Abstract
The effect of solids on the thermal conductivity of mango juice concentrate obtained from a South African supermarket was studied in a shear flow field using a coaxial cylinder apparatus with a rotating outer cylinder. The fluid was observed to be shear thinning and thermal conductivity increased with temperature, shearing rate and particle size. The thermal conductivity of the juice with coarse particles was found to be significantly different from that without particles at a given temperature and shear rate. Significantly different values were obtained when the solid concentration is 50 mg/l. Temperature and shear rate dependent models were tested with the data and were found to correlate the observed data fairly well with a correlation coefficient of above 0.95.
INTRODUCTION
Good engineering analysis and design of production systems and product quality evaluation of food substances often rely on reliable thermophysical and transport property data. The transfer of heat to and from a product has significant interest in product quality control during heating or cooling of food. The successful management of heat transfer processes depends on the availability of thermophysical data such as thermal conductivity. The thermal properties of food substances are used in quantitative analysis for various thermal processes, such as heating, cooking, sterilization, drying and extrusion cooking.[Citation1] The processing of fruit juices often requires heating and sterilization.
Non-Newtonian liquid and semi-solid food substances have varying viscosity under a shearing environment. It is therefore reasonable to suggest that there will be changes in the thermal conductivities when these substances are subjected to a shearing field. There is significant information on the effect of temperature, moisture content and composition on thermal conductivity of food substances in literature.[Citation2–10] However, not much data is available on the effect of shear stress-shear rate on the thermal conductivity of foods. This is because available data (values) for most liquid foods have been measured under static conditions. If a shear rate effect exists on the thermal conductivity such as in the case with apparent viscosity, then measurements must be made over a range of shear rates when the fluid is in motion with respect to a static boundary.
The effects of shear rate on thermal conductivity of viscoelastic polymer fluids have been fairly reported in literature. Cocci and Picot[Citation11] reported that the thermal conductivity of Dow 200 fluids increase with increase in shear rate in the region of (). Chitrangad and Picot[Citation12] and Picot et al.[Citation13] reported that at low shear rates of (
), the thermal conductivity of DOW 200 fluids and polyethylene melts increased with increasing shear rate, reached a maximum point and then decreased with increasing shear rate (
). Lee and Irvine[Citation14] observed for non-Newtonian fluids such as aqueous CMC and Separan solutions, that there were as much as 70% and 50% increases in thermal conductivities for CMC and Separan respectively depending on the shear rate, polymer concentration and temperature. Their results showed a linear relationship between shear rate and thermal conductivity. Shin and Lee[Citation15] carried out thermal conductivity measurements of polymer suspensions under a rotating couette flow conditions with a varying rotational speed of the outer cylinder. The thermal conductivity of the test suspensions in shear flow increased with shear rate and displayed asymptotic plateau values at high shear rates. Furthermore, the shear rate dependent conductivity was strongly affected by both particle size and volume concentration in shear flow field. Xu Qi Lin et al.[Citation16] and Ikhu-Omoregbe and Chen[Citation17] used similar flow conditions to measure the thermal conductivity of two fruit juices and sauces obtained from a supermarket in Auckland, New Zealand, respectively, observed trends as those for the polymer materials. That thermal conductivity increases linearly as shear rate increases.
A number of models have been proposed in literature[Citation6,Citation8,Citation18,Citation19] to predict the thermal conductivity of two-phase systems. These models were based on the geometry of the components in the two phases. Furthermore, some have been extended to predict multi-component systems. Maroulis et al.[Citation20] applied some of these models to predict the thermal conductivity of gelatinized starch at different temperatures and compositions. Two types of thermal conductivity prediction models can be identified in the literature.[Citation6] The first relates temperature to thermal conductivity while the second relates composition to thermal conductivity (a structural model). However, both cover only non-shearing cases. Empirical models will be proposed to correlate the effect of shear rate and/or temperature on thermal conductivity. The thermal conductivities of most substances are known to be functions of temperature and are of the form:
where a, b, and c are constants and T is temperature (ºC). In this paper a model of the form was used:
where is shear rate (s−1). The objectives of this investigation were to: determine the effect of shear rate on the thermal conductivity at three temperatures of mango fruit juice; investigate the effect of size and concentration of solids on the thermal conductivity of this juice in a shearing environment; and propose some empirical models to demonstrate the effect of shear rate on apparent thermal conductivity.
MATERIAL AND METHODS
Materials
A number of solution mixtures were made with the mango juice concentrate by adding known quantities and sizes of thyme solids. Both materials were obtained from a supermarket in Durban, South Africa. The mango juice concentrate (Bromo Foods (Pty) Ltd. Salt River, South Africa) consisted of mango puree, guava puree, orange juice, sugar, water, acidifying agent (E330), stabilizer (E412), sodium benzoate, sulphur dioxide, flavourants, colourants, and vitamin C.
Three different size fractions of thyme (The Spice People, South Africa) were used as with a size ranges of 750–1000 microns (coarse fraction), 300–750 microns (medium fraction) and 150–300 microns as the fines fraction. The concentrations of the solids used in this set of runs were 25.0 g per litre of juice. In another set of runs the fines particles were used but the concentrations were 10, 20, 25, 35, and 50 mg/l, respectively.
Method
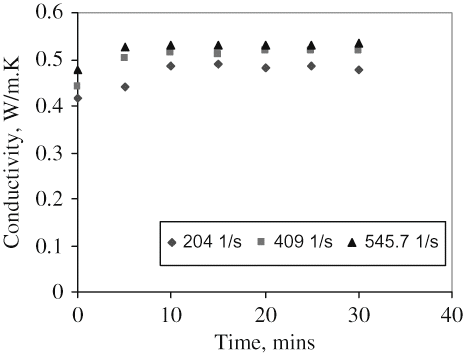
The equipment and procedure adopted in this work are similar to those described in details by Qi Lin et al.[Citation16] and Ikhu-Omoregbe and Chen.[Citation17] The equipment consists of two concentric cylinders, in which the inner cylinder is stationary and the outer one can be rotated. Both cylinders were made from materials such as copper with high thermal conductivities, so that the temperature drop across each is negligible compared to that across the test fluid. The test fluid is contained in the annular space (1.5 mm) between the two cylinders. One of the advantages of couette method is the non-existence of Taylor vortices especially if the gap is narrow[Citation21,Citation22] and the annular space of ro/ri of 1.074 satisfies the elementary requirement of ISO 3219 for narrow gap. The rest of the system consists of a constant temperature bath, a main heater, a guard heater, thermocouple to measure the inner cylinder and bath temperatures, and Pico data acquisition system. To commence the run, the speed of rotation is set and the system was allowed to reach a steady state which takes about 10–15 min. Then the temperatures, voltage and current are recorded every 10 s for 10 min through the data acquisition system; the speed of rotation of the outer cylinder was also recorded. The speed was varied as to give a shear rate of between 0 and 700 s−1. The mean value of these readings at a set shear rate was taken as one experimental point. All thermal conductivity measurements were in triplicates and showed a variation of not more than ±5%. The effect of time of shear on thermal conductivity of the pure juice was examined at shear rates of 204, 409, and 546 s−1 and at a temperature of 30ºC.
If we assume that Fourier's law of conduction is applicable, the shear rate variation across the gap (annulus) is negligible, the thermal conductivity of the liquid (test fluid) in the gap is considered to be independent of temperature, at steady state the following equation can be written for thermal conductivity, k:
where Q is heat input calculated from current and voltage measurements Q is current (A) × voltage (V); do
is outer diameter (m); di
is inner diameter (m),l is length of the testing section (m); and ΔT is temperature difference across the testing fluid (ºC). For a narrow gap” ≈1.5 mm between the cylinders, regardless of fluid type, the velocity profile can be assumed to be linear over this distance. The shear rate (), s−1
within the gap can thus be considered to be uniform and can be calculated as:
where n is rotational speed, (rpm.); do and di are as defined above. The rheological measurements (shear stress – shear rate and viscosity) of the test fluids were made using an Anton Paar MC51 rheometer (Anton Paar GmbH, Ostfilden, Germany). The measurement system used for this work consisted of a measuring cup of radius 21 mm and bob of radius 19.5 mm. The gap length was 60 mm and cone angle of the measuring bob was 120°. A constant temperature circulator (Viscotherm VT2, Anton Paar GmbH) with a temperature range of −20 and 180°C (± 0.1°C) was used to control measurement temperature. The rheological measurements were at various shear rates ranging from 1 to 800 s−1, and at a temperature of 30°C. The rheological measurements were made to demonstrate that the fluids used in this work are non-Newtonian fluids. The suitability of this apparatus set up for this study was determined in another publication.[Citation19 ]Also in that work the presence and effect of secondary flow on the measurement was explained.
RESULTS AND DISCUSSION
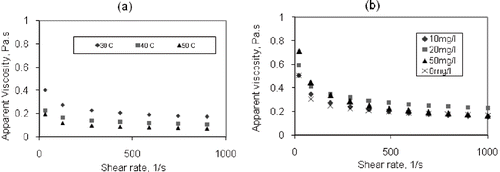
shows that the mango juice concentrate (pure and mixed with solids) is non-Newtonian and that its viscosity decreases with increasing shear rate suggesting that this fluid is shear thinning. The apparent viscosity of the pure juice varied from 0.40 Pa.s at low shear rate to 0.17 Pa.s at high shear rate at a temperature of 30°C. Furthermore, the results show that its viscosity decreases with increasing temperature as the molecules become elongated or expand. Application of the power law viscosity model to the pure mango juice gives values for consistency index, K as 0.94 and flow behaviour index, n as 0.251. The results also show a correlation coefficient, R2 > 99.6%, suggesting a good fit. There were no significant viscosity changes with the presence of solids as similar patterns and values were obtained ().
The results in show the values of thermal conductivity with shear rate for mango juice, with the fine, medium and coarse particle fractions respectively at the different temperatures of 30, 40, and 50°C. It can be observed that the thermal conductivity values for the juice without solids varied from 0.30 W/m K at zero shear rate and 30°C temperature, to 0.76 W/m K at a shear rate of 700 s−1 and at 50°C temperature. Similar values for the mixture with fines varied from 0.34 W/m K at zero shear rate and 30°C to 0.98 W/m K at a temperature of 50°C and shear rate of 615 s−1. These increased to 0.37 W/m K at 30°C and zero shear rates, and 1.43 W/m K and a temperature of 50°C for the fluid containing the coarse fraction. These values compare well with those obtained for mango pulp by Telis et al.[Citation19] The results also show that thermal conductivities for the fluids with particles are higher than that without the particles for a given shear rate. The fluid with the coarse particles also has higher conductivity values at a given shear rate. Furthermore the results show that thermal conductivity values increase significantly with increasing shear rate for a given temperature. Also for a given shear rate the conductivity increases with temperature. It can be observed from that the apparent thermal conductivity tends to increase with solids concentration for a give shear rate. However, a least significant different (LSD) analysis indicated that the effects of particle size and solids concentration are not significant (p < 0.05) except for the 50 mg/l concentration and for the coarse particles for the solid contents particle size and shear rates investigated. Furthermore, correlation analysis for the comparative effects of particle size and solids concentration does not indicate which had a more pronounced effect on thermal conductivity of the substance, although there is more variation with particle size compared to concentration.
Table 1 Thermal conductivity, W/mK values at various shear rate, particle size and temperature for mango juice
Table 2 Effect of solids concentration on thermal conductivity at various shear rates and 30ºC
Based on a critical Reynolds number of 1000 after which instabilities and turbulence in the gap start to significantly affect the results, critical shear rates were determined for the various fluid mixtures used in this work. A critical shear rate of 2970 s−1 was obtained for the least viscosity value of 0.073 Pa·s for any of the material condition. This value is much higher than the maximum shear rate of 800 s−1 experienced in the thermal conductivity measurements. Furthermore the maximum Reynolds number experienced during the experiments was 300 for the least viscous conditions. As the critical shear rate and Reynolds number were not exceeded, it is thus correct to state that instabilities due to turbulence would not have had any significant effect on the results obtained.
Correlation analysis suggests that there is a strong linear correlation between thermal conductivity and shear rate (R2 > 0.95) for all the composition and solids sizes on one hand and between thermal conductivity and temperature (R2 > 0.95) for all the composition and solids sizes investigated on the other hand. The analysis of the results also show that thermal conductivity for the conditions studied increase significantly with increasing shear rate for a given temperature. These observations are in agreement with literature[Citation11,Citation14,Citation16] that thermal conductivity of non-Newtonian fluids increase with shear rate. A number of reasons have been given for the effect of shear rate on thermal conductivity.
Cocci and Picot[Citation11] explained it to be due to either preferred orientation or cluster rotation of the polymer molecules. Shin and Lee[Citation15] suggested that the shear rate dependent conductivity was strongly affected by both particle size and volume concentration in shear flow field which is agreement with the findings of this work. Xu Qi Lin et al.[Citation16] suggested that the higher thermal conductivity at higher shear rates is due to the fluid structure becoming more aligned along the streamlines and hence becoming ordered. This was apparent because one of the fluids (mango concentrate) contained some fibres. Though the pure mango juice used in this work did not contain mango fibres, the presence of the thyme solids is thought to have possible similar effects.
A common phenomenon when a material is continuously being sheared is the occurrence of viscous dissipation resulting in local heating and temperature distribution and consequently affecting the thermal properties obtained. The contribution of viscous energy can be determined by estimating the Brinkman number and viscous heat production. Analysis of the system and results gave a Brinkman number less than 0.01 and a viscous heat contribution of 2.81% for the most viscous condition and at highest shear rate of 800 s−1. The implication of this is that viscous dissipation did not significantly affect the results obtained.
shows that the thermal conductivity of mango juice increases with the time of stress at the beginning and then remains fairly constant after about 10 min. This is a period of unsteady state with reference to thermal conductivity when shearing action is commenced. This observation suggests a realignment of the polymer structure due to shearing forces. It is also possible that the polymer structure could have become “stretched” resulting in increased rate of heat transfer across the material.
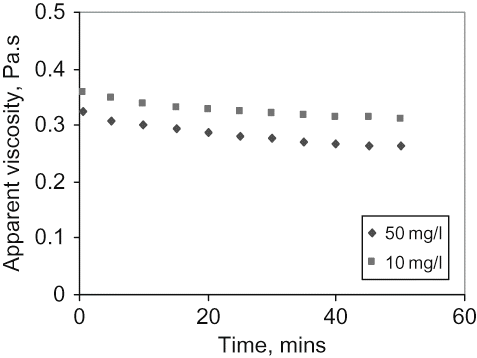
The effect of time on viscosity at constant shear rate is shown in for a shear rate of 100 s−1 and 30ºC temperature. The results show that the apparent viscosity decreases slightly with increasing duration of shear. It can be observed that while viscosity is decreasing thermal conductivity is increasing, suggesting that as the bond forces are being ‘stretched’ (sheared), the ability of the material to conduct heat is enhanced agreeing with the observations of Ikhu-Omoregbe and Chen.[Citation17] Similar shear decay curves were observed for mustard suspensions,[Citation23] chickpea flour dispersion,[Citation24] mango pulp,[Citation25] and fruit sauces.[Citation26] The effect is less pronounced for the mixture with the highest solids content.
Thermal Conductivity Models
A number of models have been proposed in literature[Citation6,Citation15] to predict the thermal conductivity of two-phase systems. These models were based on the geometry and composition of the components in the two phases. Furthermore, some have been extended to predict multi-component systems in a non-shearing field. In this paper attempt is made to account for the effect of shear rate/shear stress on the thermal conductivities of food substances. The thermal conductivity of most materials is known to be a function of temperature and is of the form of EquationEq. (1).
Correlation analysis of the relationship between thermal conductivity and shear rate suggest a linear relationship. Hence it is possible to deduce empirical models of the form of EquationEq. (2), which incorporate the effects of the rates of shear to correlate the data. From the results, the following models can be deduced to relate conductivity, temperature and shear rate for these materials.
-
Pure mango juice:
-
-
Mango juice with fine solids:
-
-
Mango juice with medium solids:
-
-
Mango juice with coarse solids
-
where s is the slope of conductivity-shear rate curve and is shear rate. (a-d) show the thermal conductivity values predicted using the three shear-rate dependent models for the pure mango juice, juice with fine particles, juice with the medium particles and juice with the coarse particles, respectively. The above results suggest that both equations A and C best correlate the data while model B did not fairly correlate the data. The models tend to correlate the data better at the lower shear rates than at the higher values. The performance of these empirical models suggests that the relationship between thermal conductivity and shear rate can be said to be linear rather than exponential.
Figure 4 Predicted thermal conductivity values for: (a) pure juice at 50ºC; (b) with fines at 30ºC; (c) with medium particles at 40ºC; and (d) with coarse particles at 50ºC.
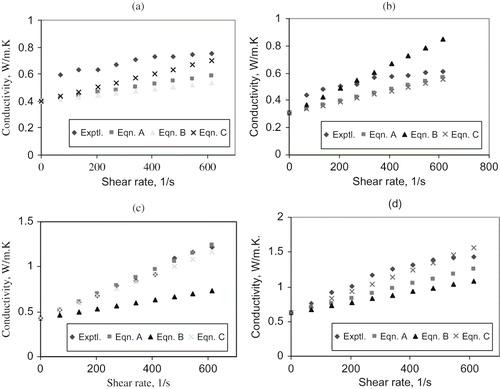
Statistical analysis of the results shows good correlation between experimental and predicted values using the models. An analysis of the standard errors and standard % error between predicted values and experimental thermal conductivities for the different solid size mixtures was carried out in order to compare the performance of the proposed models. It was observed from the analysis results that models A and C gave the lowest standard error values for all temperatures and solid content. For example whilst model A gave a standard error of 0.027 and standard % error of 3.23 at 40ºC for the mixture containing medium sized particles, model B gave respective values of 0.292 and 35.42 at the same temperature and material. This implies that the predicted values of thermal conductivity values were in closer agreement with experimental values using models A and C for the various solids contents used in this work. The performances of both models agree with earlier assertion that the relationship between shear rate and thermal conductivity is rather linear as these models are based on linear relationship between thermal conductivity and shear rate. The performance of the models for the different solid contents does not show any defined trend. The values of the standard errors and standard % errors are not significantly different for a given temperature except for the coarse solids. The solids are not dissolved in the fluid and therefore the effect of the presence of solids thought to be that of possible scattering or bouncing off heat or a type of convective transfer rather than conduction. It is apparent that this is better played by the larger particles, comparatively. The form of these models are empirical hence the specific model parameters or/and constants may not be applicable to other materials, but its form could be suitable for predicting the thermal conductivity for liquid food material in a shear fields.
CONCLUSION
The effects of solid particles on the thermal conductivity of mango juice obtained from the market have been studied in a shearing environment using a coaxial cylinder apparatus with a rotating outer cylinder. The juice concentrate was found to be shear thinning and the thermal conductivity values increased with increasing rate of shear. The thermal conductivities were also found to increase with the presence of solids in the juice. Furthermore, the thermal conductivity values were found to be significantly higher with the coarser particles and at a concentration of 50 mg/l for a given temperature and shear rate. The thermal conductivity was also observed to increase asymptotically to a constant value with time of shear. The results were not significantly affected by viscous dissipation contribution. Three temperature and shear rate dependent empirical models for the conductivities have been proposed and tested. The models were based on the assumption that the relationship between thermal conductivity and shear rate is linear. Two of these models were found to fit the data fairly well with correlation coefficient above 0.95.
ACKNOWLEDGMENT
The author would like to thank the University of KwaZulu-Natal for providing the funds for this work.
REFERENCES
- Drouzas , A.E. and Saravacos , G.D. 1988 . Effective thermal conductivity of granular starch materials . Journal of Food Science , 53 : 1795 – 1799 .
- Sweat , E.V. and Parmelee , C.E. 1978 . Measurement of thermal conductivity of dairy products and margarines . Journal of Food Process Engineering , 2 : 187 – 197 .
- Wang , N. and Brennan , J.G. 1992 . Thermal conductivity of potato as a function of moisture content . Journal of Food Engineering , 17 : 152 – 160 .
- Renaud , T. , Briery , P. , Andrieu , J. and Laurent , M. 1992 . Thermal properties of model foods in the frozen state . Journal of Food Engineering , 15 ( 2 ) : 88 – 94 .
- Reddy , C.S. and Datta , A.K. 1994 . Thermophysical properties of concentrated reconstituted milk during processing . Journal of Food Engineering , 21 : 31 – 40 .
- Rahman , S. 1995 . Food Properties Handbook , 314 – 332 . Boca Raton, FL : CRC Press .
- Tavman , I.H. and Tavman , S. 1999 . Measurement of thermal conductivity of dairy products . Journal of Food Engineering , 41 : 109 – 114 .
- Rahman , M.S. , Chen , X.D. and Perera , C.O. 1997 . An improved thermal conductivity prediction model for fruits and vegetables as a function of temperature, water content and porosity . Journal of Food Engineering , 31 : 163 – 170 .
- Krokida , M.K. , Michailidis , P.A. , Maroulis , Z.B. and Saravacos , G.D. 2002 . Literature data of thermal conductivity of foodstuffs . International Journal of Food Properties , 5 ( 1 ) : 63 – 111 .
- Fasina , O.O. , Farkas , B.E. and Fleming , H.P. 2003 . Thermal and dielectric properties of sweetpotato puree . International Journal of Food Properties , 6 ( 3 ) : 461 – 472 .
- Cocci , A.A. and Picot , J.J.C. 1973 . Rate of strain effect on thermal conductivity of a polymer liquid . Polymer Science and Engineering , 13 : 337 – 341 .
- Chitrangad , B. and Picot , J.J.C. 1981 . Similarity in orientation effects on thermal conductivity and flow birefringence for polymers: Polydimethylsiloxane . Polymer Engineering and Science , 21 : 782 – 786 .
- Picot , J.J.C. , Goobie , G.I.. and Mawhinnery , G.S. 1982 . Shear-induced anisotropy in thermal conductivity of polyethylene melts . Polymer Science and Engineering , 32 : 154 – 157 .
- Lee , D. and Irvine , T.F (Jr) . 1997 . Shear rate dependent thermal conductivity measurements of Non-Newtonian fluids . Experimental Thermal and Fluid Science , 15 : 16 – 24 .
- Shin , S. and Lee , S.H. 2000 . Thermal conductivity of suspensions in shear flow fields . International Journal of Heat and Mass transfer , 43 : 4275 – 4284 .
- Qi Lin , S.X. , Chen , X.D. , Chen , Z.D. and Bandoadhayay , P. 2003 . Shear rate dependent thermal conductivity measurement of two fruit juice concentrates . Journal of Food Engineering , 57 : 217 – 224 .
- Ikhu-Omoregbe , D.I.O. and Chen , X.D. 2005 . Thermal conductivity of two fruit sauces in a shearing environment . Proceedings of the 7th World Congress of Chemical Engineers . July 10–14 2005 , Glasgow, Scotland.
- Hsu , Chuan-Liang and Heldman , D.R. 2004 . Prediction models for the thermal conductivity of aqueous starch . International Journal of Food Science and Technology , 39 : 737 – 743 .
- Telis , V.R.N. , Telis-Romero , J. , Sobral , P.J.A. and Gabas , A.L. 2007 . Freezing point and thermal conductivity of tropical fruit pulps: mango and papaya . International Journal of Food Properties , 10 : 73 – 84 .
- Maroulis , Z.B. , Shah , K.K. and Saravacos , G.D. 1991 . Thermal conductivity of granular starch . Journal of Food Science. , 56 ( 3 ) : 773 – 778 .
- Steffe , J.F. 1996 . Rheological Methods in Food Process Engineering , 2nd , 182 East Lansing, MI : Freeman Press .
- Mezger , T.G. 2006 . The Rheology Handbook , 2nd , 173 – 178 . Hannover : Vincentz Networks .
- Choi , Y.H. and Yoo , B. 2004 . Characterisation of time-dependent flow properties of food suspensions . Int. Journal of Food Science and Technology , 39 : 801 – 805 .
- Ravi , R. and Bhattacharya , S. 2006 . The time-dependent rheological characteristics of a chicpea flour dispersion as a function of temperature and shear rate . Int. Journal ofFood Science and Technology , 41 : 751 – 756 .
- Bhattacharya , S. 1991 . Yield stress and time-dependent rheological properties of mango pulp . Journal of Food Science , 64 ( 6 ) : 1029 – 1033 .
- Ikhu-Omoregbe , D.I.O. 2007 . Thermal conductivity of South African sauces in a shear flow field . International Journal of Food Science and Technology , 42 : 753 – 761 .