Abstract
Water soluble melanoidins (WSM) from a glucose-glycine model system were obtained and separated into high (HMW) and low (LMW) molecular weight fractions. Adsorption isotherm and GAB and BET fitting parameters of WSM were determined. Physical and structural properties were investigated through thermal and rheological analysis. The LMW fraction exhibited thermal properties similar to that of oligosaccharides and the HMW fraction was compatible with a complex and large molecular size biopolymer structure, while both exhibited gel like properties. Intrinsic viscosity values were consistent with spherical hydrodynamic volumes.
INTRODUCTION
Melanoidins are macromolecules originated by the Maillard reaction and formed primarily by interactions between carbohydrates (typically reducing sugars) and compounds characterised by a free amino group, such as amino acids. The importance of melanoidins is due to their wide presence in foods and to the effects that these compounds could have on food quality. Depending on the extent of heat-induced reaction, melanoidins could impair or improve the overall quality of food products. Besides brown colour, their presence is associated with peculiar aroma compounds with a typical flavour that can be undesired (e.g., in pasteurized or sterilized milk, beverages, etc.) or, on the contrary pleasant and desired (e.g., bread, cakes, chips, chocolate, coffee, etc.). In the latter case, melanoidins often confer functional properties namely regarding structure and sensorial characteristics.
Studies on melanoidins are mainly focused on their chemical characterization[Citation1,Citation2,Citation3,Citation4 among others]; chemistry of reaction and identification of various pathways; evaluation of the impact of different reaction parameters (pH, temperature, time, sugar reactivity, reagent concentration, water activity and glass transition temperature); quantification of nutritional damage in terms of loss of availability of essential amino acids; pharmacokinetics and toxicological impact of Maillard reaction products (MRPs) and their role in metabolism.[Citation5,Citation6] The effects of physical changes on the rates of Maillard reaction,[Citation7,Citation8,Citation9,Citation10,Citation11,Citation12 , among others] and the role of MRPs as a structure making reaction[Citation13,Citation14,Citation15] have also been investigated. However, the references are scarce in the literature with regard to the physical structure of melanoidins,[Citation16,Citation17] possibly due to their complexity and heterogeneity. This is of relevance since some among the most important roles of melanoidins, as for most biopolymers, related to flavour and texture, are strongly affected by physical changes and structure transitions.[Citation9,Citation18,Citation19 , among others]
Some aspects of the physical structure of melanoidins, strongly considered to be composed mainly of cross-linked polymers, can be elucidated by both thermal and rheological methods. Among the thermal properties that can be characterized by differential scanning calorimetry, (DSC) and which can be related to the material structure at a molecular level, are the glass transition temperature (Tg), which is related both to the molecular complexity (Tg increases as molecular size and complexity increase), and the water content (Tg decreases with the increase of water content); and the so called T′g, defined as the Tg of the “maximally concentrated solution upon freezing”, which is considered a solute specific property.[Citation20,Citation21] Some thermal data related to non enzymatic browning in a glucose/glycine model system were reported.[Citation13,Citation15] Variation of thermal parameters such as Tg dry (Tg of anhydrous material), T′g and viscosity as function of heating time or polymerization degree during the reaction have been reported, and a Tg of the anhydrous polymer mixture has been extrapolated in the range from 37°C to 78°C. More detailed thermal parameters of low and high molecular weight melanoidins (in the text, LMW and HMW respectively) obtained from a glucose/glycine model system were reported,[Citation16,Citation17] which also considered state diagrams and rheological properties of melanoidins.
Material structure may also be characterised in terms of rheological behaviour under flow (e.g., Newtonian, pseudoplastic, with a yield value), and through dynamic measurements in the oscillation mode. Besides information on viscoelastic properties, oscillatory tests, and the Cox–Merz rule can discriminate among the gel like or entangled nature of the polymer.[Citation22] When dilute solutions are considered, viscosity measures allow the determination of the “intrinsic viscosity” i.e. a parameter linked to the hydrodynamic molecular volume and the chain conformation in solution.[Citation22, Citation23]
Others aspects of physical and physicochemical properties of materials could be obtained also by the water adsorption isotherm. The shape of the water activity (aw) vs. moisture content graph along with the parameters obtained by fitting (e.g. by GAB and BET equations) could contribute to understand the interactions between the polymer and water molecules.[Citation24] The aim of the present study was to investigate by thermal and rheological approach on the structure and water relations of low (LMW) and high (HMW) molecular weight melanoidins obtained from a glucose/glycine model system.
MATERIALS AND METHODS
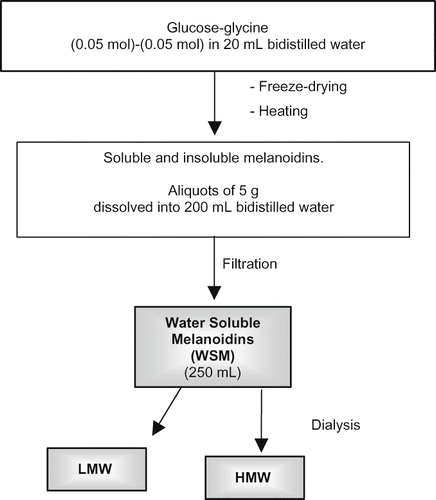
Standard melanoidins were prepared from a glucose-glycine (Sigma-Aldrich, Steinheim, Germany) model system,[Citation25], as reported in . Aliquots of 0.05 mol glucose and 0.05 mol glycine were dissolved with 20 mL bidistilled water into a 250 mL beaker, freeze dried (Mini fast 1700 freeze dryer, Edwards Alto Vuoto, Milan, Italy) and subjected to heating (125°C × 2 h) in a preheated oven (Memmert, Enco s.r.l., Venice, Italy) equipped with a fan (ventilation at the first speed). Melanoidins were cooled at room temperature in a desiccator and ground to a fine powder. Aliquots of 5 g were dissolved in 200 mL bidistilled water. The solution was stirred for 12 h at 4°C and then filtered (Whatman n. 4 filter paper, Whatman International Ltd Maidstone, England). Residue on the filter paper was washed with 2 × 20 mL bidistilled water, mixed with the original filtrate and made up to 250 mL in a volumetric flask. This mixture contains water soluble melanoidins (WSM). A UV-visible absorbance spectrum (at 280, 360, 420, and 520 nm) of WSM (1 mL diluted to 100 mL with bidistilled water) was obtained to verify that absorbance values were between 0 and 0.1 units.
Low (LMW) and high (HMW) molecular weight melanoidins from WSM were separated by dialysis (cut-off: 10000 Da). Aliquots of 50 mL of WSM were placed in a 30 cm dialysis tubing (cat. no. D-9652, 30 cm in length, Sigma-Aldrich, Steinheim, Germany) and submerged in 1 L stirred bidistilled water at 4°C for 12 h. After such time, the water surrounding dialysis tubing was replaced with 1 L fresh water and stirred for a further 12 h at 4°C. This step was repeated four times, which means five changes to collect 6 L of LMW containing dialysate.
The HMW fraction was collected together with the bidistilled water (3 × 10 mL) used to rinse dialysis tubing. LMW and HMW containing solutions were freeze-dried and stored in a desiccator over phosphorus pentoxide at –35°C until used.
Adsorption Isotherms
Adsorption data at 25°C were determined gravimetrically after exposure of freeze-dried WSM at relative humidity (RH) ranging from 11–86%. Sorption data of WSM were fitted with the BET[Citation26] and GAB equations[Citation27,Citation28] by using Statistica software (Statistica 6.1 for Windows, Stat soft, Tulsa, Okla., U.S.A.).
Thermal Analysis
Thermal analysis was performed on WSM, LMW, and HMW by differential scanning calorimetry (Mettler TA 4000 system equipped with TC11 TA Processor, DSC 30 measuring cell and Graph Ware TA 72PS.2 software, Mettler, Greinfensee, Switzerland). Heat flow calibration was performed with indium, temperature calibration was done with n-hexane, distilled water and indium. Samples for DSC were equilibrated directly into 40 μL aluminium DSC pans at selected relative humidity (0, 11%, 24%, 33%, 44%, 52%, 68%, 75%, 86%); pans were cooled to at least 40°C below the expected Tg and scanned under 10mL·min−1 dry nitrogen flow at 10°C·min−1, to at least 30°C above the end set of glass transition. Samples were scanned twice to avoid the enthalpic relaxation peak. The reported values are the average of at least two onset Tg determinations. Freezable solutions were subjected to annealing before heating in order to determine T′g and T′m (melting temperature of the maximally cryoconcentrated solution).
Image Analysis
Magnifications at 20 × 1.25 of WSM at aw 0, 0.33, 0.44, and 0.52 were taken with an optical microscope (Wild makroskop M40 Wild Heerbrugg, Switzerland). Immages were recorded in a PC by using a video camera (JVC Colour video camera head model no. TK‐1280E, Japan).
Rheological Analysis
Rheological tests were carried out using a controlled stress rheometer (StressTech rheometer, Reologica Instruments AB, Sweden) at 20°C ± 0.2°C, using cone-plate sensor geometry (cone angle 4°, 40 mm diameter) for WSM and LMW (70% w/w) and a plate-plate sensor geometry (10 mm diameter) for HMW (70% w/w). Linear viscoelastic regions were determined by stress sweeps at a fixed frequency of 1 Hz. Dynamic frequency sweeps were performed in a 0.1–10 Hz frequency range, at shear stresses of 0.08 Pa and 1.70 Pa for WSM and LMW respectively and a shear stress of 200 Pa for HMW. Prior to analysis, the sample placed in the rheometer sample cell rested for 20 minutes allowing the stress induced during loading to relax. The reported data are the average of at least three replications, except for the HMW fraction owing to the very low yield of the preparation method.
Intrinsic Viscosity Determination
Intrinsic viscosity of LMW and HMW was calculated according to Ross-Murphy[Citation22] and Lapasin and Pricl.[Citation23] Melanoidins were dissolved both in water and in NaCl 0.05 M solution and viscosity of dilute solutions (concentrations between 0.04 and 7.5 g·100 mL−1) was determined at least in triplicate. Analysis was performed by using a capillary viscometer (size 50, Cannon-Fenske Routine Viscometers, UK) at 20°C ± 0.2°C. WSM in NaCl 0,05 M showed a linear regression of time to flow (seconds) vs. concentration (g·100mL−1) with a correlation coefficient (R2) of 0,9978. Similarly, time to flow vs. concentration of LMW and HMW fractions gave both linear regressions with R2 of 0.9953 and 0.9969 respectively.
Relative viscosity, the ratio of the viscosities for the solvent and the solution (ηrel = η/ηs) and specific viscosity (ηsp = ηrel−1), were obtained and intrinsic viscosity [η] was calculated at ηrel approaching to 1.2, according to Ross-Murphy[Citation22] and Kraemer[Citation29]. For [η] determination, the Huggins and Kraemer plots i.e. of ηsp/C (reduced viscosity) and ln(ηrel)/C (inherent viscosity) vs. C (concentration expressed as g·100 mL−1) were applied and the mean intercept obtained.
The parameters K′ and K″ were also calculated and the equivalence condition of Equationequations (1) and Equation(2) K′−K″ = 1/2[Citation23] has been verified. The appropriateness of Huggins and Kraemer regression equations was evaluated with residuals analysis, the residuals were randomly scattered above and below the line at Y = 0 (Excel for Windows 9.0/2000, Microsoft Corporation).
RESULTS AND DISCUSSION
Adsorption Isotherms
shows adsorption isotherms of WSM. The GAB parameters m0, CG and k were determined. m0 is the monolayer i.e. the amount of water capable of interacting with all the available adsorption sites in a material; CG is a measure of the strength of binding of water to the primary binding sites; k is related to the properties of the multilayer molecules relative to the bulk liquid. The larger CG, the stronger water is bound in the monolayer and the larger the difference in enthalpy between the monolayer and the multilayer molecules. The more the sorbed molecules are structured in a multilayer the lower the value for k.[Citation30] When k approaces one, there is almost no distinction between multilayer molecules and liquid molecules. When CG ≈ 1 and k ≈ 1 then mono≈multi≈liquid.[Citation30] The GAB parameters of the isotherm of WSM are reported in . The m0 indicates a significant amount of water in the monolayer with corresponding aw of about 0,56 but both the low CG and the k close to 1 indicate that water does not structure firmly in a monolayer and/or in a multilayer. The BET equation has also been considered since the k value approaches to 1, and used to fit experimental data in the aw range of 0–0.55.[Citation31] As expected, the BET monolayer value is lower than that obtained with the GAB equation and corresponds to aw of about 0.40. The CBET value is still low if compared to that of other biopolymers like collagen, β-lactoglobulin, egg albumin and native wheat starch which are characterized by CBET values higher than 10.[Citation32, Citation33] Despite the remarkable m0 content, both the BET and GAB parameters would suggest weak water-polymer interactions hence reflecting water-holding more than real water-binding capacity.
Figure 1 Adsorption isotherm and Tg curve of WSM. Lines: isotherms resulting from GAB (determination coefficient R = 0.993) and BET (R = 0.992) equations fitting. Dots are experimental points.
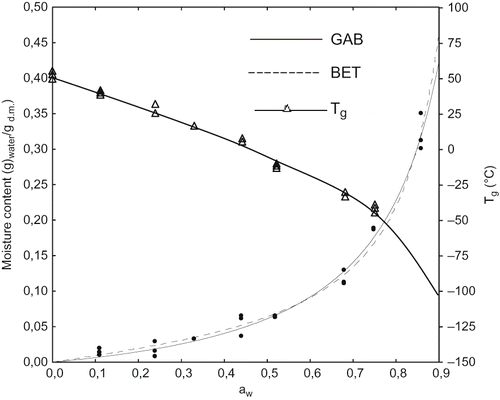
Table 1 GAB and BET parameters of WSM
Thermal Behaviour
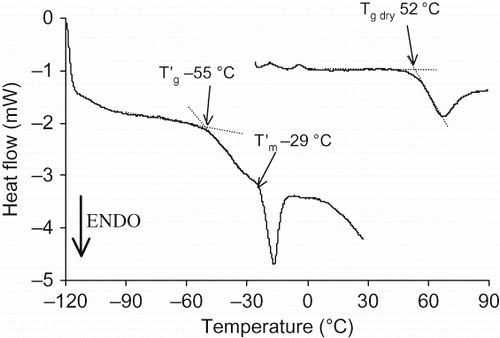
WSM and LMW fraction exhibited similar DSC traces and the following thermal parameters: glass transition temperature of the anhydrous powder (Tg dry) = 52°C, glass transition temperature of the maximally cryo-concentrated solution (T′g) = −55°C and incipient melting temperature (T′m) = −29°C (). The Tg dry and T′m values are close to sucrose ones (Tg dry = 62°C, T′m = −30°C)[Citation34] and thus typical of rather low molecular weight species.
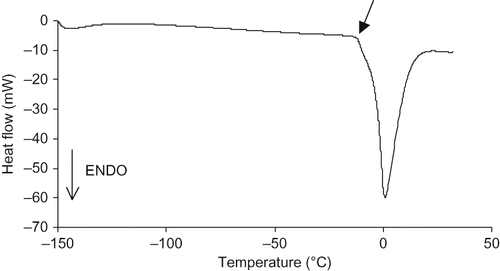
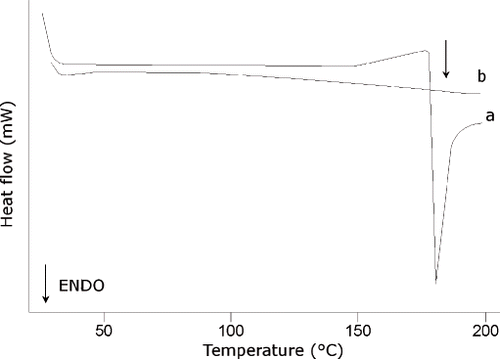
and show DSC curves of dry (scanned twice) and 27% HMW aqueous solution, respectively. The absence of a measurable Tg (as in some biopolymers e.g. proteins) (), and a higher T′m, –12°C, (as in many biopolymers, e.g. gluten, starch …) () marked HMW thermograms, whose behaviour was typical of complex high molecular weight biopolymers. The endothermic peak at 180°C (first scan in ) is ascribable to thermal degradation rather than melting; the latter should have led in the second scan to a Tg, which was not observed. Tg of WSM remained unaltered by the presence of HMW and the mobility of LMW was unaffected by the presence of high molecular weight polymers suggesting a phase separated system. Similar behaviour has been observed for sugar/biopolymer mixtures.[Citation35]
Figure 3 DSC traces of dry HMW. Arrow indicates the thermal degradation endothermic peak; (a: first scan; b: second scan).
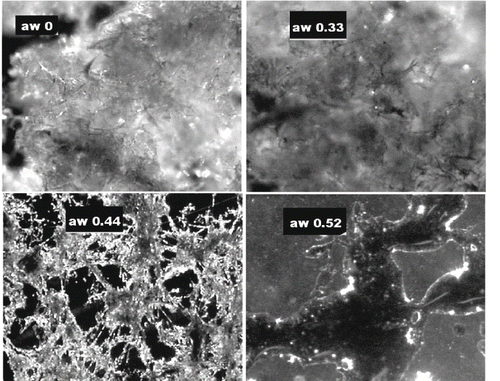
As a result of moisture sorption, Tg decreases, and in powdered samples this could induce collapse, a time dependent phenomenon whose rate is defined by the temperature difference T-Tg, where T is the reference temperature (ambient, storage)[Citation36]. In images of WSM equilibrated at aw 0, 0.33, 0.44, and 0.52 are reported. No structural changes (at 25°C) were observed up to aw 0.33 corresponding to a Tg >17°C and (T–Tg) > 8°C (see ). At aw 0.44, close to the BET monolayer value and corresponding to T-Tg of 18°C, the WSM become collapsed and at higher moisture contents the melanoidins appeared as a dispersion.
Rheological Behaviour
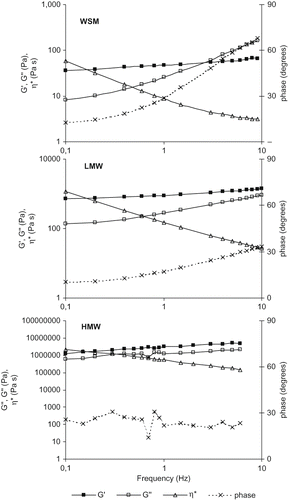
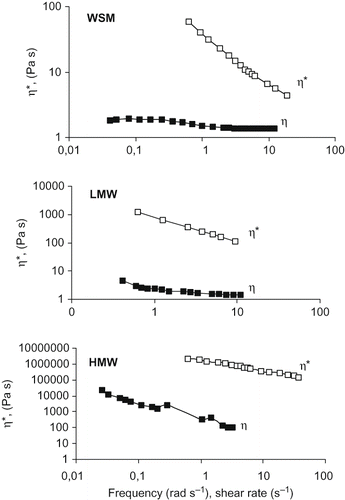
The kind of interactions among polymer chains was investigated by dynamic tests from which viscous/elastic moduli (G″, G′), phase angle and complex viscosity (η∗) were obtained. shows the mechanical spectra of WSM, LMW, and HMW (70% w/w) at shear stresses of 0.08, 1.7 and 200 Pa respectively. All samples showed G′ > G″ and a slight frequency dependence, whilst the complex viscosity decreased with frequency. Phase angle set at low values, especially for LMW and HMW (). Such mechanical spectra were consistent with gel like structures which were also confirmed by the Cox-Merz superimposition () of steady shear viscosity (η) vs. shear rate (flow curve) and complex viscosity (η∗) vs. frequency. WSM exhibited a weak gel structure behaviour where the slope of viscosities converge at high shear rates/frequencies, as in the case of weak gels.[Citation22] Conversely, for LMW and HMW samples, the two curves paralleled (), as typical of strong gels. Flow curve () of 70% w/w WSM solution presented a pseudoplastic region at low shear rates, possibly owing to cross-link bonds rupture, followed by a Newtonian plateau the last being typical of symmetrical particles. Differently from what expected, the WSM which contains both LMW and HMW, exhibited a weak gel structure and was also characterised by lower moduli (G′ = 48 Pa, G″ = 26 Pa, at 1 Hz) in respect to both HMW (G′ = 3.15 MPa, G″ = 1.27 MPa, at 1 Hz) and LMW (G′ = 900 Pa, G″ = 280 Pa, at 1 Hz) ().
Intrinsic Viscosity
shows reduced viscosity (ηsp/C) vs. concentration (g·100 mL−1) of WSM dissolved both in water and NaCl 0.05M solution. For the water-polymer solution the reduced viscosity increased very rapidly at high dilution and a complex dependence upon concentration was observed whilst the NaCl 0.05 M-polymer solution gave a linear trend. According to Lapasin and Pricl,[Citation23] such peculiar behaviour is typical of polyelectrolytes whose dilute solutions display unique dependence on concentration distinguishing them from nonionic polymers. The electrostatic forces have important effects on the configuration of macroions and the related physicochemical properties, such as viscosity. Many polysaccharides exist in aqueous solution in the form of macroions. With regard to melanoidins, Migo and coworkers[Citation37] reported a schematic diagram showing the net electrical charge of melanoidins synthesized by autoclaving glucose/glycine/Na2CO3 or glucose/n-butylamine liquid solutions at various pHs. They observed that melanoidins at pHs lower than 2.5 have a net electrical charge of +1; at pH 2.5 they are at the isoelectric point and hence the net electrical charge is 0; at pH 7 the net electrical charge is −1 and at higher pH the net electrical charge becomes more negative. In the present work, the pH of WSM, LMW and HMW solutions set in a range of 3.30 to 4.00 where solutes are not at their isolelectric point but behave as macroions, as evidenced from the reduced viscosity data in . In these cases, the intrinsic viscosity of polymer may be determined by dilution at constant ionic strength.[Citation23]
Figure 8 Reduced viscosity of WSM dissolved in water as function of concentration. Inner rectangle: reduced viscosity of WSM dissolved in NaCl 0.05 M.
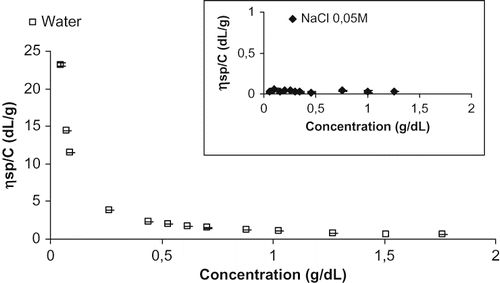
Intrinsic viscosity [η] (dL·g−1) is a characteristic property of an isolated polymer molecule in a given solvent, and is related to its hydrodynamic volume and shape. For macromolecules and rigid particles, [η] is strictly connected with the geometrical parameters characterizing their conformation.[Citation23] According to Einstein's hydrodynamic theory of rigid spheres, [η] of spheres is 2.5·10−2 dL·g−1 and it is independent of size (particle diameter) and therefore molecular weight. Other molecular shapes are characterised by significantly larger values.[Citation22] Bicerano and coworkers[Citation38] reported [η] values of 3-5·10−2 dL·g−1 for angular but equant particles and 4–10 10−2 dL·g−1 for rods or fibers. The Huggins constant K′ (from Equationequation (1)) has a magnitude characteristic of the system under consideration and depends on the sizes, shapes and cohesion properties of both solvent molecules and solute submolecules, but not on the number of submolecules (i.e., the length) of the polymer chain.[Citation39] K′ has been reported both as a measure of the hydrodynamic interactions between the macromolecules,[Citation23] and as a predictor of the degree of interaction between polymer and the solvent.[Citation40,Citation41,Citation42] According to Bit et al.,[Citation40] the sign of K′ is often taken as a measure of the type of interactions in the polymer chain and, in general, positive K′ values indicate enhanced interunit attractive interactions. From the dependence of intrinsic viscosity on weight average molar mass (Mw) the molecular shape of polymers can be inferred, as reported for inulin Wolff et al.[Citation43] The critical concentration C∗ (g·100 mL−1) represents the amount of polymer contained in a given volume in conditions of total occupancy, i.e., without interpenetration of the macromolecules and can be derived from intrinsic viscosity with C∗≈ 1/[η].[Citation22]
Intrinsic viscosity, K′ and critical concentrations of LMW, HMW and WSM are reported in . For all samples, the intrinsic viscosity was in the range of 0.021 dL g−1 to 0.025 dL g−1 indicating quasi-spherical shapes, such as for globular polymers. Furthermore, as typical of spherical particles, an increase of [η] with increasing molecular weight, i.e., from LMW to HMW was not observed. With regard to molecular mass (M), an approximated estimation for WSM obtained with the same method used in this work and separated into seven M ranges, has been reported by Hoffmann,[Citation25] who also reported the yield of molecular weight fractions. According to Hoffmann,[Citation25] LMW melanoidins are mostly represented, ∼92%, by M < 103 Da while HMW are mostly represented by M > 3·104 Da. Only a minor percentage of HMW is in between 104 and 3·104 Da. Hence, molecular weight differences between LMW and HMW are on the average of at least one order of magnitude and this should have led, for non-spherical particles, to a significant increase of [η], as observed for, e.g., inulin[Citation39] and/or others polymers.[Citation44,Citation45 among others]
Table 2 Intrinsic viscosity, K′ and critical concentrations of LMW, HMW fractions, and WSM
In the Huggins constants (K′) for LMW, HMW, and WSM are reported. According to Lapasin and Pricl,[Citation23] K′ ranges roughly from 0.3–0.4 in good solvents to 0.5–0.8 in ideal solvents. According to Simon and coworkers[Citation46] and Rotureau and coworkers,[Citation47] K′ may be higher than 1 reflecting an increase in the polymer-polymer interactions and a decrease in the polymer-solvent interactions. An increase in Huggins constant with the crosslink density has been observed for microgels by Omari et al.[Citation48] The increased K′ value of HMW suggests an increase in the crosslink density and interunit attractive forces so accounting for the enhanced gel strength of HMW with respect to LMW (). When spherical particles are concerned, the critical concentration (C∗) is directly related to the hydrodynamic volume of macromolecules. C∗ of WSM, LMW and HMW reported in were only slightly different and resulted 40.0 for WSM, 47.6 for LMW and 43.5 g dL−1 for HMW.
CONCLUSION
Adsorption isotherm parameters of WSM suggest weak water polymer interactions reflecting water holding more than water binding capacity. WSM collapsed upon hydration at about aw 0.44, close to the BET monolayer value, indicating the adequacy of the BET rather than the GAB model. WSM moistened at aw 0, aw 0.11, and aw 0.24 were in the glassy state (at 25°C). Tg dropped below reference temperature at higher aw′s. Rheological behaviour and intrinsic viscosity indicate that LMW and HMW melanoidins are electric charged, globular shaped polymers. Both the mixture and the two fractions can give gel networks that are strong for the HMW fraction. The thermal behaviour of LMW was not affected by the presence of HMW, which is consistent with a phase-separated system. By converse, the interactions among LMW and HMW melanoidins in the WSM determine a weakening of the gel structure with respect to the single fractions. The increase of the Huggins constant (k′) with increasing molar mass from LMW to HMW would indicate enhanced interunit attractive interactions. The reported data may contribute to an insight into both the relations with water and the physical nature, structure and behaviour of food melanoidins.
REFERENCES
- Cämmerer , B. and Kroh , L.W. 1995 . Investigation of the influence of reaction conditions on the elementary composition of melanoidins . Food Chemistry , 53 : 55 – 59 .
- Hofmann , T. 2001 . “ Isolation, separation and structure determination of melanoidins ” . In Melanoidins in food and health , Edited by: Ames , J.M. Vol. 1 , Belgium : European communities . Proceedings of the COST Action 919 workshop, University of Reading, UK, Dec 3, 4, 1999
- Kato , H. and Hayase , F. 2002 . An approach to estimate the chemical structure of melanoidins . International Congress Series , 1245 : 3 – 7 .
- Yaylayan , V.A. and Kaminsky , E. 1998 . Isolation and structural analysis of Maillard polymers: caramel and melanoidin formation in glycine/glucose model system . Food chemistry , 63 ( 1 ) : 25 – 31 .
- Finnot , P.A. 2005 . Historical perspectives of the Maillard reaction in food science . Annals of the New York Academy of Sciences , 1043 : 1 – 8 .
- Gerrard , J.A. 2006 . The Maillard reaction in food: progress made, challenges ahead- Conference report from the eighth international symposium on the Maillard reaction . Trends in Food Science and Technology , 17 ( 6 ) : 324 – 330 .
- Bell , L.N. , Touma , D.E. , White , K.L. and Chen , Y.H. 1998 . Glycine loss and Maillard browning as related to the glass transition in a model food system . Journal of Food Science , 63 ( 4 ) : 625 – 628 .
- Buera , M.P. and Karel , M. 1995 . Effect of physical changes on the rates of nonenzymic browning and related reactions . Food Chemistry , 52 : 167 – 173 .
- Karmas , R. , Buera , M.P. and Karel , M. 1992 . Effect of glass transition on the rates of nonenzymatic browning in food systems . Journal of Agricultural and Food Chemistry , 40 ( 5 ) : 873 – 879 .
- Lievonen , S.M. , Laaksonen , T.J. and Roos , Y.H. 1998 . Glass transition and reaction rates: nonenzymatic browning in glassy and liquid systems . Journal of Agricultural and Food Chemistry , 46 ( 7 ) : 2778 – 2784 .
- Roos , Y.H. and Himberg , M.J. 1994 . Nonezymatic browning behaviour, as related to glass transition, of a food model at chilling temperatures . Journal of Agricultural and Food Chemistry , 42 : 893 – 898 .
- White , K.L. and Bell , L.N. 1999 . Glucose loss and Maillard browning in solids as affected by porosity and collapse . Journal of Food Science , 64 ( 6 ) : 1010 – 1014 .
- Manzocco , L. and Maltini , E. 1999 . Physical cahnges induced by the Maillard reaction in a glucose-glycine solution . Food Research International , 32 : 299 – 304 .
- Manzocco , L. , Nicoli , M.C. and Maltini , E. 1999 . DSC analysis of Maillard browning and procedural effects . Journal of Food Processing and Preservation , 23 ( 4 ) : 317 – 328 .
- Maltini , E. and Manzocco , L. Oct 4–5, 2001 2002 . “ Structural and thermophysical aspects of non-enzymatic browning ” . In Melanoidins in food and health , Edited by: Fogliano , V. and Henle , T. Vol. 3 , Oct 4–5, 2001 , Belgium : European communities . Proceedings of Cost action 919 workshops, Napoli, Italy March 30–31 and Dresden, Germany
- Anese , M. , Manzocco , L. and Maltini , E. 2001 . “ Determination of the glass transition temperatures of “solution A” and HMW melanoidins and estimation of viscosities by the WLF equation: a preliminary study ” . In Melanoidins in food and health , Edited by: Ames , J.M. Vol. 2 , Belgium : European communities . Proceedings of Cost action 919 workshops, Berlin, Germany Apr 7–8 and Prague, Czech Republic Sept 22–23, 2000
- Maltini , E. , Anese , M. and Pittia , P. 2002 . “ Thermal and mechanical properties of melanoidins from model solutions ” . In Melanoidins in food and health , Edited by: Fogliano , V. and Henle , T. Vol. 3 , Belgium : European communities . Proceedings of Cost action 919 workshops, Napoli, Italy March 30-31 and Dresden, Germany, Oct 4–5, 2001
- Roos , Y.H. , Joupilla , K. and Zielasko , B. 1996 . Nonenzymatic browning-induced water plasticization . Journal of Thermal Analysis , 47 : 1437 – 1450 .
- Roos , Y.H. 1995 . Phase transitions in foods , 360 San Diego, California : Academic Press Inc .
- Levine , H. and Slade , L. 1988 . Principles of “cryostabilization” technology from structure/property relationships of water-soluble food carbohydrates- a review . Cryo-Letters , 9 ( 1 ) : 21 – 63 .
- Roos , Y.H. and Karel , M. 1991 . Phase transitions of mixtures of amorphous polysaccharides and sugars . Biotechnology Progress , 7 : 49 – 53 .
- Ross-Murphy , S.B. 1995 . “ Rheology of biopolymers solution and gels ” . In New Physico-chemical techniques for the characterization of complex food systems , Edited by: Dickinson , E. 139 – 156 . Great Britain : Blackie Academic & Professional .
- Lapasin , R. and Pricl , S. 1995 . Rheology of industrial polysaccharides - theory and applications , 620 Great Britain : Blackie Academic & Professional .
- Iglesias , H.A. , Chirife , J. and Boquet , R. 1980 . Prediction of water sorption isotherms of food models from knowledge of components sorption behaviour . Journal of Food Science , 45 ( 3 ) : 450 – 452 . 457
- Hofmann , T. 2001 . “ On the preparation of glucose/glycine standard melanoidins and their separation by using dialysis, ultrafiltration and gel permeation chromatography ” . In Melanoidins in food and health , Edited by: Ames , J.M. Vol. 2 , Belgium : European communities . Proceedings of Cost action 919 workshops, Berlin, Germany Apr 7–8 and Prague, Czech Republic Sept 22–23, 2000
- Brunauer , S. , Emmett , P.H. and Teller , E. 1938 . Adsorption of gases in multimolecular layers . Journal of the American Chemical Society , 60 : 308 – 319 .
- Anderson , R.B. 1946 . Modifications of the Brunauer, Emmett and Teller equation . Journal of the American Chemical Society , 68 : 686 – 691 .
- Guggenheim , E.A. 1966 . Application of statistical mechanics , Oxford : Clarendon Press .
- Kraemer , E.O. 1938 . Molecular weights of celluloses and cellulose derivatives . Industrial and Engineering Chemistry , 30 ( 10 ) : 1200 – 1203 .
- Quirijns , E.J. , van Boxtel , A.J.B. , van Loon , W.K.P. and van Straten , G. 2005 . Sorption isotherms, GAB parameters and isosteric heat of sortpion . Journal of the Science of Food and Agriculture , 85 ( 11 ) : 1805 – 1814 .
- Chirife , J. and Iglesias , H.A. 1978 . Equations for fitting water sorption isotherms of foods. Part 1- A review . International Journal of Food Science and Technology , 13 : 159 – 174 .
- Timmermann , E.O. , Chirife , J. and Iglesias , H.A. 2001 . Water sorption isotherms of foodstuffs: BET or GAB parameters? . Journal of Food Engineering , 48 ( 1 ) : 19 – 31 .
- Timmermann , E.O. 2003 . Multilayer sorption parameters: BET or GAB values? Colloids and Surfaces . 220 ( 1 ) : 235 – 260 .
- Roos , Y.H. 1993 . Melting and glass transition of low molecular weight carbohydrates . Carbohydrate Research , 238 : 39 – 48 .
- Kasapis , S , Mitchell , J. , Abeysekera , R. and MacNaughtan , W. 2004 . Rubber to glass transitions in high sugar/biopolymer mixtures. Trends in Food Science and Technology . 15 ( 6 ) : 298 – 304 .
- Levine , H. and Slade , L. 1986 . A polymer physico-chemical approach to the study of commercial starch hydrolysis products (SHPs) . Carbohydrate Polymers , 6 : 213 – 244 .
- Migo , V.P. , Del Rosario , E.J. and Matsumura , M. 1997 . Flocculation of melanoidins induced by inorganic ions . Journal of Fermentation and Bioengineering , 83 ( 3 ) : 287 – 291 .
- Bicerano , J. , Douglas , J.F. and Brune , D.A. 1999 . Model for the viscosity of particle dispersions . Journal of Macromolecular Science: part C: reviews in macromolecular chemistry , C39 : 561 – 642 .
- Huggins , M.L. 1942 . The viscosity of dilute solutions of long-chain molecules. IV. Dependence on concentration . Journal of the American Chemical Society , 64 ( 11 ) : 2716 – 2718 .
- Bit , G. , Debnath , B. and Saha , S.K. 2006 . Dilute solution behaviour of progressively hydrolyzed polyacrylamide in water-N,N dimethylformamide mixtures . European Polymer Journal , 42 : 544 – 552 .
- Mathlouthi , M. , Larreta-Garde , V. , Xu , Z.F. and Thomas , D. 1989 . Solute-solvent and water activity of small carbohydrates: application to the study of enzyme stability in aqueous sugar solutions . Journal of Carbohydrate Chemistry , 8 ( 2 ) : 233 – 245 .
- Kemp , S.E. , Birch , G.G. , Portmann , M.O. and Mathlouthi , M. 1990 . Intrinsic viscosities and apparent specific volumes of amino acids and sugars. “Effective size” and taste of sapid molecules . Journal of the Science of Food and Agriculture , 51 ( 1 ) : 97 – 107 .
- Wolff , D. , Czapla , S. , Heyer , A.G. , Radosta , S. , Mischnick , P. and Springer , J. 2000 . Globular shape of high molecular mass inulin revealed by static light scattering and viscometry . Polymer , 41 ( 22 ) : 8009 – 8016 .
- Pavlov , G.M. , Korneeva , E.V. , Harding , S.E. and Vichoreva , G.A. 1998 . Dilute solution properties of carboxymethylchitins in high ionic-strength solvent. Polymer . 39 ( 26 ) : 6951 – 6961 .
- Pavlov , G.M. , Errington , N. , Harding , S.E. , Korneeva , E.V. and Roy , R. 2001 . Dilute solution properties of lactosylated polyamidoamine dendrimers and their structural characteristics . Polymer , 42 ( 8 ) : 3671 – 3678 .
- Simon , C. , Dugast , J.Y. , Le Cerf , D. , Picton , L. and Muller , G. 2003 . Amphiphilic polysaccharides. Evidence for a competition between intra and intermolecular associations in dilute system . Polymer , 44 ( 26 ) : 7917 – 7924 .
- Rotureau , E. , Dellacherie , E. and Durand , A. 2006 . Viscosity of aqueous solutions of polysaccharides and hydrophobically modified polysaccharides: application of Fedors equation . European Polymer Journal , 42 : 1086 – 1092 .
- Omari , A. , Tabary , R. , Rousseau , D. , Leal Calderon , F. , Monteil , J. and Chauveteau , G. 2006 . Soft water-soluble microgel dispersions: structure and rheology . Journal of Colloid and Interface Science , 302 : 537 – 546 .