19); the values obtained were 12.93 kJ/mol for the F samples, and 22.57 kJ/mol for the PT samples. These values are very close to those found in the assessment of the color change in peach and plums subjected to thermal treatment over a 56–80°C temperature range.[Citation16]
CONCLUSIONS
The drying kinetics was efficiently described by Fick's second law of diffusion. During the dehydration, only the falling rate period was present. This confirmed that diffusivity controls the movement of moisture into the cactus pear. The water effective diffusivity (Dw ) varied from 1.51 × 10−10to 5.32 × 10−10m2/s as a function of the temperature range (40–70°C); both the polynomial model and the Arrhenius model satisfactorily described this variation. The vitamin C loss and the color change, at all four drying temperatures, behaved as a first-order reaction, and the degradation constants k1 and k2 were affected mainly by the temperature. According to the constant k1 , the best vitamin C retention was obtained in the pretreated samples dried at 40 and 50°C, while k2 indicated that the osmotic pretreatment brings about the greater color change in the samples during drying.
ACKNOWLEDGMENTS
The authors wish to acknowledge the financial support of the Fundación Produce San Luis Potosí, A.C. (Project Research 2004), and we would also like to thank O. Beltrán-Guevara for the revision of the English version.
REFERENCES
- Krokida , M.K. , Maroulis , Z.B. and Saravacos , G.D. 2001 . The effect of the method of drying on the colour of dehydrated products . International Journal of Food Science and Technology , 36 : 53 – 59 .
- Ho , J.C. , Chou , S.K. , Chua , K.J. , Mujumdar , A.S. and Hawlader , M.N.A. 2002 . Analytical study of cyclic temperature drying: effect on drying kinetics and product quality . Journal of Food Engineering , 51 : 65 – 75 .
- El-Aouar , A.A. , Azoubel , P.M. and Murr , F.E.X. 2003 . Drying kinetics of fresh and osmotically pre-treated papaya (Carica papaya L.) . Journal of Food Engineering , 59 ( 1 ) : 85 – 91 .
- Park , K.J. , Bin , A. and Reis-Brod , F.P. 2002 . Drying of pear d'Anjou with and without osmotic dehydration . Journal of Food Engineering , 56 : 97 – 103 .
- Mendoza , R. and Schmalko , M.E. 2002 . Diffusion coefficients of water and sucrose in osmotic dehydration of papaya . International Journal of Food Properties , 5 ( 3 ) : 537 – 546 .
- Sablani , S.S. and Rahman , M.S. 2003 . Effect of syrup concentration, temperature and sample geometry on equilibrium distribution coefficients during osmotic dehydration of mango . Food Research International , 36 : 65 – 71 .
- Shi , J. and Le Maguer , M. 2002 . Osmotic dehydration of foods: mass transfer and modeling aspects . Food Reviews International , 18 ( 4 ) : 305 – 335 .
- Lahsasni , S. , Kouhila , M. , Mahrouz , M. and Jaouhari , J.T. 2004 . Drying kinetics of prickly pear fruit (Opuntia ficus indica) . Journal of Food Engineering , 61 : 173 – 179 .
- Moreno-Castillo , E.J. , Abud-Archila , M. , González-García , R. , Grajales-Lagunes , A. and Ruiz-Cabrera , M.A. 2005 . Water transfer and color retention analysis in cactus pear fruits (Opuntia ficus indica) subjected to osmotic dehydration . International Journal of Food Properties , 8 : 323 – 336 .
- Secretaría , Salud de . 2004 . Farmacopea de los Estados Unidos Mexicanos, 8th . Edited by the Secretaria de Salud; Publicaciones e impresiones de Calidad S.A. de C.V., México, México , Vol. 1 : 874 – 875 .
- Pedreschi , F. , Hernández , P. and Figueroa , C. 2005 . Modeling water loss during frying of potato slices . International Journal of Food Properties , 8 ( 2 ) : 289 – 299 .
- Corradini , M.G. and Peleg , M. 2006 . Predictions of vitamin loss during non-isothermal heat process and storage with non-linear kinetic models. Trends in Food Science . Technology , 17 : 24 – 34 .
- Polydera , A.C. , Stoforos , N.G. and Taoukis , P.S. 2005 . Quality degradation kinetics of pasteurised and high pressure processed fresh navel orange juice: Nutritional parameters and shelf life . Innovative Food Science & Emerging Technologies , 6 : 1 – 9 .
- Uddin , M.S. , Hawlader , M.N.A. , Ding , L. and Mujumdar , A.S. 2002 . Degradation of ascorbic acid in dried guava during storage . Journal of Food Engineering , 51 : 21 – 26 .
- Ramallo , L. A. and Mascheroni , R. H. 2004 . Prediction and determination of acid ascorbic content during pineapple drying . Drying 2004–Proceedings of the 14thInternational Drying Symposium (IDS2004) . August 22–25 2004 , Sao Paulo, Brazil. Vol. C , pp. 1984 – 1991 .
- Lozano , J.E. and Ibarz , A. 1997 . Colour changes in concentrated fruit pulp during heating at high temperatures . Journal of Food Engineering , 31 : 365 – 373 .
- Kaymak-Ertekin , F. 2002 . Drying and rehydrating kinetics of green and red peppers . Journal of Food Science , 64 ( 1 ) : 168 – 174 .
- Maroulis , Z.B. , Saravacos , G.D. , Panagiotou , N.M. and Krokida , M.K. 2001 . Moisture diffusivity data compilation for foodstuffs: effect of material moisture content and temperature . International Journal of Food Properties , 4 ( 2 ) : 225 – 237 .
- Madamba , S.P. , Driscoll , R.H. and Buckle , K.A. 1996 . The thin-layer drying characteristics of garlic slices . Journal of Food Engineering , 29 : 75 – 97 .
- Akgun , N.A. and Doymaz , I. 2005 . Modelling of olive cake thin-layer drying process . Journal of Food Engineering , 68 ( 4 ) : 455 – 461 .
- Ramallo , L.A. , Pokolenko , J.J. , Balmaceda , G.Z. and Schmalko , M.E. 2001 . Moisture diffusivity, shrinkage, and apparent density variation during drying of leaves at high temperatures . International Journal of Food Properties , 4 ( 1 ) : 163 – 170 .
- Castro , I. , Teixera , J.A. , Salengke , S. , Sastry , S.K. and Vicente , A.A. 2004 . Ohmic heating of strawberry products: electrical conductivity measurements and ascorbic acid degradation kinetics . Innovative Food Science and Emerging Technologies , 5 : 27 – 36 .
- Burdurlu , H.S. , Koca , N. and Karadeniz , F. 2006 . Degradation of vitamin C in citrus juice concentrates during storage . Journal of Food Engineering , 74 ( 2 ) : 211 – 216 .
- Barreiro , J.A. , Milano , M. and Sandoval , A.J. 1997 . Kinetics of colour change of double concentrated tomato paste during thermal treatment . Journal of Food Engineering , 33 : 359 – 371 .
- Mayor , L. and Sereno , A.M. 2002 . Kinetics of color changes during continuous osmotic of pumpkin . Drying 2002–Proceedings of the 13thInternational Drying Symposium (IDS2002) . August 27–30 2002 , Beijing, China. Vol. B , pp. 978 – 985 .
- Chua , K.J. , Mujumdar , A.S. , Hawlader , M.N.A. , Chou , S.K. and Ho , J.C. 2001 . Batch drying of banana pieces-effect of stepwise change in drying air temperatures on drying kinetics and product colour . Food Research International , 34 : 721 – 731 .
- Koyuncu , T. , Tosun , I. and Ustun , N.S. 2003 . Drying kinetics and color retention of dehydrated rosehips . Drying Technology , 21 ( 7 ) : 1369 – 1381 .