Abstract
Porcine pancreatic α-amylase was separated on DEAE-cellulose into two isoforms, i.e., PPA-I and PPA-II and their molecular weight was found to be ∼ 55 kDa by gel filtration. The pH and temperature optima of PPA and its isoforms were 6.9 and 45°C. Chlorides of metal ions Ca2+, Ba2+, Co2+, and Mg2+ enhanced the enzyme activity, whereas Al3+ and Hg2+ completely inactivated the enzyme. Oxalic and citric acids inhibition of PPA and its isoforms is concentration dependant. EDTA was found to be the most effective inhibitor. PPA and its isoforms released maltotriose and maltotetraose from starches.
INTRODUCTION
Alpha amylases (α-1, 4 glucan-4-glucanohydrolase; EC 3.2.1.1) are a class of hydrolases widely distributed in microbes, plants and animals.[Citation1] α-Amylase is an important industrial enzyme used in starch liquefaction to produce glucose, maltose, and also in brewing, baking, textile, paper, detergent and sugar industries.[Citation2] Hithertoknown α-amylases are metalloenzymes containing at least one Ca2+ ion per enzyme molecule, which is essential for their activity and stability.[Citation3] Calcium is required to maintain the structural integrity of amylases. Removal of calcium ions leads to either decreased thermostability or decreased enzymatic activity or both.[Citation4]
The three-dimensional structure of porcine pancreatic α-amylase is extremely similar to human pancreatic α-amylase. Pancreatic α-amylase consists of three domains: a catalytic core domain (A) comprising a (β/α)8 barrel, an extended loop inserted between the third β-strand and the third α-helix (B domain) and a C-terminal eight stranded β-sheet domain (C domain).[Citation5] Molecular cloning and primary structure analysis of porcine pancreatic α-amylase showed highest homology to the human pancreatic α-amylase sequence (87.1%) among all the amylases known.[Citation6,Citation7]
Pancreatic α-amylase is mainly responsible for in vivo degradation of starch.[Citation8] In humans starch digestion is initiated by the salivary amylase in the mouth, wherein starch is degraded into oligomers which are further degraded by pancreatic α-amylase into maltose, maltotriose and low molecular weight maltooligosaccharides in the small intestine.[Citation9] Combination of soft ionization methods like Electron Spray Ion-Mass Spectrometry (ESI-MS) has been developed as a powerful technique to analyze the sequence and linkage positions of oligosaccharides.[Citation10]
Porcine pancreatic amylase was separated into two components PPA-I and PPA-II and the measured molecular masses of PPA-I and PPA-II were 55400 ± 6Da and 55395 ± 6Da respectively.[Citation11,Citation12] Optimum pH values for amylases were neutral which is in tune with several mammalian amylases.[Citation13]
Even though the sequencing of PPA and its isoforms has been elucidated in detail, the kinetic parameters of PPA in comparison with that of PPA-I and PPA-II was not studied. Hence, a detailed study was undertaken to (a) separate porcine pancreatic α-amylase isoforms; (b) compare the kinetic parameters of PPA with that of PPA-I and PPA-II; and (c) study the mode of action of PPA and its isoforms on cereal and millet starches by identifying the released products by ESI-MS.
MATERIALS AND METHODS
Materials
Finger millet, ragi (Eleusine coracana, variety Indaf-15), rice (Oryza sativa, variety Jaya), and maize (Zea mays, variety NAC - 6002) were procured from Zonal Agricultural Research Centre, Mandya of UAS, Bangalore, Karnataka, India. Wheat (Triticum aestivum, variety DPW- 225 was procured from the local market.
The following special chemicals were obtained from various agencies: Sugar standards (malto-oligosaccharides), diethyl amino ethyl (DEAE)-cellulose, porcine pancreatic α-amylase, ethylenediaminetetraacetic acid (EDTA), 3,5,d-nitro salicylic acid (DNS) were procured from Sigma Chemicals Company, St. Louis, MO, USA.
Column for HPLC analysis (μ Bondapak-NH2 column) was obtained from Waters Associates, Milford, MA, USA. General chemicals used in this study were all of analytical grade and obtained from E-Merck, BDH, SRL and SD fine chemicals.
DEAE-Cellulose Chromatography
PPA (25 mg) was loaded onto a DEAE-cellulose column (φ 2.8 × 40 cm) pre-equilibrated with Tris-buffer (500 mL, 20mM, pH 8.0) at a flow rate of 30 mL/h and washed with the same buffer to remove the unbound proteins. A linear NaCl gradient (0–0.6 M) in equilibrating buffer was used to elute the bound protein which was collected (5 mL each) and monitored for protein (280 nm) as well as for amylase activity. In this present work, resolution of PPA-I and PPA-II components are obtained by DEAE-cellulose under slightly modified conditions.[Citation14,Citation15] The two activity peaks were designated as PPA-I and PPA-II in the order of their elution. PPA-I and PPA-II were concentrated and individually loaded on Sephadex G-100 glass column (φ 0.9 × 70 cm) pre-equilibrated with phosphate buffer (pH 6.9, 50 mM) and fractions (6 mL/h) were collected, monitored for protein and amylase activity.
Enzyme Assay
Amylases (PPA, PPA-I, and PPA-II) were assayed using gelatinized soluble starch (1g/100 mL, 1 mL) as substrate in sodium phosphate buffer (50 mM, pH 6.9), incubated with enzyme (50 μl) at 37°C for 60 min. The reaction was stopped by adding DNS reagent (1mL).[Citation16] The tubes were heated in a boiling water bath for 10 min cooled to room temperature and absorbance was measured after appropriate dilution at 540 nm.[Citation17] One unit of enzyme activity was defined as μmol of maltose equivalent released per min under the assay conditions.
Effect of Substrate Concentration (Kinetic Parameters)
Kinetics of PPA, PPA-I and PPA-II using starches isolated from different sources were studied at pH 6.9 and at 37°C.[Citation18] The initial velocity was measured at various substrate concentrations [S], which were expressed as percentage for comparison. The values of Michaelis constants (Km) and the maximum velocity (Vmax) were determined from the Lineweaver-Burk plots.[Citation19]
Effect of pH
Amylase activities were determined at various pH values using different buffers (50 nM concentration) such as sodium acetate (pH 4.0–5.5), sodium phosphate (pH 6.0–7.5) and Tris-HCl (pH 8.0–9.0) at 37°C. The maximum activity was taken as 100% and relative activities were plotted against different pH values.[Citation20]
Temperature Optima
Amylase activities were determined at a temperature range of 30–70°C (with an interval of 5°C) with 1% soluble starch as substrate in 50 mM sodium phosphate buffer. The maximum activity was taken as 100% and relative activities were plotted against different pH values.[Citation20]
pH Stability
Stability of amylases were determined using different buffers (ranging in their pH values from 3.0–9.0) such as phthalate-HCl (2.0–3.0), sodium acetate (4.0–5.0), sodium phosphate (6.0–8.0), Tris-HCl (8.0–9.0) at 50mM concentration. The residual activity was measured after pre-incubating the enzyme at pH 3.0–9.0 for 4 h (ranging from 15–240 min) at 37°C. The assay conditions were otherwise similar to that given above. The original activity was taken as 100%, and the relative activity was plotted against different time intervals.[Citation21]
Thermal Stability
PPA and its isoforms in sodium phosphate buffer (50mM, pH 6.9) were incubated at a temperature range of 30–70°C for 15 min. The residual activity was estimated taking original activity as control (100%) and relative activity was plotted against different temperatures.[Citation21]
Metal Ion Effect
PPA and its isoforms were incubated with 5 mM solution of salts of metal ions (chlorides of Ca2+, Ba2+, Co2+, Mg2+, Zn2+, Cu2+, Al3+, Hg2+) and their activities were determined. The enzyme activities without metal ions were taken as 100% and relative activities determined in the presence of metal ions were calculated.[Citation20]
Effect of Inhibitors
The inhibitors such as citric acid (1–15 mM), oxalic acid (1–15 mM), and EDTA (2.5–150 μm) were incubated with PPA and its isoforms at 37°C for 15 min and the residual activity was measured. The enzyme activities without inhibitors were taken as 100% and relative activities were plotted against different inhibitor concentrations.[Citation21]
Digestibility
ESI-MS of maltooligosaccharides
Starches were isolated according to the procedure adopted by Mohan, et.al.[Citation22] Isolation of starches were similar to that of starch isolation from legumes.[Citation23] Starches were gelatinized and subjected to digestion by PPA, PPA-I and PPA-II for 60 min and solutions were precipitated with three volumes of absolute ethanol and kept overnight at 4°C. The undigested residual starches were separated by centrifugation and the supernatants consisting of oligosaccharides were resolved and identified by HPLC (LC-8 A CR4A, Shimadzu) on μ-Bonda pack amino column (4.1 mm × 30 cm) using acetonitrile : water (70:30) as eluent with a flow rate of 1 mL / min.[Citation24,Citation25,Citation26]
Structural characterization of maltooligosaccharides by Electron Spray Ion-Mass Spectrometry (ESI-MS)
The fragmentation pattern of the maltooligosaccharides produced by PPA and its isoforms are described using ESI-MS.[Citation27,Citation28] Mass spectra of the oligosaccharides obtained by pancreatic α-amylase digestion of the starches were measured by Alliance, Waters 2695 mass spectrometer using positive mode electrospray ionization. Capillary voltage was 3.5 kV, core voltage 100 V, source 80°C, desolvation temperature 150°C, core gas (Argon) 35 L h−1 and desolvation gas (Nitrogen) 500 L h−1.[Citation29]
RESULTS AND DISCUSSION
DEAE-Cellulose Chromatography
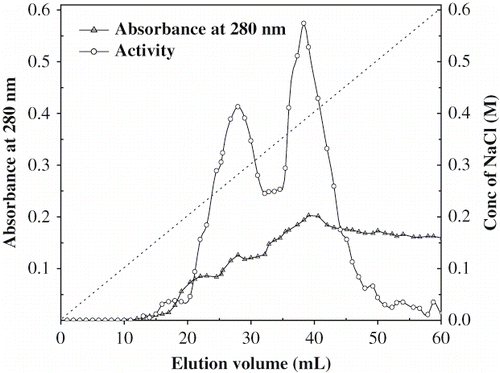
Porcine pancreatic α-amylase was separated into two activity peaks on DEAE cellulose column and designated as PPA-I and PPA-II () with a linear gradient of NaCl (0–0.6 M). PPA-I and PPA-II were eluted at concentrations of 0.25 and 0.36 M NaCl respectively. Porcine pancreatic α-amylase (PPA) was resolved to homogeneity from tissue extracts into two forms PPA-I and PPA-II as reported by Granger, et. al.[Citation30] These two forms have the same kinetic properties and molecular masses with distinct pI of 7.5 and 6.4, respectively.[Citation31,Citation12]
Effect of Substrate Concentration (Kinetic Parameters)
The effect of different concentrations of cereal and millet starches on the initial velocity was calculated. Km and Vmax for PPA, PPA-I, and PPA-II were found out from the Line-Weaver double reciprocal plots.[Citation19,Citation32] The Km values of PPA for ragi, rice, wheat and maize starches varied between 0.8–3.3% and the Vmax was between 2841–6212 U/mg protein, respectively. For PPA-I, Km values for ragi, rice, wheat, and maize ranged between 1.3–2.5% whereas Vmax was found to be between 2740–4201 U/mg protein. Km values of PPA-II for ragi, rice, wheat and maize were in the range of 1.0–2.96% and the Vmax values ranged between 2755–5102 U/mg protein (). Maize starch was the most difficult to digest followed by ragi, wheat and rice starches as indicated by the relatively high Km value.
Table 1 Kinetic constants of PPA, PPA-I, and PPA-II for cereal and millet starches. (Km and Vmax)
pH and Temperature Optima
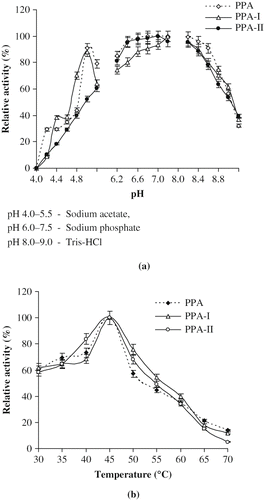
The optimum pH for the hydrolytic activity of PPA under physiological conditions has long been believed to be around neutrality on the basis of the study on soluble starch.[Citation33] Effect of pH on the activity of amylase is influenced by the kind of substrate and the assay conditions.[Citation34] In the present study soluble starch was taken as the substrate at 1% concentration. pH optima of PPA, PPA-I and PPA-II were 6.9 (). The activities of PPA and its isoforms are maximum at pH 7.0 compared to the activities observed in the acidic pH. The activities of PPA, PPA-I and PPA-II in sodium acetate buffer at pH 5.0 were 80, 85, and 65% respectively. PPA and its isoforms showed maximum activity in sodium phosphate buffer at pH 6.9. Tris-HCl buffer had a stabilizing effect on the activities of PPA, PPA-I and PPA-II. PPA retained about 80% activity at pH 8.5. PPA-I and PPA-II had maximum of 80 and 60% activities at pH 8.0. The results obtained in the present investigation are in accordance with the published literature.[Citation33]
PPA, PPA-I, and PPA-II showed temperature optima at 45°C. All the three enzymes retained up to 50% activity at 50°C. The activity increased sharply with gradual increase in temperature up to 45°C, whereas it showed decrease with further increase in temperature, indicating loss in the active conformation of the enzyme. PPA, PPA-I and PPA-II showed only 20–30% activity at 70°C ().
pH and Temperature Stability
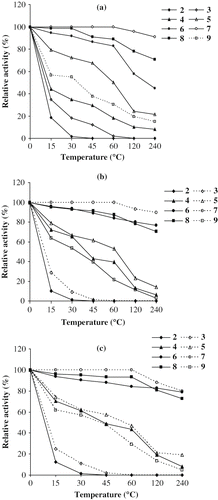
PPA was found to be stable in the pH range of 7.0–8.0 with 80% activity after 4 h incubation whereas PPA-I and PPA-II were stable in the pH range of 6.0–8.0 with almost 80% activities after 4 h incubation. However, the activity of these amylases decreased drastically at pH 2.0–3.0 after pre-incubation for 30 min ( a,b,c).
α-Amylases are generally stable in the pH range of 5.5–8.0. Exceptions exist on both sides of the pH scale as reported for bacterial amylases.[Citation35,Citation36] An acidophilic α-amylase from a Bacillus sp. has a pH optima of 2.0[Citation35] and an alkalophilic α-amylase from another Bacillus sp. has pH optima of 10.5.[Citation36] This broad range of optimum pH values for activity of individual α-amylases indicated their evolutionary adaptability to environmental circumstances.[Citation37]
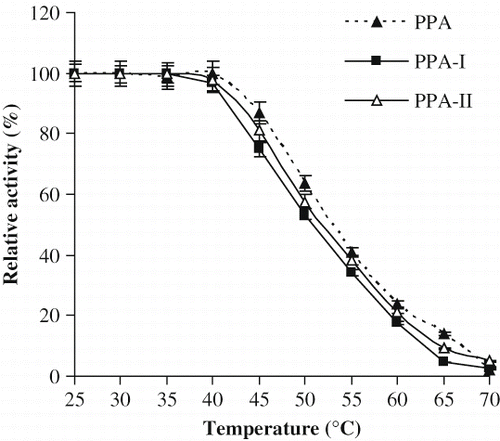
More than 95% activity was retained by PPA, PPA-I and II up to 45°C (). At 50°C the activities of PPA, PPA-I and PPA-II dropped to 50, 63, and 65% respectively. However, at 70°C, PPA and its isoforms have completely lost their activity.
The lowest temperature optimum was reported as 25°C for Fusarium oxysporum α-amylase,[Citation3,Citation38] whereas the highest for Bacillus licheformis α-amylase (90°C).[Citation39] The temperature optima of α-amylases, are observed between 50 and 60 °C. The extra thermostability of thermophilic Bacillus licheniformis α-amylase has been found to be mainly due the additional salt bridges involving a few specific lysine residues.[Citation40] Bacillus stearothermophilus α-amylase,[Citation41] as well as a mutant α-amylase from Bacillus amyloliquefaciens[Citation42] have been suggested to be stable against thermal denaturation through ionic interaction.
Effect of Metal Ions
Effect of di- and trivalent ions on the enzyme activities of PPA, PPA-I, and PPA-II are given in . Activity of PPA has increased in the presence of divalent ions such as Ca2+, Co2+, Mg2+ and Ba2+. Hg2+ and Al3+ completely inhibited the activities of all the three amylases, i.e. PPA, PPA-I and PPA-II. Cu2+ ions also inhibited the activities of the all the three enzymes to a larger extent.
Table 2 Effect of metal ions on the activities of PPA, PPA-I and PPA-II
The inactivation is attributed to the binding of this metal ion either to the catalytic residue or by replacing the Ca2+ from the substrate-binding site of the enzyme. Ca2+ had stabilizing effect on PPA-I and PPA-II. Enhancement of amylase activity by Ca2+ ions is based on its ability to interact with negatively charged amino acid residues such as aspartic acid and glutamic acids, which resulted in stabilization, as well as maintenance of enzyme conformation. In addition, calcium is known to have a role in substrate binding.[Citation43] The presence of Ca2+ is needed for the optimum activity and stability of the enzyme. The inhibition by other metal ions might be due to the competition for calcium binding site. With respect to the other metal ions, Al3+ completely inactivated PPA, PPA-I and PPA-II.
Alpha-amylases have long been known to require at least one Ca2+ ion per enzyme molecule for maintaining their tertiary structure and catalytic activity.[Citation44,Citation45] PPA was reported to have a calcium ion-binding site in which Ca2+ creates an ionic bridge between two β-structures. The essential Ca2+ ion appears to stabilize the active site cleft by inducing an ionic bridge between domains A and B. Role of Mg2+ in maintaining the stability and structure of the α-amylase is well documented.[Citation34] Significant increase in the activity of α-amylase from B. amyloliquifaciens from a commercial source was reported by the exogenous addition of Co2+.
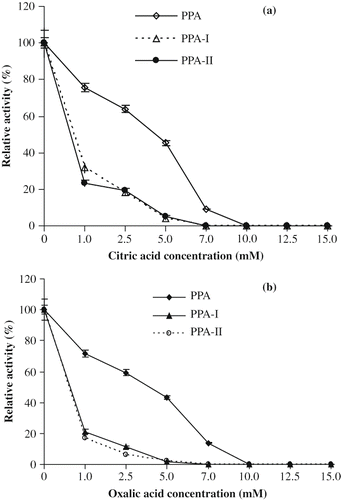
Effect of Citric and Oxalic Acids
Carboxylic acids such as citric and oxalic acids inhibit α-amylases by selective removal of Ca2+ present in the enzyme. The effect of citric and oxalic acids on the activities of PPA, PPA-I, and PPA-II were tested in different concentrations (0–15.0 mM). Citric acid (10 mM) completely inhibited the enzyme activities of PPA and PPA-II, whereas 7.5 mM concentration completely inhibited PPA-I activities (). Oxalic acid (10mM) completely inhibited PPA activity whereas, PPA-I and PPA-II were inhibited at 5.0 mM and 7.5mM respectively (). The differential inhibition effect of citric/oxalic acid on the activities of PPA, PPA-I, and PPA-II may be attributed to relative binding of Ca2+ to the enzymes.
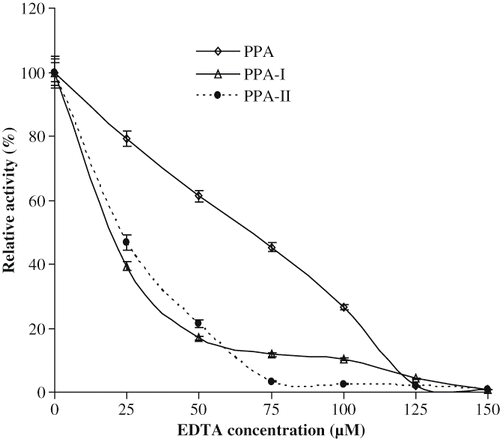
Effect of EDTA
EDTA at 125 μm concentration completely inhibited the activity of PPA and its isoforms (). The inhibition by EDTA is due to its chelating of bound calcium present in PPA and its isoforms.[Citation46,Citation43] Calcium ions appear to stabilize the active site cleft by inducing an ionic bridge between the domains both in the native enzyme and its isoforms. However, EDTA inhibited isoforms I and II much more strongly than PPA. This may be perhaps due to the presence of more amount of bound calcium in the native enzyme than its isoforms, which may require higher concentration of EDTA to chelate the bound calcium present in the native enzyme.
Inhibition of amylase activity by EDTA indicated that the enzyme is a metallo-protein. EDTA binds most divalent metal ions. Removal of Ca2+ from the enzyme exposes the 2-sulphdryl groups and thereby the enzyme loses its activity. The inhibition may be due to the preferential and rapid formation of enzyme-inhibitor complex over substrate-enzyme complex indicating this to be a competitive type of inhibition.[Citation46]
Digestibility
PPA, PPA-I, and PPA-II released mainly oligosaccharides of varying degree of polymerization (G2 to G7) and negligible amounts of higher oligosaccharides (DP = >8) from cereal and millet starches. The pattern obtained from cereal starches differ especially with respect to amounts of G3 and G4 oligosaccharides (Data not shown). The in vitro digestibility pattern of Curcuma starch was carried out using α-amylase from Bacillus sp. The digestibility of tuber starch from Curcuma was high when compared to cereal starches.[Citation47]
Structural Characterization of Maltooligosaccharides by Electron Spray Ion-Mass Spectrometry (ESI-MS)
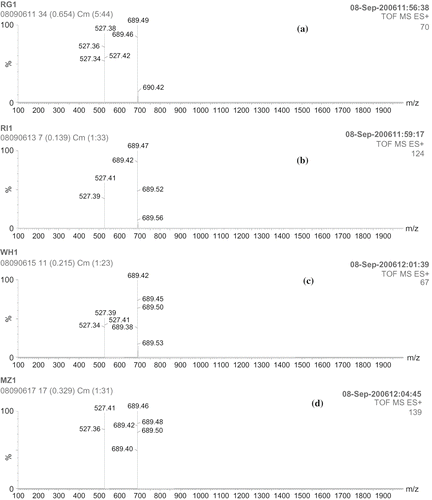
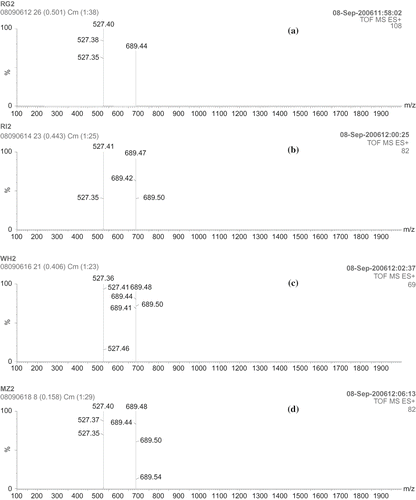
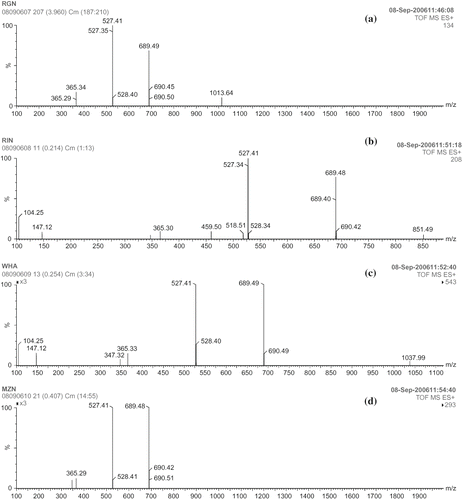
The fragmentation pattern of the starches by pancreatic α-amylase was confirmed by ESI-MS (, , ). PPA digested starches of ragi showed the MS spectrum of the ion at 365.3 m/z (maltose: fragmentation, 180 × 2 = 360 − 18 = 342 + 23 = 365), 527.41 m/z (maltotriose: fragmentation, 180 × 3 = 540 − 36 = 504 + 23 = 527) and 689.4 m/z (maltotetraose: fragmentation, 180 × 4 = 720−54 = 666 + 23 = 689) and trace of maltohexaose at 1013 m/z (fragmentation, 180 × 6 = 1080−90 = 990 + 23 = 1013). PPA digested starches of rice showed the MS spectrum of the ion at 365.3, 527.41, and 689.4 m/z and small amount of maltopentaose at 851 m/z (fragmentation, 180 × 5 = 900 – 72 = 828 + 23 = 851). 23 is the sodium adducts [M+ Na]+ that is detected in all the samples. With respect to PPA digestion of wheat and maize starches, MS spectrum of the ion was observed at 365.3, 527.41 and 689.4 m/z, which correspond to maltose, maltotriose, and maltotetraose. Digestion of starches by PPA-I and PPA-II showed similar pattern of fragmentation at 527.41 (maltotriose: fragmentation, 180 × 3 = 540 − 36 = 504 + 23 = 527) and 689.4 (maltotetraose: fragmentation, 180 × 4 = 720 − 54 = 666 + 23 = 689) m/z.
Figure 7(i) ESI-MS spectra of maltooligosacharides obtained by PPA digestion of starches: (a) ragi; (b) rice; (c) wheat; (d) maize.
CONCLUSIONS
PPA was separated into two isoforms i.e. PPA-I and PPA-II on DEAE-cellulose and their molecular weight was found to be 55 kDa. They were inactivated below pH 4.0 and above 70°C. Ca2+ was enhancing the activities of PPA and its isoforms. EDTA, oxalic acid and citric acid inhibited PPA and its isoforms. Kinetic studies revealed a similar degradation pattern of starches by PPA, PPA-I, and PPA-II. PPA and its isoforms released preponderantly maltotriose and maltotetraose from the cereal and millet starches as identified by ESI-MS.
ACKNOWLEDGMENTS
The authors thank Dr V. Prakash F.R.Sc. Director C.F.T.R.I., for his kind encouragement and support. AG thanks the Council of Scientific Industrial Research (C.S.I.R) New Delhi for the Senior Research Fellowship.
REFERENCES
- Muralikrishna , G. and Nirmala , M. 2005 . Cereal α-amylases- an overview . Carbohdr. Polym. , 20 : 1 – 11 .
- Eliasson , A.C. 1996 . Carbohydrates in Food , 347 – 553 . New York : Marcel Dekker, Inc .
- Janecek , S. and Balaz , S. 1992 . α-Amylases and approaches leading to their enhanced stability . FEBS Lett. , 1 : 1 – 3 .
- Saboury , A.A. and Karbassi , F. 2000 . Thermodynamic studies on the interaction of calcium ions with alpha amylase . Themochemica Acta , 362 : 121 – 129 .
- Qian , M. , Ajandouz , E.H. , Payan , F. and Nahoum , V. 2005 . Molecular basis of the effect of chloride ions on the acid acid-base catalyst in the mechanism of pancreatic α-amylase . Biochemistry , 44 : 3194 – 3201 .
- Darnis , S. , Juge , N. , Guo , X.-J. , Marchis-Mouren , G. , Purigserver , A. and Chaix , J.-C. 1999 . Molecular cloning and primary structure analysis of porcine pancreatic α-amylase . Biochim. Biophys, Acta , 1430 : 281 – 289 .
- Roux , G.F. , Perrier , J. , Forest , E. , Marchis-Mouren , G , Purigserver , A. and Santimone , M. 1998 . The human pancreatic α-amylase isoforms: isolation, structural studies, and kinetics of inhibition by acarbose . Biochim. Biophys. Acta , 1388 : 10 – 20 .
- Andersson , L. , Rydberg , U. , Larsson , H. , Andersson , R. and Aman , P. 2002 . Preparation and characterization of linear dextrins and their use as substrates in in vitro studies of starch branching enzymes . Carbohdr. Polym. , 47 : 53 – 58 .
- Marchel , L.M. , Larr , J. VanDe , Goetheer , E. , Chimmelpennink , E. , Bergsma , J. , Beeftink , H.H. and Tramper , J. 1999 . Effect of temperature on the saccharide composition obtained after α-amylolysis of starch . Biotechnol. Bioeng. , 63 : 344 – 355 .
- Tuting , W. , Adden , R. and Mischnick , P. 2004 . Fragmentation pattern of regioselectively O-methylated maltooligosaccharides in electrospray ionization-mass spectrometry / collision induced dissociation . Int. J. Mass Spect. , 232 : 107 – 115 .
- Mouren , M. and Pasero . 1967 . Isolation of two amylases in porcine pancreas . Biochim. Biophys. Acta , 140 : 366 – 368 .
- Alkazaz , M. , Desseaux , V. , Marchis-Mouren , G. , Payan , F. , Forest , E. and Santimone , M. 1996 . The mechanism of porcine pancreatic α-amylase kinetic evidence for two additional carbohydrate-binding sites . Eur. J. Biochem. , 241 : 787 – 796 .
- Kazuhiko , I. , Matsui , I. and Honda , K. 1995 . Optimum pH control mechanism for porcine pancreatic α-amylase . Biosci. Biotech. Biochem. , 59 : 1175 – 1176 .
- Robyt , J.F. , Chittenden , C.G. and Lee , C.T. 1971 . Structure and function of amylases . Arch. Biochem. Biophys. , 144 : 160 – 167 .
- Cozzone , P. , Pasero , L. and Marchis-Mouren , G. 1970 . Characterization of porcine pancreatic isoamylases: separation and amino acid composition . Biochim. Biophys. Acta , 200 : 599 – 593 .
- Bernfeld , P. 1955 . “ Amylases α and β. Methods in enzymology ” . Edited by: Colobick , S.P. and Kalpan , N.O. Vol. 1 , 149 – 158 .
- Miller , G.L . 1959 . Use of dinitrosalicylic acid reagent for determination of reducing sugars . Anal. Chem. , 31 : 426 – 428 .
- Noman , A.S.M. , Hoque , M.A. , Sen , P.K. and Karim , M.R. 2006 . Purification and some properties of α-amylase from post-harvest Pachyrhizus erosus l. tuber . Food Chem. , 99 : 444 – 449 .
- Lineweaver , H. and Burk , D. 1934 . The determination of enzyme dissociation constants . J. Amer. Chem. Soc. , 56 : 658 – 666 .
- Nirmala , M. and Muralikrishna , G. 2003 . Three α-amylases from malted finger millet (ragi, Eleusine coracana): purification and partial characterization . Phytochem. , 62 : 21 – 30 .
- Nirmala , M. and Muralikrishna , G. 2003 . Properties of three α-amylases from malted millet (ragi, Eleusine coracana, indaf-15) amylases . Carbohdr. Polym. , 54 : 43 – 50 .
- Mohan , B.H. , Anitha , G. , Malleshi , N.G. and Tharanathan , R.N. 2005 . Characteristics of native and enzymatically hydrolyzed ragi (Eleusine coracana) and rice (Oryza sativa) starches . Carbohdr. Polym. , 59 : 43 – 50 .
- Singh , B. and Nagi , H.P.S. 2005 . Studies on the functional characteristics of flour/starch from wrinkled peas (Pisum sativum) . Int. J. Food Prop. , 8 : 49 – 59 .
- Nirmala , M. and Muralikrishna , G. 2003 . In vitro digestibility studies of cereal flours and starches using purified finger millet (Eleusine coracana, ragi Indaf-15) amylases . Carbohdr. Polym. , 52 : 275 – 283 .
- Nikolov , V. , Jakovljevic , J.B. and Boskov , Z.M. 1984 . High performance liquid chromatographic separation of oligosaccharides using amine modified silica columns . Starch/Stärke , 36 : 97 – 100 .
- Faulks , R.M. and Bailey , A.L. 1990 . Digestion of cooked starches from different food sources by porcine amylase . Food Chem. , 36 : 191 – 203 .
- Fernandez , L.E.M. , Obel , Scheller , V. and Roepstorff , P. 2004 . Differentiation of isomeric oligosaccharide structures by ESI tandem mass MS and GC-MS . Carbohdr. Res. , 339 : 655 – 664 .
- Levigne , S.V. , Ralet , M.-C.J. , Quemener , B.C. , Pollet , B.N.L. and Lapierre , C. 2004 . Thibault, J.-F.J. Isolation from sugar beet cell walls of arabinan oligosaccharides esterified by two ferulic acid monomers . Plant Physiol. , 134 : 1173 – 1180 .
- Careri , M. , Bianchi , F. and Corradini , C. 2002 . Recent advances in the food application of mass spectrometry in food-related analysis . J. Chromatography A , 970 : 3 – 64 .
- Granger , M. , Abadie , B. , Mazzei , Y. and Marchis-Mouren , G. 1975 . Enzymatic activity of TNB blocked porcine pancreatic α- amylase . FEBS Lett. , 50 : 276 – 278 .
- Ajandouz , E.H. and Marchis-Mouren , G. 1995 . Subsite mapping of porcine pancreatic alpha-amylase I and II using p-nitrophenyl- α-maltooligosaccharides . Carbohydr. Res. , 268 : 267 – 277 .
- Kazuhiko , I. , Matsui , K. and Honda , K. 1990 . Substrate-dependent shift of optimum pH in porcine pancreatic α-amylase-catalyzed reactions . Biochemistry , 29 : 7119 – 7123 .
- Wakim , J. , Robinson , M. and Thoma , J. A. 1969 . The active site of porcine pancreatic alpha amylase: factors contributing to catalysis . Carbohydr. Res. , 10 ( 4 ) : 487 – 503 .
- Witt , W. and Slauger , J.J. 1996 . Purification and characterization of α-amylase from poplar leaves . Phytochem. , 41 : 365 – 372 .
- Uchino , F. 1982 . A Thermophilic and unusually acidophilic amylase produced by a thermophilic acidophilic Bacillus sp . Agric. Biol. Chem , 46 : 7 – 13 .
- Horikosi , K. 1971 . Production of alkaline enzymes by alkalophilic microorganisms . Agric. Biol. Chem. , 234 : 556 – 561 .
- Toda , H. , Kondo , K. and Narita , K. 1982 . The complete amino acid sequence of Taka-amylase A . Proc. Japan Acad , 58 ( B ) : 208 – 212 .
- Chary , S.J. and Reddy , S.M. 1985 . Starch degrading enzymes of two species of Fusarium . Folia Microbiol. , 30 : 452 – 457 .
- Krishanan , T. and Chandra , A.K. 1983 . Purification and characterization of alpha-amylase from Bacillus licheniformis CUMC305 . Appl. Environ. Microbiol. , 46 : 430 – 437 .
- Tomazic , S.J. and Klibanov , A.M. 1988 . Why is one Bacillus alpha-amylase more resistant against irreversible thermoinactivation than another? . J. Biol. Chem. , 263 : 3092 – 3096 .
- Brumm , P.J. and Teague , W.M. 1989 . Effect of additives on the thermostability of Bacillus stearothermophilus a-amylase . Biotechnol. Lett. , 11 : 541 – 544 .
- Suzuki , Y. , Ito , N. , Yuuki , T. , Yamagata , H. and Udaka , S. 1989 . Amino acid residues stabilizing a Bacillus alpha-amylase against irreversible thermoinactivation . J. Biol. Chem. , 264 : 18933 – 18938 .
- Bush , D.S. , Sticher , L. , Hwystee , R.V. , Wegner , D. and Jones , R.L. 1989 . The calcium requirement for stability and enzymatic activity of two isoforms of barley aleurone α-amylase . J. Biol. Chem. , 264 : 19392 – 19398 .
- Buisson , G. , Duee , E. , Haser , R. and Payan , F. 1987 . Three dimensional structure of porcine pancreatic α-amylase at 2.9 Å resolution . Role of calcium in structure and activity. The EMBO J , 69 : 3909 – 3916 .
- Vallee , B. , Stein , E. , Sumerwell , W. and Fischer , E. 1959 . Metal content of alpha-amylases of various origins . J. Biol. Chem. , 234 : 2901 – 2905 .
- Boyer , E.W. and Ingle , M. B. 1972 . Extracellular alkaline amylase from a Bacillus species . J. Bacteriol. , 110 : 992
- Jyothi , A.N. , Moorthy , S.N. and Vimala , B. 2003 . Physicochemical and functional properties of starches from two species of Curcuma . Int. J. Food Prop. , 6 : 135 – 145 .