Abstract
In this research, two kinds of filling methods (block and pieced) in two container types (pot and plastic) were used for the preparation of Otlu cheeses. The uses of different containers influenced (P < 0.01) acidity, pH, dry matter, fat contents and water soluble nitrogen. Degradation of α s1- and β-casein were higher in pieced cheeses in pot (P1F2). Also, breakdown products of casein were higher in P1F2 cheeses compared to others. The amounts of α s1- and β-caseins of all treatments decreased during ripening, while the amounts of α s1-I casein and breakdown products increased. The acceptability of cheeses in block form was higher.
INTRODUCTION
In Turkey, although there are many traditional cheese varieties, their production is largely based on small-scale dairies and family farms. Recently, the industrial production of Otlu (herby) cheese (cheese added different herbs) has been introduced.[Citation1] Otlu cheese, a semi-hard, salty and herb added, is manufactured in small family businesses for their needs and commercial purposes from raw sheep's and cow's milk between May and June in the Eastern and South-Eastern of Turkey. For produce of Herby cheese milk, is filtered through a coot-cloth and is heated up to 30–35°C. Then it is coagulated by using rennet. After coagulated, curd is cut into small cubes. The herbs were added on the curd weight. The herbs are commonly used in cheese as follows; Allium sp., Chaerophyllum macropodum, Antriscus nemorosa, Silene vulgaris, Ferula sp., Prangos sp., Tymus sp., and Mentha sp.,[Citation1,Citation2,Citation3,Citation4,Citation5,Citation6] they are added to cheese at different ratios. These plants give the cheese its flavor, as well as aiding in the preservation of cheese. The curd are drained and pressed for 3–5 hours. Then cheeses are cut into different widths. Cheese forms are salted with dry salt or brine. Cheeses are filled in pot or plastic container by mixed different ratios cacik (yoghurt curd) or lor (whey curd). The containers are closed after covering the surface with salt and placed upside-down 30–80 cm underground ripening for 3–8 months. Then the salty-surface layer removed and finally the cheese is stored at room temperature throughout consumption. There is no scientific reason for burying herby cheese in soil rather than in pots and plastic containers, but it is believed that ripening cheese under soil is easier, cheaper, and produces better flavor and body. Since the production method of Herby cheese is not standardized, the quality of cheese available in the market varies a lot.
Proteolysis of cheese in general is influenced by several factors including chymosin, pH, and moisture levels in curds, store temperature, time, and salt content. Proteolysis is probably the most important biochemical event, having a major impact on flavor and texture of most cheese varieties. Proteolysis contributes to the softening of cheese texture during ripening due to hydrolysis of the casein matrix of the cure and through a decrease in the water activity of the cure due to changes in water binding by the new carboxylic acid and amino groups formed on hydrolysis.[Citation7] The proteinases from starter and nonstarter microorganisms can also be active in the degradation of cheese proteins and peptides. Lactic acid bacteria are weakly proteolytic, but possess a very comprehensive range of proteinases/peptidases capable of hydrolyzing casein-derived peptides to small peptides and amino acids.[Citation8] Proteolysis and lipolysis are two primary processes in cheese ripening with a variety of chemical, physical, and microbiological changes that take place usually under controlled environmental conditions. Coskun[Citation9] indicated that more proteolysis and lipolysis occurred in the cheese samples made from raw milk and herb than the cheese samples made from pasteurized and cultured milk. Increase of the herb ratio (Allium sp.) in Van herby cheese caused more proteolysis and lipolysis. It has been reported that Prangos sp.,[Citation5] Ferula sp.,[Citation4] and Chaerophyllum sp.[Citation1] herbs affect the WSN, TCA-SN/TN and PTA-SN/TN ratios of cheeses. Proteolysis in cheese involves a complex and dynamic series of events and, in order to better understand the development of proteolysis in cheese, it is necessary to investigate the nitrogen fractions formed during ripening. [Citation10]
Using the abovementioned traditional method, it is very difficult to produce a high quality product due to lack of standardization of most manufacturing steps, especially the ripening conditions. Since there is negligible study about the effect of packaging materials (pot and plastic) and filling methods (block and pieced) on Herby cheese ripens and the changes taking place during ripening, this research was aimed at standardizing a method for production of Herby cheese, to compare the packaging of materials and filling methods, and to examine chemical, biochemical, microbiological and sensorial changes during ripening.
MATERIALS AND METHODS
Cow's milk was supplied from Yuzuncu Yil University Agriculture Faculty dairy plant, its content is shown in the . Totally, 1000 L of milk was used for experimental cheese production in each trial, commercial animal rennet (strength 1: 12 000) was obtained from Pinar Company, Istanbul. Herbs known as “sirmo” (Allium sp.) in region, in pickled form were obtained from dairy products market in Van. Lactic culture (Lactococcus lactis subsp. lactis and Lactococcus lactis subsp. cremoris) in lyophilized form obtained from “Wiesby GmbH & Co.KG.” (Niebüll, Germany) was used as starter culture. Five kg pot container, production cooked soil and 5 kg plastic container, produced from polyethylene was purchased from Van market. Lor (whey curd) was obtained from Van dairy fabric, its content is shown in the .
Table 1 Contains milk, lor (whey curd) and fresh cheese uses in this study
Experimental Cheeses
The necessity quantities raw cow's milk was pasteurized at 65°C for 30 min, cooled to 32°C, and divided into three groups. Before being used in cheese production, lyophilized culture were subcultured in sterile reconstituted skim milk individually, and a mixture (1/1, v/v) of bacteria was applied in the cheese production at a rate of 10 g/L and incubated for 60 min. Milks were coagulated with rennet for 90 min. After coagulation, curds were cut into 8–10 mm cubes with a wire knife. The herb (sirmo, Allium sp.) was added to the all groups 1% milk weight. The herbs were thoroughly mixed into the curds. Curds transferred to a cheesecloth to drain; pressure (80–90 kg/m2 for 180 min.) was then applied. After the removal of the whey, the curd was portioned into blocks of 7 × 7 × 7 cm. Cheeses were salted with dry salt (the amount of dry salt used was 5 of cheese weight) and held 14 h and this cheese have content in . At the end of this time, cheese samples were packed in pot (soil cup, P1) and plastic (P2) containers. In this research, two filling methods were applied. In the first method (F1), one layer lor (30%), one layer block cheese (70%) was used until the containers completely full. In the second method (F2), cheese (70% partially pieced cheese) and lor (30%) were mixed and filled in to the containers and were marked P1F1, P1F2, P2F1, and P2F2 codes to cheeses. After that, cheese containers were stored in the soil to ripe the experiment cheese. The cheese samples were analyzed for some chemical, biochemical, microbiological and sensorial properties after 2, 90, 120 and 150 days of the ripening period. Herby cheese was made in triplicate and average compositions of fresh cheeses are given in the .
Physicochemical Analyses
The cheese samples were analyzed titrable acidity (%lactic acid) with titrable 0.1 N NaOH and pH with a pH meter (NEL®-890), for total nitrogen using micro Kjeldahl digestion and distillation units (VELP Scientifica, DK 20, Milano, Italy), salt content using Mohr method, ash content using a muffle furnace at 550°C for 24 h. Fat content of cheese was measured by the Gerber method; dry matter content was determined by weight difference using a drying oven (Nüve, Ankara). The ripening index (water soluble nitrogen, WSN/TN,%) was determined by the Kjeldahl method as described follows; 10 g cheese was homogenized with 50 mL distilled water and filtered. The water-soluble nitrogen (WSN) content of the extracted cheese was expressed as a percentage of total nitrogen (TN), which was described as a ripening index [Citation11].
Lipolysis Analysis
Lipolysis was done using the BDI method and measured as Acid Degree Value (ADV). For this test, 10 g of finely ground sample were placed in a lipolysis butyrometer. Twenty ml of BDI reagent (30 g triton X-100 70 g sodium tetra phosphate in 1 L distilled water) were added and the butyrometers were placed in a boiling water bath for 20 min to extract the fat. The mixture was centrifuged for 1 min and enough aqueous methanol was added to bring the fat into neck of butyrometer and centrifuged for 1 another min. Then, the fraction of liquid fat was transferred into a 50 ml glass and weighed. Five ml fat solvent (4:1 petroleum ether and n-propanol) were added to the flaks. This was titrated with 0.02 KOH and total free fatty were calculated.[Citation12]
Preparation of Cheese Proteins and Electrophoresis
The electrophoretical analysis of protein patterns was conducted with the method given by Creamer.[Citation13] Samples buffer (pH = 8.4) was prepared with EDTA 0.0925 g, Tris 1.08 g, boric acid 0.55 g, and urea 36.0 g made up to 100 mL. A cheese sample (0.5 g) was homogenized in 25 mL samples buffer, and then centrifuged at 3000xg for 30 min; 2 mL of central portion was transferred into a small tube and stored at -20°C until required. Casein standard was prepared using sodium caseinate obtained from cow milk by dissolving in urea; resolving gel buffer was prepared with Tris 9.2 g, Urea 54 g and solved in 100mL of distilled water, made up to 200mL (pH 8.8); 15mL of 30% acrylamide/bis-acrylamide (37.5:1) solution, 35 mL of separating gel buffer and 15 μL TEMED were used for resolving gel solution. After degassing, 70 μL of ammonium persulphate (APS) solution (0.1 mg/L) was added and immediately poured into gel apparatus. A volume of 0.5 mL of distilled water was placed on gel solution. After polymerization of the resolving gel, water was removed and the comb was inserted. Stacking gel solution was prepared with Tris 1.08 g, urea 36.0 g, boric acid 0.55 g, EDTA 0.092 g and 5 g of acrylamide/bis-acrylamide (37.5:1), and made up to 100 mL (pH = 8.4); 15 mL of this solution was taken, and 15 μL of TEMED was added. After degassing, 50 μL of APS was added. This solution was poured on the previous gel, and after polymerization, the comb was removed. Finally, the gel was placed in the electrophoresis unit (Hoefer Scientific Instrument SE-600). To the frozen cheese samples, 3% mercaptoethanol and 2 % bromophenol blue (0.1 %) were added; 40 μL of cheese samples were taken and placed into the slots. Stock chamber buffer was prepared with EDTA 3.7 g, Tris 43.2 g and boric acid 22 g and made up to 1L (pH = 8.4), and the buffer was diluted with distilled water (1:4) before use. The condition of electrophoresis were (10 ± 1°C), maximum 280 V, maximum 70 mA and 20 W. Protein bands were stained with Coomassie BBR-250 solution (1 g of Coomassie brilliant blue R-250, 500 mL of isopropanol and 200 mL of glacial acetic acid, and made up to 2 L). Then, bands were destained with destaining solution (200 mL of isopropanol and 200 mL acetic acid made up to 2 L). The gels were scanned with a PC scanner and pictures were transferred to the PC.
Microbiological Analyses
Total aerobic mesophilic bacteria (TAMB) were enumerated on plate count agar (Oxoid®) following the pour-plate method and incubated at 32oC for 48 h. Psychrotrophs were grown on plate count agar (Oxoid ®) incubated at 7 ± 1oC for 10 d. Yeast and mould were determined on potato dextrose agar (Oxoid®) acidified with 10 % lactic acid (Merck®) following plate method, with incubated at 24oC for 5 d. [Citation14,Citation15]
Sensorial Analysis
Organoleptic assessment of the cheeses after 90, 120, and 150 d of ripening was carried out by a ten-member panel of the University staff selected on the basis of interest and experience in sensory evaluation of herby cheeses. The samples were presented to panelists under fluorescence light to avoid visual bias. Prior to assessment, each cheese was cut into 10 g cubes, equilibrated to room temperature (20°C) after waiting for 4 h room temperature and presented in randomized. Overall sensory quality was assessed using a scaling method (1–10 points), where 1 reflected a very bad, and 10 reflected a very good score for appearance and color, body, and texture, flavor, and acceptability.[Citation16,Citation17] A set of three to four samples was given to each assessor, together with instructions on the evaluation procedure. Panel members were also instructed to report any defects in appearance (wet, dry, cracks), body and texture (hard, soft, pasty, crumbly, grainy) and flavour (rancid, salty, bitter, sour). Water was provided for mouth washing between evaluations of samples.
Statistical Analysis
Statistical analysis of data regarding the effects cheese types (block and pieced cheeses ripened in pot and plastic containers) and ripening times on some chemical, biochemical, microbiological and sensory properties were performed by ANOVA procedures using SAS® PROC GLM/STAT.[Citation18] The differences between means were evaluated by the Duncan's multiple range tests.
RESULTS AND DISCUSSION
Chemical Changes
Chemical changes belonging two filling methods in pot and plastic containers on Herby cheese samples during ripening are presented in . It can be seen from that pieced cheeses in plastics (P2F2) contained the highest mean titratable acidity, as block cheeses in pots have lower acidities (P < 0.01). Effects of filling methods on acidity of cheeses were shown differences in all times. These values are similar to those reported by Prieto et al. [Citation19] on Quesucos de Liébana cheese from cow milk, but higher than the results reported by Coskun and Tuncturk [Citation20] on effect of Allium sp. Van herby cheese. The titratable acidity value of fresh cheese samples was found as 0.77, and it reached the highest point at the end of the 90 days (as P1F1 samples was highest points cheeses in 120 days) after that it was started to decrease (P < 0.01). The pH values of cheeses were effected statistically (P < 0.01) merely at end time of ripening. The mean pH values significantly (P < 0.01) decreased until 90 days of ripening and then increased. Titratable acidity increases and pH decreases due to the production of organic acids (primarily lactic acid), which LAB responsible for most of the sugar fermentation. [Citation21] Increase in the pH and decrease in the titratable acidity after 60 days of ripening may be due to formation of alkaline nitrogenous compounds.[Citation21] The effect of packaging materials and filling methods on the dry matter contents of cheese samples was significant (P < 0.01). The dry matter contents of cheese in the pots were lower than cheese ripened on the plastic containers, as method pieced filling has higher dry matter contents statistically (P < 0.01) throughout ripening, except cheeses in 2 days. Dry matter content of cheese samples significantly (P < 0.01) increased during the ripening period. These differences might be due to water-holding capacity of herb, which is in agreement with the results of Coskun and Tuncturk,[Citation20] Ekici et al.[Citation3] Ripening time and packaging materials affected significantly fat content of cheese samples (P < 0.01). The average fat content of cheese samples in plastic containers was higher than that cheeses in pot containers, as filling methods had no significant differences (P > 0.01). The protein content of cheeses in of plastic containers was higher than that of cheeses in pot containers. Fat and protein contents of experimental cheeses increased continuously during the ripening period. Increases in dry matters of samples had effects positive to fat and protein contents. Effect of packaging materials and filling methods on salt content of cheeses had not significant differences for all during ripening (P > 0.01). Initial salt content (3.07%) increased rapidity until 90 days of ripening and these changes increases continued importantly (P < 0.01) in different rates throughout ripening. The rate of salt absorption was very high at the first month due to a movement of NaCl molecules because of the osmotic pressure and difference moisture contents of cheeses.[Citation22] Salting of cheese can influence the cheese pH due to its effect on microbial activity. Low levels of salt can stimulate bacterial activity; however, concentrations >2.5 % have a negative effect.[Citation23] Ash content in cheeses was not shown different statistically by the packaging materials and filling methods as salt content, but ash content increased importantly (P < 0.01) depended upon the increasing of salt and dry matter contents of cheese samples during the ripening period.
Table 2 Chemical changes of Otlu (herby) cheese pot and plastic containers during ripening
Biochemical Changes
Proteolysis in cheese involves a complex and dynamic series of events and, in order to better understand the development of proteolysis in cheese, it is necessary to investigate the nitrogen fractions formed during ripening.[Citation10] The WSN fraction contains whey proteins, proteose-peptone (soluble proteins, peptides, amino acids, amines, urea, ammonia), low molecular weight peptides (<15000 Dalton molecular mass) derived from casein hydrolysis.[Citation23] The soluble nitrogen compounds are mainly produced by the action of the coagulant.[Citation24] A comparison of the proteolysis data for the cheeses belonging to days of 2, 90, 120, and 150 of ripening were given in . The WSN values of the cheeses were affected significantly (P < 0.01) from the ripening period. The lowest ripening degree of cheese samples was found at 2 day (3.76) and the highest ripening degree was found at 150 day (17.78%). While the mean WSN ratio of pieced filling cheeses in pot containers was higher and differing significantly (P < 0.01) from the other cheeses in containers, were no significant differences between filling methods. These showed that the proteolytic activity of the cheese resulted in a high moisture content and TAMB organism's counts, compared to cheeses in plastics. This is in good agreement with the fact that higher moisture cheeses in pot containers that contribute to water soluble nitrogen result from the cumulative and combined action of rennet (mainly), microorganisms and the indigenous milk enzymes (mainly plasmin), on the caseins.[Citation5] Thus, the value is expected to increase with the ripening time. Similar results were already observed by Tarakci et al.[Citation1] for the effect of different ratios Chaerophyllum sp. herb on herby cheese, but higher than the results reported by Agboola And Radovanovic-Tesic[Citation25] for herby cheese and cheese added Australian Native Herbs and lower than the results reported Dervisoglu and Yazici[Citation26] for the Kulek cheeses of ripened in soil under, Ekici et al.[Citation3] for herby cheeses and Sengul et al.[Citation27] for civil cheeses. Differences in proteolysis parameters in cheeses might be due to differences of the milk used, herb addition, manufacturing procedure, and packaging materials, filling method, and ripening conditions. The environment conditions of these cheeses (low acidity, high moisture content) favors the activity of chymosin on αs1-casein resulting in the rapid production or water soluble peptides.[Citation28] These results show that Herby cheese does not undergo an excessive proteolysis at a period of five months.
Table 3 Ripening degree (WSN ratios) and lipolysis changes of herby cheeses during ripening
The triglycerides in all cheese varieties undergo hydrolysis through the action of indigenous, endogenous, and/or exogenous lipases, which results in the liberation of fatty acids in cheese during ripening. The triglycerides of ruminant milk fat are rich in short-chain fatty acids that, when liberated, have low flavour thresholds that contribute significantly to the flavour of many cheese varieties. Lipolytic agents in cheese generally originate from the milk, the coagulant.[Citation7] Lipolysis changes of herby cheeses ripened pot and plastic containers in 90, 120, and 150 days are presented . It was found that packaging materials and filling methods significantly not affected lipolysis values (P > 0.01). Even if all empty spaces in cheese vacuum-packets were attempted to be filled, some air might remain inside the cheese and cause the growth of moulds, resulting in lipolytic activity.[Citation1] Lipolysis degrees of all cheese samples were increased during ripening period. This irregular change may indicate that free fatty acids are converted to lower fatty acids and methyl ketones or some fatty acids are utilized to supply energy for mould growth.[Citation25,Citation26] Lipolytic agents in the cheese generally originate from the milk, the coagulant (in the case of rennet paste) and the cheese microflora; starter, nonstarter and adjunct microorganisms.[Citation7]
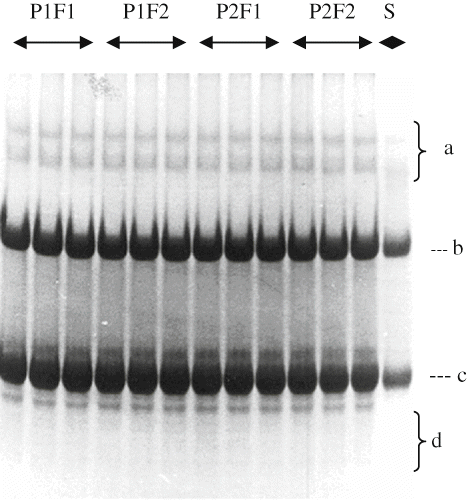
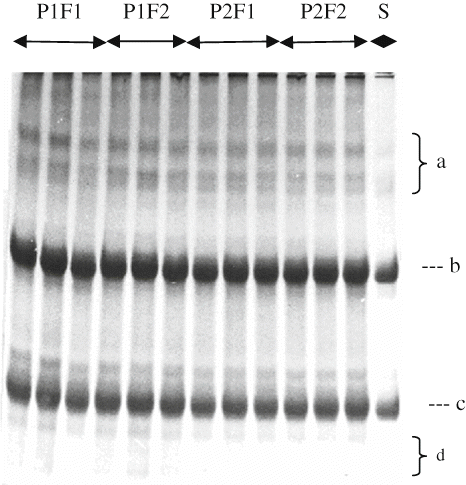
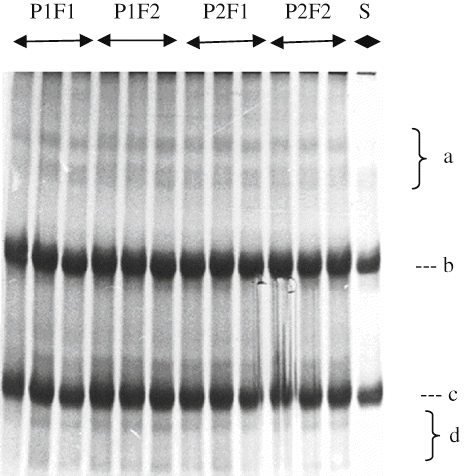
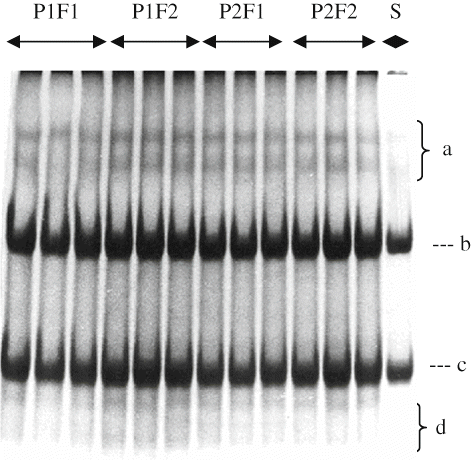
Electrophoretograms Changes
Primary proteolysis in cheese may be defined as an alteration in β-, γ-, αs-caseins, peptides other minor bands, which can be detected by poly-acrylamide gel electrophoresis (PAGE).[Citation29] show the electrophoretical effect of filling methods and packaging materials on casein fraction of αs1-, β-, γ-, and other degradation products of Herby cheeses during ripening. It was clearly seen from the electrophoretograms that β- and αs 1-casein degradation and the accumulation of γ-caseins and other degradation products increased with increasing ripening time. At the end of ripening, αs1-casein hydrolysis was not observed different in point of bands intensity between cheeses ripened in pot and plastic containers. αs1-casein was observed with a high intensity at the beginning of ripening, and then its intensities decreased during ripening because of proteolysis by milk plasmin, microbial enzymes, and the clotting enzyme used.[Citation30] These results are in good agreement with the findings of Irigoyen et al.,[Citation31] Poveda et al.,[Citation32] and Cagno et al.[Citation33] Different in α s1-casein degradation in the literature are possible due to differences of milk used, manufacturing procedure, and ripening.[Citation34,Citation35] As ripening progressed, hydrolysis of the caseins became clearer. With increasing ripening during, in all cheese samples were characterized by the extensive hydrolysis of αs1-casein. Proteolysis may be mainly attributed to chymosin activity in the model cheeses, which manufactured without cooking the curd[Citation33] since the activity of plasmin was weak at the lower pH degrees and proteinase activity of starter culture was insufficient. As known, chymosin activity is higher in cheese body at the lower pH.[Citation33] Another reason of higher degradation rate of αs1 -casein in cheeses may be enhanced activity of starter and non-starter bacteria depending on decrease of pH in these cheeses. Different findings about αs1-casein degradation rate in literature are possible due to differences of milk used, manufacturing procedure, and ripening conditions.[Citation34]
It has been established that β-casein is more resistant to proteolysis, especially in cheese matrix, either calf rennet or starter enzymes, owing to their structure and particularly, their tendency to associate. Plasmin is an alkaline milk protease plays a major role in proteolysis.[Citation36]Plasmin dissociates from casein micelles as the pH is decreased[Citation37] and its activity in cheese increases.[Citation34] This may have more plasmin activities and therefore more proteolysis. The principal proteolytic agents are the residual coagulant and enzymes from starters or the indigenous microflora. Extensive plasmin activity is not expected in this type of cheese due to its acidity and salt content, both of which are unfavorable for plasmin action.[Citation38] The β-casein showed lower breakdown degree than αs1-casein in all cheese samples. Breakdown of β-casein in cheeses was not observed intensely among packaging materials and filling methods at the end of the ripening period, as in the case of αs1-casein degradation of all cheeses. β-casein was degraded slowly during ripening as a result of its resistance to hydrolysis as in other brine-ripening cheeses.[Citation36,Citation37] The β-casein band intensities decreased continuously in the experimental cheeses during ripening, as seen in Figures. The progress of the β-casein degradation in this study is in agreement with the findings by Yazici and Dervisoglu[Citation39] and higher than those found in soft share cheese.[Citation40]
Some of γ-casein patterns can be observed from , however, their density is low and bands are not clearly separated. Since γ-caseins are degradation products of β-casein,[Citation41] increments in the γ-caseins during the ripening period are closely depend on the degradation of β-casein by plasmin. Casein breakdown products were seen more in the cheeses pieced filling in pot containers than other cheeses in 150 days of ripening. This trend was in good agreement with the indices of proteolysis reported previously (). This may be explained by the fact that growth of microorganisms was better in cheese packed (pot) produce from soil due to its higher permeability to air and moisture. In addition, pot cheese samples had higher ripening index (WSN ratios) and moisture than cheeses in plastic containers.
Protein breakdown products also contribute to the background savoury, nonspecific cheesy flavour of all cheeses, but an increased level of specific low molecular weight peptides in cheeses can result in flavour defects, such as bitterness. The fat fraction of cheese is important for the perception and development of cheese flavour. The fat in cheese can be degraded by lipolysis due to lipase activity from the somatic cells and microorganisms or oxidation. [Citation42]
Microbiological Changes
Changes in some microbiological properties of cheese samples are shown in . Total aerobic mesophilic bacteria (TAMB) counts were higher in block cheeses in pot than those of the other cheeses and these difference were significant (P < 0.01) for all days of ripening period, expect fresh cheeses. In addition to, filling methods were not effect TAMB counts. The TAMB counts had shown decreases continuously during ripening, these changes were significant (P < 0.01) through at ripening period and were agree with the results reported by Pérez-Elortondo et al.,[Citation43] Agboola and Radovanovic-Tesic,[Citation25] and Ekici et al.[Citation3] Yeast and mould levels in the samples were high and these count changed statistically (P < 0.01) from 7.28 to 6.57 log cfu/g. Yeast and moulds may grow in the higher acidity levels. Yeast and mould counts of the cheeses were not affected by packaging materials and filling methods. Similar changes are by Pérez-Elortondo et al.[Citation43] The proteolytic enzymes of yeast and mould may contribute to a small increase in WSN during storage. [Citation44] As can be seen in , the highest mean count for psychrotrophs were found in pieced cheeses in pot of 90 days and, while the lowest mean count was obtained with the block cheeses in plastic (P < 0.01). Psychographs in all samples decreased continuously during ripening, were ≤104 cfu/g at the end of ripening. These values are similar to those reported by Dervisoglu and Yazici[Citation26] for Kulek cheese, but lower than the results reported by Coskun[Citation9] Van herby cheese.
Table 4 Microbiological changes of herby cheeses in pot and plastic containers during ripening (log cfu/g)
Sensory Evaluation
Sensory analyses of cheese samples are shown in . All sensorial scores of cheese samples increased continuously during ripening (P < 0.01). The appearances and color of the experimental cheeses ripened in pot containers was considered good at both samples types, and did not significantly differ from that of the cheeses in plastics containers. The body and texture scores for the block cheeses were, however, no significantly (P > 0.01) higher than those of pieced cheeses. These results may be attributed to the higher level of proteolysis found in the block cheese, as shown by the higher levels of soluble nitrogen fractions (). Enhanced breakdown of the casein fractions has been associated with improved texture and smooth body. The block cheeses ripened containers had similar taste and flavor scores and greater than the other cheese all during ripening. The all cheeses in 150 days of ripening exhibited a slight bitterness, which may be partly explained by the high moisture content. The peptidolytic enzymes contributed by different cultures were probably responsible for the increasing of flavor of cheeses. These results could be attributed to the greater levels of soluble nitrogen fractions (water-soluble nitrogen) and lipolytic changes higher cheeses in end of ripening (). The saltiness scores during ripening times had a significant (P < 0.01) in all samples, but other scores were no different among packet containers and filling methods. The acceptability scores (averages quality) of block cheeses was greater than priced cheeses. Ripening time acceptability scores for herby cheeses were positive, these improvement were significant (P < 0.01).
Table 5 Sensorial properties of herby cheeses different times in pot and plastic
CONCLUSION
In this study, cheeses in plastic containers had higher titratable acidity, dry matter, fat, protein, salt, and ash contents than cheese ripened in pots. Using the pieced cheese method with pots increased higher than ripening degree (WSN ratios) those other cheeses with ripening. Breakdown of casein fraction was higher in pots, and this effected proteolysis of cheese samples. The cheese samples ripened in plastic containers had higher amount of dry matter, as a result, the acceptability was higher in these cheeses than the others. The block filling method affected significantly the protein content of cheese samples. Pieced filling method increased importantly titratable acidity of cheese samples. Using pieced method in pots increased ripening degree, and breakdown products of casein were clearly seen in the cheese samples. During the ripening period, dry matter, fat, salt, and ash contents were statistically increased. Titratable acidity increased until 90 days of ripening and then decreased. The ripening degree and lipolysis of cheese samples increased during storage time. The important changes were seen on caseins fractions. During ripening period, bacterial count decreased. The desirable flavour of cheese samples were formed end of the storage, and the acceptability scores of cheeses is raised.
ACKNOWLEDGMENT
The present study has been supported by a grant from the Yuzuncu Yil University Scientific Research Fund (Project No: 1996-ZF-049).
Notes
REFERENCES
- Tarakci , Z. , Sagun , E. and Durmaz , H. 2006 . The effect of mendi (Chaerophyllum sp.) on ripening of vacuum-packed Herby cheese . International Journal of Dairy Technology , 59 : 35 – 39 .
- Coskun , H. and Tuncturk , Y. 2000 . The effect of Allium sp. on the extension of lipolysis and proteolysis in Van Herby cheese during maturation . Nahrung , 44 : 52 – 55 .
- Ekici , K. , Coskun , H. , Tarakci , Z. , Ondul , E. and Sekeroglu , R. 2006 . The contribution of herbs to the accumulation of histamine in Otlu cheese . Journal of Food Biochemistry , 30 : 362 – 371 .
- Tarakci , Z. , Durmaz , H. and Sagun , E. 2005 . Siyabonun (Ferula sp.) Otlu peynirin olgunlasmasi uzerine etkisi, YYU Tarim Bilimleri Dergisi . 15 : 53 – 56 .
- Tarakci , Z. 2004 . The influence of helis (Prangos sp.) on characteristics of vacuum-packed Van herby cheese during ripening . Milchwissenschaft , 59 : 619 – 623 .
- Sagun , E. , Durmaz , H. , Tarakci , Z. and Sagdıc , O. 2006 . Antibacterial activities of the extracts of some herbs used in Turkish herby cheese against Listeria monocytogenes serovars . International Journal of Food Properties , 9 : 255 – 260 .
- McSweeney , P.L.H. 2004 . Biochemistry of cheese ripening . International Journal of Dairy Technology , 57 : 127 – 144 .
- Hayaloglu , A.A. , Guven , M. , Fox , P.F. , Hannon , J.A. and Mcsweeney , P.L.H. 2004 . Proteolysis in Turkish White-brined cheese made with defined strains of Lactococcus . International Dairy Journal , 14 : 599 – 610 .
- Coskun , H. 1998 . Microbiological and biochemical changes in Herby cheese during ripening . Nahrung , 42 : 309 – 313 .
- McSweeney , P.L.H. and Fox , P.F. 1993 . “ Cheese: methods of chemical analysis ” . In Cheese: Chemistry, Physics and Microbiology. General Aspects , Edited by: Fox , P. F. Vol. 1 , 341 – 388 . London : Chapman and Hall .
- Kurt , A. , Cakmakci , S. and Caglar , A. 1993 . “ Sut Ve Mamulleri Muayene ve Analiz Metotlari Rehberi ” . In Ataturk Univ. Ziraat Fak. Yay , Vol. 18 , 1 – 238 . Turkey : Erzurum .
- Case , R.A. , Bradley , R.L. and Williams , R.R. 1985 . “ Chemical and Physical Methods ” . In Standard Methods for the Examination of Dairy Products , 15th , Edited by: Richardson , G.H. 327 – 404 . Baltimore : American Public Health Association .
- Creamer , L.K. 1991 . Electrophoresis of Cheese. Bulletin of the IDF No:261/ . : 14 – 28 .
- Frank , J.F. , Hankin , L. , Koburgen , J.A. and Marth , E.H. 1985 . “ Tests for group of microorganisms ” . In Standard Methods for the Examination of Dairy Products , 15th , Edited by: Richardson , G.H. 189 – 201 . Washington, DC : American Public Health Association .
- Nunez , M. , Garcia , A.C. , Rodriguez , M.M.A. , Medina , M. and Gay , A. P. 1986 . The effect of ripening and cooking temperatures on proteolysis and lipolysis in Manchego cheese . Food Chemistry , 21 : 115 – 123 .
- Aston , J.W. , Giles , J.E. , Durward , I.G. and Dulley , J.R. 1985 . Effect of elevated ripening temperatures on proteolysis and flavour development in Cheddar cheese . Journal of Dairy Research , 52 : 565 – 572 .
- Tuncturk , Y. 1996 . “ Kasar peynirinin starter kultur, proteinaz ve lipaz enzimleri ilavesiyle hizli olgunlaştirilmasi uzerinde bir arastirma ” . In Doktora Tezi. Yuzuncu Yil Univ. Fen bilimleri Enstitüsü , 1 – 140 . Turkey : Van .
- SAS/STAT Software . 1998 . Changes and Enhancements through Release 6.12 , Cary, NC : SAS Institute Inc. .
- Prieto , B. , Urdiales , R. , Franco , I. , Fresno , J.M. and Carballo , J. 2000 . “Quesucos de Liébana” cheese from cow's milk: biochemical changes during ripening . Food Chemistry , 70 : 227 – 233 .
- Coskun , H. and Tuncturk , Y. 1998 . Van Herby cheese . Traditional Dairy Products, (Ed. M Demirci), Ankara MPM , 621 : 20 – 32 .
- Goncu , A. and Alpkent , Z. 2005 . Sensory and chemical properties of white pickled cheese produced using kefir, yoghurt or a commercial cheese culture as a starter . International Dairy Journal , 15 : 771 – 776 .
- Guinee , T.P. and Fox , P.F. 1993 . Cheese: Chemistry, Physics and Microbiology, General Aspects , Edited by: Fox , P.F . Vol. 1 , 257 – 302 . London : Chapman and Hall .
- Guven , M. , Yerlikaya , S. and Hayaloglu , A.A. 2006 . Influence of salt concentration on the characteristics of Beyaz cheese, a Turkish white-brined cheese . Lait , 86 : 73 – 81 .
- Roseiro , L.B. , Garcia-Risco , M. , Barbosa , M. , Ames , J.M. and Wilbey , R.A. 2003 . Evaluation of Serpa cheese proteolysis by nitrogen content and capillary zone electrophoresis . International Journal of Dairy Technology , 56 : 99 – 104 .
- Agboola , S.O. and Radovanovic-Tesic , M. 2002 . Influence of Australian native herbs on the maturation of vacuum-packed cheese . LWT-Food Science and Technology , 35 : 575 – 583 .
- Dervisoglu , M. and Yazici , F. 2001 . Ripening changes of Kulek cheese in wooden and plastic containers . Journal of Food Engineering , 48 : 243 – 249 .
- Sengul , M. , Gurses , M. , Dervisoglu , M. and Yazici , F. 2006 . A survey on the some chemical and biochemical properties of civil cheese, a traditional Turkish cheese . International Journal of Food properties , 9 : 791 – 801 .
- Romeih , E.A. , Michaelidou , A. , Bibiaders , C.G. and Zerfiridis , G.K. 2002 . Low-fat white-brined cheese made from bovine milk and two commercial fat mimetics: chemical, physical and sensory attributes . International Dairy Journal , 12 : 525 – 540 .
- Rank , T.C. , Grappin , R. and Olson , N.F. 1985 . Secondery proteolysis of cheese during ripening . Journal of Dairy Science , 68 : 801 – 805 .
- Carmona , M.A. , Sanjuan , E. , Gomez , R. and Fernanandez-Salguero , J. 1999 . Effect of starter cultures on the physico-chemical and biochemical features in ewe cheese made with extracts from flowers of Cynara cardunculus . Journal of the Science of Food and Agriculture , 79 : 737 – 744 .
- Irigoyen , A. , Izco , J.M. , Ibanez , F.C. and Torre , P. 2001 . Influence of rennet milk-clotting activity on the proteolytic and sensory characteristics of an ovine cheese . Food Chemistry , 72 : 137 – 144 .
- Poveda , J.M. , Sousa , M.J. , Cabezas , L. and Mcsweeney , P.L.H. 2003 . Preliminary observations on proteolysis in Manchego cheese made with a defined-strain starter culture and adjunct starter (Lactobacillus plantarum) or a commercial starter . International Dairy Journal , 13 : 169 – 178 .
- Cagno , R.D. , Quinto , M. , Corsetti , A. , Minervini , F. and Gobetti , M. 2006 . Assessing the proteolytic and lipolytic activities of single strains of mesophilic lactobacilli as adjunct cultures using a Caciotta cheese model system . International Dairy Journal , 16 : 119 – 130 .
- Fox , P.F. , Law , J. , McSweeney , P.L.H. and Wallace , J. 1993 . “ Biochemistry of cheese ripening ” . In Cheese: Chemistry, Physics and Microbiology , Edited by: Fox , P.F. Vol. 1 , 389 – 439 . London : Chapman and Hall .
- Dervisoglu , M. , Tarakci , Z. , Aydemir , O. , Temiz , H. and Yazici , F. 2009 . A survey on some chemical, biochemical and sensory properties of kes cheese, a traditional Turkish cheese . International Journal of Food Properties , 12 : 358 – 367 .
- Lawrence , R.C. , Creamer , L.K. and Gilles , J. 1987 . Texture development during cheese ripening . Journal of Dairy Science , 70 : 1748 – 1760 .
- Stepaniak , L. 2004 . Dairy enzymology . International Journal Dairy Technology , 57 : 153 – 171 .
- Hayaloglu , A.A. , Guven , M. , Fox , P.F. and Mcsweeney , P.L.H. 2005 . Influence of starters on chemical, biochemical, and sensory changes in Turkish white-brined cheese during ripening . Journal of Dairy Science , 88 : 3460 – 3474 .
- Yazici , F. and Dervisoglu , M. 2003 . Effect of pH adjustment on some chemical, biochemical, and sensory properties of Civil cheese during storage . Journal of Food Engineering , 56 : 361 – 369 .
- Martin-Harnandez , M.C. , Juarez , M. and Ramos , M.A. 1988 . Ripening and storage study of soft goat cheese with Penicillium candidum on the surface . Food Chemistry , 30 : 191 – 203 .
- White , S.R. , Broadbent , J.R. , Oberg , C.R. and Mcmahon , D.J. 2003 . Effect of Lactobacillus helveticus and Propiyonibacterium freudenrichii ssp. shermanii compositions on property for split defect in Swiss cheese . Journal of Dairy Science , 86 : 719 – 727 .
- Guler , Z. and Uraz , T. 2004 . Relationships between proteolytic and lipolytic activity and sensory properties (taste–odour) of traditional Turkish white cheese . International Journal of Dairy Technology , 57 : 237 – 242 .
- Pérez-Elortondo , F.J. , Albisu , M. and Barcina , Y. 1999 . Brining time effect on physicochemical and microbiological parameters in Idiazábal cheese . International Journal of Food Microbiology , 49 : 139 – 149 .
- Kucukoner , E. , Tarakci , Z. and Sagdic , O. 2006 . Physicochemical and microbiological characteristics and mineral content of herby cacik, a traditional Turkish dairy product . Journal of the Science of Food and Agriculture , 86 : 333 – 338 .