Abstract
In-vitro experiments were conducted to provide predictive indices for the hypoglycemic effect of ashgourd, radish, pea peel, and cardamom peel fibers and its action of particle size and pH (related to human physiology) on the major functional properties. Ashgourd and radish fibers demonstrated significantly (p ≤ 0.05) higher water binding capacity and swelling capacity in stomach pH, i.e., 1.8; whereas pea peel and cardamom peel fibers exhibited higher hydration capacity at duodenal pH, i.e., 8.7. Ashgourd fiber (30 mesh) showed higher glucose adsorption capacity (452.1 μm/g) and exhibited maximum retarding effect on the flow of glucose across the dialysis bag for 12 h in comparison with other fiber sources. The 30 mesh (250–380 μm) and 60 mesh (150–230 μm) fiber particles showed better hydration properties as well as hypoglycemic effect as compared to 100 mesh (40–110 μm) particles. These fibers showed an excellent performance in relation to functional properties and hypoglycemic effect thus can be incorporated as low calorie bulk ingredient in high fiber foods to reduce calorie level and to help in controlling blood glucose concentration.
INTRODUCTION
Dietary fiber (DF) is of late gaining increasing importance in view of its close relationship with the gastrointestinal (GI) disorders, diabetes mellitus, obesity and cardiovascular diseases, frequently occurring now a days, have a very low incidence among people consuming recommended amounts of fiber.[Citation1] DF thus enjoys a very positive connation as a food component ingredient with consumers, food scientists and nutrition health professionals, as it plays an important role in human health and is considered as multifunctional substance affecting the functions in the human body. The USDA recommendations of the food pyramid consumption of DF range from 25–30 g/day.[Citation2] It is one of the natural constituents of fruits, vegetables, grains, legumes, cereals etc, which is not enzymatically degraded within the human alimentary digestive tract. DF is a chemical complex capable of reaction and interaction within a food matrix and within the human digestive system.[Citation3] It is the component of diet that can affect the gut directly and also have indirect metabolic actions by altering the pattern of digestion and absorption.[Citation4] DF exists in soluble and insoluble forms, which differ in their physicochemical properties and physiological effects. Soluble dietary fiber (SDF) such as pectin, gums etc are characterized by their viscosity and water solubility. The soluble fibre fraction modestly lower blood cholesterol and triacylglyceride concentrations as well as attenuate the post-prandial glucose response, whereas insoluble dietary fiber (IDF), such as cellulose, hemicellulose and lignin are characterized by their physical characteristics (porosity, density) and by their ability to increase fecal bulk and decrease intestinal transit time, hence enhancing intestinal peristalsis.[Citation5] The most likely explanation for the reduction of postprandial hyperglycemia by DF is direct delaying effect on glucose absorption in the GI tract, due to alteration in the diffusion of the digestion end product within the lumen.[Citation6] Thus, viscous forms of DF are most able to alter events (such as the rate of absorption of glucose) occurring within the GI tract. The functional properties may explain the functionality of these unexplored sources in food products as well as provide clue to their physiological effects with extension to industrial applications. However, merely adding fiber to food may not guarantee desirable physiological effectiveness. It has been reported that incorporation of finely ground wheat bran in a low-fiber diet causes severe constipation in human subjects.[Citation7] Thus, particle size is a very critical parameter for maintaining homogeneity of the products. It affects the functional properties and has relevance on the physicochemical properties thereby on the application in the food products.
The main objective of the present investigation was to study the effect of particle size and pH (related to human physiology), on the major functional properties such as water binding capacity (WBC), swelling capacity (SC), and cation exchange capacity (CEC) of ashgourd (Benincasa hispida), radish (Raphanus sativus), pea peels (Pisum sativum) and cardamom peels (Elettaria cardamomum), under the simulated conditions of pH in human system. The systematic approach for understanding the behavior of fibers in the human digestive tract still needs the attention of the researchers, thereby this in-vitro study was taken up, where the major functional properties of these novel fibers were determined with respect to various conditions existing in different parts of the human digestive tract (pH value, time of passage). These characteristics may have important physiological and/or technological implications by relating the functional properties of fibers to physiological effects. The literature on the effect of particle size on the hypoglycemic effect of these fibers is not available and these fibers from new origins will probably widen the fields of application as a dietary fiber source.
MATERIALS AND METHODS
Sample Collection and Preparation
Ashgourd, radish, peas with pods and cardamom, were purchased from the Mysore local market. Ashgourd and radish were washed, peeled, and cut into small pieces. These vegetables were then blanched in boiling hot water for 2 min and 4 min, respectively. The juice was extracted (three times) and the residue obtained was further dehydrated in a cabinet drier (Everflow Tray Drier, Chennai, India) at 60–70°C to obtain a moisture content of 5–7%. The green peas and cardamom were dehulled and the pea peels were blanched in boiling water (containing 0.1% MgO + 0.1% NaHCO3 + 0.4% KMS) for 3 min. The peels were further dried as reported above, to obtain a moisture content of 6–8%. The cardamom peels were dried for 2 h to remove surface moisture and facilitate grinding. All the dehydrated fiber samples were ground in a flour mill and then passed through standard sieves using a sieve shaker (Jayant Scientific, Mumbai), equipped with 30 mesh, 60 mesh and 100 mesh sieves to get 250–380 μm, 150–230 μm, and 40–110 μm particle size samples, respectively. The various samples (500 g) were packed in paper (42 GSM)/Al foil (0.02 mm)/polyethylene 60 μ pouches. All the chemicals and reagents used were of analytical grade.
Functional Properties
The Water binding capacity (WBC) and cation exchange capacity (CEC) were determined as described by Gorecka et al.,[Citation8] while the swelling capacity (SC) was measured as outlined by Sowbhagya et al.[Citation9] The methods were modified to study these functional properties under experimental pH conditions which may be encountered in particular part of human digestive tract, i.e., oral cavity: pH 6.6, passage time 7 min; stomach pH 1.8 for 135 min; and duodenum pH 8.7 for 60 min.
Three different water-binding capacities were measured by transferring fixed amount of fiber into a plastic centrifuge tubes and 30 ml of the buffers with pH 6.6, pH 1.8, and pH 8.7, were added in these tubes. The tubes were kept in the water bath at 37°C and shaken for 7 min, 135 min, and 60 min, respectively. The soaked fiber was then centrifuged at 3000 g for 15 min and supernatant was carefully removed. The tubes were kept in a slant position for 30 min to drain excess of water. A portion of wet sample was removed, weighed, and dried to constant weight (±0.05 mg) at 100°C. The results were expressed as gram of water bound per gram of dry initial sample.
Three different swelling capacities were measured by transferring 1 g fiber in each graduated glass cylinder and the initial volume was noted. Thereafter, 30 ml of the buffers maintained at pH 6.6, pH 1.8, and pH 8.7, were added and the samples were allowed to swell for 7 min, 135 min, and 60 min, respectively. The final volumes were noted and expressed as ml of swollen sample per gram of dry initial sample.
Three different cation exchange capacities were measured by converting the fiber samples to acidic form by treatment with excess of 2 M HCl for 48 h. After filtration, 100 ml of 5% NaCl in buffers (pH 6.6, pH 1.8, and pH 8.7) was added to 0.5 g of wet residue. The flasks were shaken during incubation at 37°C for 7 min, 135 min, and 60 min and the solutions were titrated with NaOH/HCl. The results were expressed as meq per gram dry initial sample.
Separation of Water insoluble Solids (WIS)
Prior to these in-vitro studies, it was necessary to remove the glucose and other sugars from the prepared samples. The water-insoluble solids (WIS) were prepared according to the method of Chau, et al.[Citation10] WIS was separated from the prepared fiber samples, by homogenizing the fiber sample in distilled water (fiber:water ratio of 1:20, w/v) using a high-speed vortex for 2 min. After filtration, WIS was thoroughly washed with 70% ethanol and dried at 45°C.
Glucose Diffusion Assay
Based the method of Ou, et al.[Citation11] with slight modification, 0.5 g of fiber sample was well mixed in 25 ml of glucose solution (100 mM L−1) and dialyzed against 200 ml distilled water (pH 7.0) in an thermostatic orbital shaker cum incubator at 37°C for 24 h, using a dialysis membrane with a cutoff molecular weight of 12,000. The beaker was given a gentle shaking motion (to simulate intestinal peristalsis) for 3 h, followed by 3 h without shaking. This continued for initial 12 h incubation. For the remaining 12 h, the incubation was done without shaking. The glucose content in the dialysate was determined spectrophotometrically at regular intervals of 3 h for the first half of total incubation period using a glucose oxidase-peroxidase method (Peridochrom Glucose, GOD-POD, Boehringer Mannheim, Germany). At the end of 24 h, the glucose content in the dialysate was again measured. A control test was carried out without addition of fiber.
Determination of Glucose Adsorption Capacity
According to the method described by Ou et al.,[Citation11] the glucose-adsorption capacity was determined by mixing 1 g of fiber sample with 100 ml of glucose solution (50 Mm L−1) and incubated at 37°C for 6 h. After centrifugation at 4000 g for 20 min, the final glucose content in the supernatant was measured using the glucose assay kit (Peridochrom Glucose, GOD-POD, Boehringer Mannheim, Germany), to estimate the amount of glucose adsorbed by the fiber sample.
Chemical Analysis
Neutral Detergent Fiber (NDF), acid detergent fiber, cellulose, hemicellulose and lignin contents (%) in these plant fibers were estimated according to the methods described by Gorecka et al.[Citation8] The cell wall or neutral detergent fiber (NDF) comprises the hemicellulose, cellulose and lignin components of fiber, while acid detergent fiber (ADF) measures the lignin and cellulose content generally known as ‘Lignocellulose.’ Thus, the hemicellulose content was calculated as the difference between NDF and ADF, while cellulose was calculated as the difference between ADF and lignin content. Pectin content was estimated by the method described by Ranganna.[Citation12] The results were expressed as percentage calcium pectate.
Statistical Analysis
All the analyses were carried in triplicate, and mean ± standard deviation (SD) values have been presented. Data were statistically evaluated by one-way analysis of variance using Statistical Program for Social Science (SPSS) (version 10) for significance (p ≤ 0.05) of the various treatments and sources.
RESULTS AND DISCUSSION
The particle size, hydration properties, viscosity, and cation exchange capacity are common quality of concern when considering plant fibers as food ingredients. The viscosity and entrapment capacity contribute to the glucose and lipid metabolic effects, whereas particle size and hydration properties are strongly involved in the effects of colonic functions. These properties are in turn related to the microstructure of materials, which change during processing.
Effect of Particle Size and pH on Water Binding Capacity (WBC)
The measurement of WBC is considered as a useful parameter for predicting the fecal bulking ability of a fiber source and is generally related to its structure, particle size and also the number and nature of its water binding sites.[Citation13] WBC is more a function of fiber structure than chemical composition,[Citation14] which was also reflected in the present study, where larger particle could significantly (p ≤ 0.05) bind more water as compared to finer particles (). In all the four fibers, 30 mesh and 60 mesh particles size exhibited significantly (p ≤ 0.05) higher WBC as compared to 100 mesh particles. Sangnark and Noomhorm[Citation15] also reported a decrease in water retention capacity (12.17 to 7.35 g/g) with decrease in particle size from 500 μm to 75 μm. On the other hand, Champ and Guillon[Citation16] suggested that grinding might affect the hydration properties of DF because of an increase in surface area, which may cause the fiber to hydrate faster. However, in certain cases it might cause alteration and collapsing of fiber matrix, which traps water and results in decrease in water uptake.[Citation15,Citation17] Considering 60 mesh fiber particles, ashgourd and radish fiber, which contained more of pectin content (), demonstrated higher WBC (12.27 and 11.47 g/g, respectively) in acidic pH, i.e., 1.8, as compared to alkaline pH, i.e., 8.7, where the WBC was 11.82 g/g and 10.78 g/g, respectively (). The WBC of ashgourd and radish fiber was significantly (p ≤ 0.05) low in 6.6 pH (oral cavity), where the fiber particles were in contact with water for just 7 min (oral cavity: passage time). This indicated that although ashgourd and radish had high WBC, these fibers need some time for absorption of water from the surrounding. The pea peel and cardamom peel fiber, containing significantly (p ≤ 0.05) higher amount of cellulose (), exhibited higher WBC (9.51 and 12.43 g/g) at duodenum pH (8.7) as compared to stomach pH (). The results of the present study revealed that the DF with more soluble fiber components such as pectin and hemicellulose (partially soluble) could bind more water in the acidic pH, while the insoluble DF component such as cellulose could bind more water at alkaline pH, i.e., 8.7. Similar conclusions have been reported by Gorecka et al.,[Citation8] who studied the effect of pH on the WBC wherein the lupine flour containing high hemicellulose and lower cellulose content was characterized by higher WBC at acidic pH 1.8, while lupine hull containing more of cellulose, exhibited high WBC at alkaline pH 8.7. The 30 and 100 mesh particles of the fiber samples also exhibited similar trend with different pH, as in case of 60 mesh (). Fleury and Lahaye[Citation5] also studied the DF content of algal seaweed using physiologically simulated conditions, where the gastric and intestinal phases were simulated by allowing algal pieces to incubate at 37oC with agitation first for 2 h at pH 2 followed by 20 h at pH 7.5. They also reported that soluble DF components were more functional at pH 2, and thus, they are potentially solubilized in the stomach. Goni and Carron[Citation18] reported that water retention capacity was highly correlated with the amount of soluble DF fraction, the products with the highest soluble fiber fractions exhibited highest water retention values. Considering these information and the results achieved in the present study, it can be assumed that pectin and cellulose are the major components responsible for the hydration capacity. The difference in the WBC can thus be explained by various fractional composition of DF, found in these materials.
Table 1 Functional properties of the natural fibres under simulated conditions of digestive tract.*
Table 2 Fractional composition of selected dietary fiber (% of dry matter).*
Effect of Particle Size and pH on Swelling Capacity (SC)
Swelling capacity tends to set the volume occupied by the fiber in the hydrated state in the GI tract and is the function of the chemical composition and the physical structure of the fiber matrix.[Citation19] SC of the samples represent the volume of hydrated fiber under gravity force, while WBC represents the volume of hydrated fiber measured under centrifugal forces. The results () of the present study revealed that larger particle (30 and 60 mesh) could significantly (p ≤ 0.05) exhibit higher SC as compared to the finer particles (100 mesh). Sangnark and Noomhorm[Citation15] also reported a decrease in SC with decrease in particle size, which they attributed towards the consequence of the grinding process. The hydration capacity of these fibers varied as a function of particle size, pH and time of incubation. The 60 mesh particles of ashgourd and radish fibers showed higher SC (15.54 and 13.96 ml/g, respectively) in acidic pH, i.e., 1.8, as compared to alkaline pH, where relatively lesser SC was recorded (14.87 and 13.31 ml/g, respectively) for these fiber samples. Change in acidity towards neutral and alkaline pH resulted in lowering of SC. The pea peel and cardamom peel fiber (60 mesh) were characterized by higher SC in alkaline pH (10.52 ml/g and 13.81 ml/g, respectively) in comparison with acidic pH (9.77 ml/g and 12.91 ml/g). The 30 and 100 mesh particles also exhibited similar trend with different pH, as in case of 60 mesh particles (). Ashgourd and radish are mainly composed of primary cell walls, characterized by a higher content of pectin and a loose network of polysaccharides which confer hydrophilic and elastic properties to the fibers, while pea peel fiber is largely composed of secondary cell walls, rich in crystalline cellulose and relatively higher lignin, which gives the fiber rigidity and poor hydroscopic properties (). Sundberg et al.[Citation20] and McConnell[Citation21] also reported that lignin do not hydrate well and had hydrophobic properties, thus binds significantly lower amount of water in comparison to hydrophilic polysaccharide. Amongst the four fiber sources, highest SC (16.83 ml/g) corresponded to 30-mesh ashgourd fiber in buffer with 1.8 pH, while the lowest value was obtained for 100-mesh pea peel fiber in 1.8 pH (7.82 ml/g) (). Thus, the gastric acidification, duodenal alkalinization, and exposure to ionic constituents of the gut may modify the properties of the fibers.[Citation6] Difference in swelling between the fibers seems to be the result of the properties of their individual components and physical structure (porosity, crystallinity) of the fiber matrix. If the physical structure of fibers is altered, the spaces available for free water will be decreased and so will be the water imbibing properties.[Citation5] Thus, the alteration in particle size affected the physical structure, which directly reflects on differential water imbibing capacity. This study confirms that the WBC is dependent on the fiber source, fractional composition, particle size as wellas on pH.
Effect of Particle Size and pH on Cation Exchange Capacity
DF is a chemical complex capable of reacting and interacting within a food matrix and within the human digestive system. A major negative effect of increased consumption of DF is reported to be a reduction in the bioavailability of minerals and trace elements to the body from the GI tract.[Citation22] One mechanism by which DF may influence mineral availability is through cation exchange, which measures the ability of the fiber matrix to bind and hold ions on its surface. In the present study, the cation exchange capacity increased with decrease in acidity (). Increasing the pH at which the incubation of fibers occurred from pH 1.8 to pH 8.7, increased the CEC of the fiber samples. Similar results were observed by Goreka et al.,[Citation8] where amongst the legume sources, highest CEC was found in pH 8.7. (alkaline conditions), while the lowest one at pH 1.8 (acidic conditions). Valiente et al.[Citation3] studied the cadmium binding capacity of cocoa fiber under physiological pH conditions and reported that the binding capacity rose with increasing pH. Torre et al.[Citation23] also reported that the mineral binding capacity of the fiber components increased with increase in pH and can be attributed to increased ionization of functional groups of these polymers with increasing pH. However, as the particle size decreased, the CEC decreased. This is in accordance with results reported by Michel et al.,[Citation17] where the finer particles of sugar beet fiber showed lower CEC as compared to the coarse particles. According to him, CEC is related to the free carboxyl group content in the pectin. This explains the fact that the smaller value for the fraction with the finest particle size owes to its lower pectin content. All the samples behaved as weak monofunctional cation exchangers. At 60 mesh, under alkaline pH conditions, i.e., pH 8.7, ashgourd fiber (1.33 meq/g) showed higher CEC followed by radish (1.26 meq/g), pea peel (1.18 meq/g), and cardamom peel fiber (1.03 meq/g) (). These results can be correlated to the pectin content of the individual fiber, where ashgourd fiber exhibited highest pectin, followed by radish and pea peel fiber (). According to Jimense et al.,[Citation24] pectin suffers considerable de-esterification yielding macromolecules with increased ability to interact with cations ionically or by forming coordination complexes. In these fibers, the highest CEC was found in 30 mesh particles of ashgourd fiber at pH 8.7 (1.38 meq/g), while the lowest one was observed in 100 mesh particles of cardamom peel fiber at pH 1.8 (0.69 meq/g). Thus, the percentage of fiber fractions and the change in pH, altered the degree of ionization of constituents in the fiber matrix. Valiente et al.[Citation3] reported that mineral binding capacity of cocoa increased with increase in pH and was maximum at alkaline pH conditions. This can be attributed to increased ionization of functional groups of these polymers with increasing pH. Studies conducted by Gorecka et al.[Citation8] also supported our results and revealed that CEC is related with pH, time and source material. Depending on the GI pH, the functional groups may become charged, thus the proportion of lignin, cellulose, hemicellulose and pectin, change between the cell wall so do the proportion of various constituents and their associated linkages.[Citation25]
Effect of Dietary Fiber on Glucose Diffusion in a Glucose-Dietary Fiber System
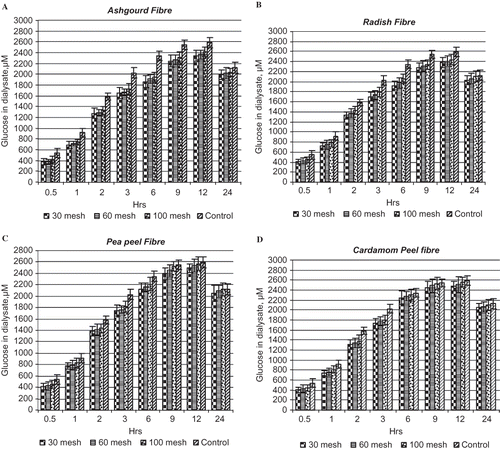
Glucose diffusion assay, is an useful in-vitro method for the prediction of the effect of fiber on the delay in glucose absorption in the GI tract.[Citation26] In this study, glucose diffusion assay and glucose adsorption capacity was determined for four fibers with different particle size. This dialysis experiments mimic events occurring along the GI tract. Movement in this system is not by true diffusion but is assisted by the convective activity of the intestinal contraction in-vivo or by the stirring, which simulates the biological system more closely than an unstirred system. This experiment has been carried out to assess the relative effects of the natural fibers in inhibiting glucose movement and evaluating these results in predicting the action of these polysaccharides in lowering the postprandial serum glucose level. The inhibition of glucose diffusion by the fiber samples was studied for 24 h, with gentle shaking motion (to stimulate intestinal peristalsis) for 3 h, followed by 3 h without shaking, so as to study the trend of glucose diffusion for 24 h. Diffusion rates of glucose varied with different DF sources and particle size. Although the difference in values was non-significant (p ≥ 0.05), 30 mesh and 60 mesh particles of ashgourd fiber diffused out lesser glucose (375.4–693.2 and 381.6–711.2 μmol, respectively) as compared to 100 mesh particles (422.3–748.8 μmol) at initial 30 min and 60 min, where the control (without sample) ranged from 542.5 to 917.4 μmol. Ashgourd fiber (30 mesh particles) showed significantly (p ≤ 0.05) high retarding effect on the flow of glucose across the dialysis bag for 9–12 h (). In case of radish fiber, a similar trend was observed in the diffusion of glucose for 24 h. At initial 30 and 60 min, the 30 mesh and 60 mesh fiber particles of radish fiber diffused out lesser glucose (412.2–722.8 and 426.3–747.2 μmol, respectively) as compared to 100 mesh particles (451.4–781.4 μmol) where the control (without sample) ranged from 542.5 to 917.4 μmol (). The diffused glucose content in the dialysate gradually increased till 12 h, recording maximum glucose in the dialysate in 100 mesh radish fiber (2439.2 μmol) (). Radish fiber could significantly (p ≤ 0.05) retard the glucose diffusion upto 9 h (2173.2 μmol), as compared to the control (2466.4 μmol) (). Although, the pattern of glucose diffusion in case of ashgourd and radish fiber was quite similar, it was observed that the rate of glucose diffusion in the dialysate was significantly (p ≤ 0.05) lesser in case of ashgourd fiber, may be due to higher pectin content in this fiber. Pea peel fiber could significantly (p ≤ 0.05) retard the glucose diffusion for only 3 h (1747.2 μmol) as compared to the control (2025.6 μmol) (). At 9 h, the glucose diffusion rate for 100 mesh was 2522.8 μmol that is almost similar to that of control sample 2546.1 μmol (). In the case of cardamom peel fiber, glucose diffusion across the dialysis membrane was faster as compared to that in pea peel fiber. The cardamom peel fiber also significantly (p ≤ 0.05) retarded the glucose diffusion after 3 h (). This trend may be due to the reason that the insoluble fiber components in case of pea peel and cardamom peel fiber, initially hindered the diffusion of glucose, due to their glucose adsorption ability and entrapment of glucose within the network of these fibers. After 3 to 4 h, however, the rate of diffusion increased as these fibers do not contribute towards the increase in viscosity (). Due to the complete imbibitions and saturation of insoluble fibers in initial 3 h, further retention of glucose could not occur and the diffusion rate increased almost similar to the control sample. This may be the reason why 100 mesh particles could not bind glucose efficiently as compared to 30 and 60 mesh particles due to breakdown of fiber network. Chau et al.[Citation10] also reported that the insoluble fiber rich fractions (FRFs) could adsorb glucose, delay the glucose diffusion, thus postpone the glucose adsorption in the GI tract. In case of all the fibers, after 12 h, a decreasing trend was observed in the fiber samples, which was probably due to the equilibrium attained by the system. Ou et al.[Citation11] have reported that soluble fiber components are relatively more effective in retarding the glucose diffusion across the membrane as compared to insoluble fiber components. This may be one of the reasons why ashgourd and radish fibers (with higher pectin content) showed greater inhibition of glucose diffusion as compared to other fiber samples. Goni and Carron[Citation18] also reported that gel forming properties of certain fibers, slow down the intestinal digestion of carbohydrate and lipids. Although the viscosity of the fibers was not measured, it is well established that the ability of these fibers to retard the absorption of glucose in the GI tract is a function of their viscosity. In-vivo and in-vitro studies of glucose absorption have shown that the delay in glucose absorption in the GI tract is determined mainly by the viscosity of soluble polysaccharides.[Citation27] Thus, DF with more soluble fiber component exhibit better results in effects of hampering the diffusion of glucose and postponing the absorption and digestion of carbohydrates.
Effect of Particle Size on the Glucose Adsorption Capacity (GAC) of Plant Fibers
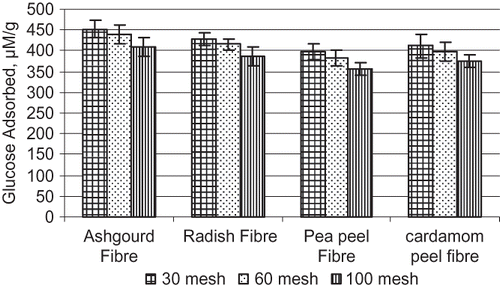
The ability of the DF to adsorb glucose indicated that they could act as functional dietary supplements for decreasing the rate of glucose absorption as well as concentration of serum glucose Yeh.[Citation28] Chau et al.[Citation13] have reported that the fibers of different composition, sources and preparation might vary in their effectiveness in controlling hyperglycemia. Amongst the four fibers, ashgourd fiber (30 mesh) could significantly (p ≤ 0.05) adsorb maximum glucose (452.1 μmolg−1) followed by radish fiber (4.27.5 μmolg−1), cardamom peel fiber (411.2 μmolg−1) and pea peel fiber (396.1 μmolg−1) (). The ability of these natural fibers to adsorb glucose would be beneficial to the reduced amount of available glucose in the small intestine. The effect of glucose adsorption by fiber samples showed that 30 mesh and 60 mesh fiber particles adsorbed significantly (p ≤ 0.05) higher amount of glucose as compared to 100 mesh particles. The glucose adsorption capacity was thus higher with larger particle size of these fibers. It may be due to the entrapment of glucose within the fiber network and reduction of water mobility on the fiber surface as a result of increased hydration capacity of larger particles, which might contribute to the retaining of glucose on the fiber surface.[Citation29] Brennan and Samyue[Citation30] postulated that the digestion of starch and sugar release from foods might be delayed due to the DF constituents adhering to the starch granules and possibly increasing digesta viscosity. The mechanisms of fibers such as hindering diffusion of glucose and glucose adsorbing, reduce the concentration of the glucose available in the small intestine, could help in lowering the postprandial serum glucose level.[Citation4] Thus, DF can have an impact on food by reducing the rate of glucose breakdown and absorption, hence avoiding an excess of glucose in the body and facilitating the steady breakdown of carbohydrates and release of glucose.
Composition of Fibers
The functional properties of DF are based not only on pH, particle size, source of fiber but also on the major components of fiber. The functional properties of DF and its technological functionality and nutritional effects are highly determined by the composition of the fiber fractions according to Lecumberri et al.[Citation31] Incorporation of coarse fiber particles (30 mesh) into food products reduces the overall food quality, while the in-vitro experiments in this study emphasized that finely ground fiber (100 mesh and above) altered the functional properties. Therefore, the 60-mesh particle size of these fibers was considered to be most appropriate for incorporation in the food products and was further studied for its major fractional composition. Significantly (p ≤ 0.05) high amount of NDF and ADF content were recorded in cardamom peel fiber (60.25% and 46.24%). Lignin was minor fraction of DF, amongst all the fiber fractions. The pea peel fiber (3.36%) and cardamom peel fiber (4.78%) showed significantly (p ≤ 0.05) higher lignin content as compared to radish fiber and ashgourd fiber, i.e., 2.03% and 1.32%, respectively. Radish and ashgourd fiber exhibited significantly (p ≤ 0.05) higher percentage of hemicellulose content (24.07% and 19.84%, respectively) as compared to pea peel and cardamom peel fiber (11.62 and 14.01%). The cellulose percentage is dependent on ADF (lignocellulose) and lignin content and was maximum in cardamom peel fiber (41.46%). The pectin content i.e. SDF content was significantly (p ≤ 0.05) high in ashgourd fiber (12.10%) followed by radish fiber (8.81%) and pea peel fiber (3.45%). Cardamom peel fiber exhibited negligible amount of pectin content (0.68%) as it mainly consist of IDF fraction. The results () clearly show that the chemical composition of the fiber samples varied between samples. Goreka et al.[Citation8] have brought out varietal variations in components of lupine flour. However, Heller[Citation32] reported that samples ground finely would yield lower values for total cell wall. A reduction of 14 to 20% of NDF was observed with decrease in particle size (20 mesh to 60 mesh), but particle size had not much effect on cellulose, hemicellulose of peanut hulls, as it is calculated only by difference.
CONCLUSION
The results of this study confirm that the functional characteristics of the dietary fiber from different origin depend on the intrinsic qualities of the fiber such as solubility, viscosity, hydration properties and the pH conditions prevailing in the different sections of gut. Dietary fiber with high pectin, i.e., ashgourd and radish fiber, plays its role mainly at stomach pH (1.8), where the hydration capacity of these fibers was maximum. This may lead to increased viscosity in the stomach and slow down the gastric emptying. While the cardamom peel and pea peel fibers, containing more of insoluble components (cellulose, hemicellulose and lignin) exhibited their action at duodenum pH (8.7), where it binds more water and helps in fecal bulking. The particle size has a greater influence on the functional properties of fiber as well as on the characteristics of the products prepared. The hydration properties and glucose adsorption capacity were found to be significantly (p ≤ 0.05) higher in 30 and 60 mesh in the fiber samples as compared to 100-mesh particle size. The hydration capacity and the CEC were related to fiber source, particle size and the environmental conditions (pH, time). The functional properties of DF are thus, strongly related to the variation in its physical and chemical environment. This study demonstrated that these DF's will certainly have an impact on food, by reducing the rate of glucose breakdown and absorption hence avoiding an abrupt increase in blood glucose level and facilitating the steady breakdown of carbohydrates and release of glucose. The four fibers showed an excellent performance in relation to functional properties and hypoglycemic effect, thus these fibers can be incorporated as low-calorie bulk ingredient in high-fiber foods to reduce calorie level and help control blood glucose concentration.
ACKNOWLEDGMENT
The authors wish to thank Dr. A. S. Bawa, Director, Defence Food Research Laboratory, for his keen interest and encouragement in carrying out the present study.
REFERENCES
- Sreenath , H.K. , Kadambi , R. , Sudarshanakrishna , Prasad, N.N. and Santhanam , K. 1996 . Characteristics of some fibre incorporated cake preparations and their dietary fibre content . Starch/Starke , 48 ( 2 ) : 72 – 76 .
- Sangnark , A. and Noomhorm , A. 2004 . Effect of dietary fibre from sugarcane bagasse and sucrose ester on dough and bread properties . Lebensm.-Wiss. U.-Technol. , 37 : 697 – 704 .
- Valiente , C. , Molla , E. , Martin-Cabrejas , M.M. , Lopez-Andreu , F.J. and Esteban , R.M. 1996 . Cadmium binding capacity of cocoa and isolated total dietary fibre under physiological pH conditions . Journal of the Science of. Food and Agriculture , 72 : 476 – 482 .
- Schneeman , B.O. 1998 . Dietary fibre and GI function . Nutrition Research , 18 : 625 – 632 .
- Fleury , N. and Lahaye , M. 1991 . Chemical and physicochemical characteristics of fibres from Laminaria digitata (Kombu Breton): A Physiological approach . Journal of the Science of. Food and Agriculture , 55 : 389 – 400 .
- Gourgue , C. , Champ , M , Guillon , F. and Laval , J.D. 1994 . Effect of extrusion-cooking on the hypoglycemic properties of citrus fibre: An in vitro study . Journal of the Science of. Food and Agriculture , 64 : 493 – 499 .
- Wricks , K.L. , Robertson , J.B. , Van Soest , P.J. , Lewes , B.A. , Rivers , J.M. , Roe , D.A. and Hackler , L.R. 1983 . The influence of dietary fibre source on human intestinal transit and stool output . Journal of Nutrition , 113 : 1464 – 1479 .
- Gorecka , D. , Lampart-Szczapa , E. , Janitz , W. and Sokolowska , B. 2000 . Composition of fractional properties of dietary fibre of lupines (L.luteus and L. albus) . Nahrung , 44 ( 4 ) : 229 – 232 .
- Sowbhagya , H.B. , Florence Suma , P. , Mahadevamma , S. and Taranathan , R.N. 2007 . Spent residue from cumin—a potential source of dietary fibre . Food Chemistry , 104 : 1220 – 1225 .
- Chau , C.F. , Huang , Y.L. and Lee , M.H. 2003 . In-vitro hypoglycemic effects of different insoluble fibre-rich fractions prepared from the peel of Citrus Sinensis L cv. Liucheng . Journal of Agriculture and Food Chemistry , 51 : 6623 – 6626 .
- Ou , S. , Kwok , K. , Li , Y. and Fu , L. 2001 . In-vitro study of possible role of dietary fiber in lowering postprandial serum glucose . Journal of Agriculture and Food Chemistry , 49 : 1026 – 029 .
- Ranganna , S. 2005 . “ Pectin ” . In Handbook of analysis and quality control for fruits and vegetable products , 2nd , 40 – 42 . New Delhi : Chapter 2. Tata McGraw-Hill Publishing Company Ltd .
- Chau , C.F. and Huang , Y.L. 2004 . Characterization of passion fruit seed fibres—a potential fibre source . Food Chemistry , 85 : 189 – 194 .
- Robertson , J.A. and Eastwood , M.A. 1981 . An examination of factors, which may affect the water holding capacity of dietary fibre . British Journal of Nutrition , 45 : 83 – 88 .
- Sangnark , A. and Noomhorm , A. 2004 . Chemical, physical and baking properties of dietary fibre prepared from rice straw . Food Research International , 37 : 66 – 74 .
- Champ , M. and Guillon , F. 2000 . Structural and physical properties of dietary fibres and consequences of processing on human physiology . Food Research International , 33 : 233 – 245 .
- Michel , F. , Thibault , J.F. and Barry , J.L. 1988 . Preparation and characterization of dietary fibre from sugar beet pulp . Journal of the Science of. Food and Agriculture , 42 : 77 – 85 .
- Goni , I. and Carron , N. M. 1998 . In-vitro fermentation and hydration properties of commercial dietary fiber-rich supplement . Nutrition Research , 18 ( 16 ) : 1077 – 1089 .
- Izydorczyk , M.S. , Chornick , T.L. , Paulley , F.G. , Edward , N.M. and Dexter , J.E. 2008 . Physico-chemical properties of hull-les barley fibre-rich fraction varying in particle size and their potential as functional ingredients in two-layer flat bread . Food Chemistry , 108 : 561 – 570 .
- Sundberg , B. and Aman , P. 1994 . Fractionation of different type of barley by roller milling and sieving , 19 : 179 – 184 .
- McConnell , A.A. , Easewood , M.A. and Mitchell , W.D. 1974 . Physical characteristics of vegetable foodstuffs that could influence bowel function . Journal of the Science of. Food and Agriculture , 25 : 1457 – 1964 .
- Mongeau , R. and Brooks , P.J. 2003 . “ Properties, sources and determination ” . In Encyclopedia of Food Science and Nutrition , 2nd , Edited by: Caballero , B , Turgo , L.Z. and Finglas , P.M. Vol. 3 , 1813 – 1832 . Academic Press .
- Torre , M. , Rodriguez , A.R. and Saura-Calixto , F. 1992 . Study of the interactions of calcium ions with lignin . Journal of Agriculture and Food Chemistry , 40 : 1762 – 1766 .
- Jimenez , A , Rodriguez , R. , Fernandez , I. , Guillen , R. , Bolanos , J.F. and Heredia , A. 2000 . Dietary fibre content of table olives processed under different European styles: study of physico-chemical characteristic . Journal of Agriculture and Food Chemistry , 80 : 903 – 1908 .
- Mc Burney , M.I. , Soest , P.J. and Chase , L.E. 1983 . Cation exchange capacity and buffering capacity of Neutral- detergent fibres . Journal of the Science of. Food and Agriculture , 34 : 910 – 916 .
- Stevonson , A. , Buchanan , C.J. and Eastwood , M.A. 1994 . Does the method of drying a hydrated poly starchy polysaccharide affect in vitro analyses to predict physiological functions . Journal of the Science of. Food and Agriculture , 66 : 111 – 116 .
- Adiotomre , J. , Eastwood , M.A. and Edward , C.A. 1990 . Brydon. Dietary fibre: in vitro methods that anticipate nutrition and metabolic activity in humans . American Journal of Clinical Nutrition , 52 : 128 – 134 .
- Yeh , H.Y. , Su , N.W. and Lee , M.H.. 2005 . Chemical compositions and physico-chemical properties of the fiber-rich materials prepared from Shoyu mash residue . Journal of Agriculture and Food Chemistry , 53 : 436 – 4366 .
- Lopez , G. , Ros , G. , Rincon , F. , Periago , M.J. , Martinez , M.C. and Ortuno , J. 1996 . Relationship between physical and hydration properties of soluble and insoluble fiber of Artichoke . Journal Agriculture and Food Chemistry , 44 : 2773 – 2778 .
- Brennan , C.S. and Samyue , E. 2004 . Evaluation of starch degradation and textural characteristics of dietary fibre enriched biscuits . International Journal of Food Properties , 7 : 647 – 757 .
- Lecumberri , E , Mateos , R. , Izquierdo-Pulido , M. and Ruerez , P . 2007 . Goya, Luis; Bravo, L. Dietary fibre composition, antioxidant capacity and physico-chemical properties of a fibre-rich product form cocoa . Food Chemistry , 104 : 948 – 954 .
- Heller , S.N. , Rivers , J.M. and Hackler , L.R. 1977 . Dietary fibre: The effect of particle size and pH on its measurement . Journal of Food Science , 42 ( 2 ) : 436 – 439 .