Abstract
The thermophysical properties of reconstituted whole milk, skim milk, and whey were measured at various temperatures and concentrations. A new method for the simultaneous determination of thermophysical properties using a modified version of current probe theory method was proposed. Two new correction coefficients were introduced, and using the values of these coefficients and an approximate solution of the heat conduction equation, the thermal conductivity and thermal diffusivity of each sample were determined. The specific heat of each sample was estimated from the definition of thermal diffusivity. These properties were expressed as a function of concentration and temperature.
Nomenclature
| β | = |
Constant, |
| γ | = |
Euler's constant, 0.5772 (−) |
| Δθ | = |
Probe temperature difference (°C) |
| κ | = |
Thermal diffusivity (m2 s−1) |
| λ | = |
Thermal conductivity (W m−1 °C−1) |
| ρ | = |
Density of the sample (kg m−3) |
| cp | = |
Specific heat (kJ kg−1 °C−1) |
| C | = |
Total solid content (%) |
| E | = |
Voltage (V) |
| I | = |
Current, 0.130 (A) |
| Ic | = |
Correction coefficient, −0.3711 (°C) |
| N | = |
Number of data set |
| Pi | = |
Calculated value |
| Pc | = |
Correction coefficient, 8.666 (m−1) |
| q | = |
Heat quantity per unit length of the probe and unit time (W m−1) |
| q′ | = |
Heat quantity of the probe per unit time (W), |
| R | = |
Resistance, 14.8 (Ω) |
| r | = |
Outer diameter of the probe, 0.0005 (m) |
| t | = |
Elapsed time (s) |
| T | = |
Temperature (°C) |
| Yi | = |
Measured value |
| Y | = |
Average value of the measured data |
| A | = |
Constant (°C) |
| B | = |
Constant (°C) |
| F | = |
Constant (°C) |
| G | = |
Constant (°C) |
| a 1 | = |
Constant (W m−1 °C−1%−1) |
| b 1 | = |
Constant (W m−1 °C−2) |
| d 1 | = |
Constant (W m−1 °C−1) |
| a 2 | = |
Constant (m2 s−1%−1) |
| b 2 | = |
Constant (m2 s−1) |
| a 3 | = |
Constant (kJ kg−1 °C−1%−1) |
| b 3 | = |
Constant (kJ kg−1 °C−2) |
| d 3 | = |
Constant (kJ kg−1 °C−1) |
INTRODUCTION
Thermal treatments such as pasteurization, concentration, drying, and cooling are frequently used in food processing, transportation, storage, and cooking. Knowledge of the thermophysical properties of foods is thus important not only for process design but also for the prediction and control of various changes that occur in food during thermal processing. The thermophysical properties of foods, such as thermal conductivity, thermal diffusivity, and specific heat, have been reviewed by Singh,[Citation1] Sweat,[Citation2] Saravacos and Maroulis,[Citation3] and others.
Thermal conductivities of some foods, such as pumpkin seed,[Citation4] dairy products and margarines,[Citation5] milk,[Citation6] and apple juice[Citation7] have been measured by a transient line source technique or probe method. In those reports, only the thermal conductivity of the material was determined from the temperature change in the line heat source or probe. We measured the thermal conductivity of powdered food[Citation8] by a twin probe method. The twin probe method is a relative probe measurement. In the twin probe method, the temperature changes of the probes inserted in the reference material (for example, water) and sample are simultaneously measured, and the thermal conductivity of the sample is calculated by multiplying the thermal conductivity of the reference material by the ratio of the temperature rises of the two probes. The twin probe method can measure thermal conductivity with a high accuracy.[Citation8,Citation9] However, this method can only measure the thermal conductivity, and it has some disadvantages; for example, two probes, and two amplifiers are needed, which increases the cost of the apparatus. Some researchers have reported the simultaneous determination of thermophysical properties such as thermal conductivity and thermal diffusivity using the probe method. Choi and Okos[Citation10] measured the thermal conductivity and thermal diffusivity of tomato juice concentrates by Nix's method. The effective thermal conductivities, effective thermal diffusivities, and specific heats of beans[Citation11] were predicted simultaneously from probe temperature changes by using Blackwell's equation. These estimation methods are useful because some thermophysical properties can be simultaneously determined from the temperature change data. However, one disadvantage of these methods is that they require complex calculations.
The thermal conductivities of milk were measured by a steady-state, parallel-plate method[Citation12] or line heat-source method.[Citation6] Oguntunde and Akintoye[Citation13] measured the specific heat of cow's milk. Mattar et al.[Citation14] reported the empirical models for the thermal conductivity, specific heat, and density of milk. There has been no report that the thermal conductivity, thermal diffusivity, and specific heat of milk were simultaneously measured under the same condition. In this study, the thermophysical properties (thermal conductivity, thermal diffusivity, and specific heat) of milk were measured under same conditions (total solid content levels and temperatures). Here, we proposed a new method for the simultaneous determination of thermophysical properties using a modified version of current probe theory method, and it easily determined the relevant thermophysical properties with sufficient accuracy. The objectives of this study were: (1) to develop a new method for the simultaneous determination of the thermophysical properties using the modified probe method; (2) to confirm the validity of this estimation method, and to carry out the simultaneous estimation of three kinds of thermophysical properties; (3) to represent these properties of the samples as a function of temperature and concentration.
MATERIALS AND METHODS
Samples
Three kinds of liquid foods adjusted to various total solid content levels were used in this study, i.e., whole milk (total solid content levels of 10, 20, 30, and 40%), skim milk (10, 20, 30, and 40%), and whey (10, 20, and 30%). Different solid content levels were obtained by dissolving commercially available whole milk powder (Snow Brand Milk Products Co., Ltd., Tokyo, Japan), skim milk powder (Snow Brand Milk Products Co., Ltd. Tokyo, Japan), and whey powder (Snow Brand Milk Products Co., Ltd., Tokyo, Japan) with distilled water. The moisture content of the powder was determined using the oven drying method in which 2 g of the powder was dried under forced hot air oven (Yamato Scientific Co., Ltd., DX-600, Tokyo, Japan) at 100°C for 5 h.[Citation15] Based on the results of these moisture content measurements, the solid content levels of the sample solutions were adjusted. To obtain the desired solid content levels of the sample solutions, the distilled water of a predetermined quantity was added to about 50 g of the powder, and the powder was dissolved by using a stirrer (AS ONE Co., Ltd., SM-101, Osaka, Japan) at 1500 rpm for 10 min. In this study, natural convection was avoided by using 1% agar to gel the sample solutions. Because the 40% whey solution could not gel, the thermophysical properties of 10–30% whey solutions were measured. The compositions of these sample solutions were calculated from the standard table of food compositions in Japan,[Citation16] and given in .
Table 1 Compositions (mass fraction) of each component for each sample
Measurement Apparatus
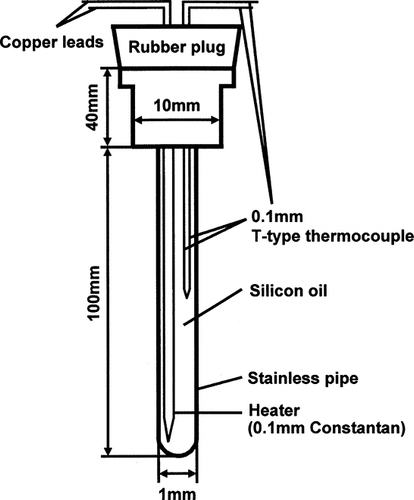
shows a schematic of the heat probe (Tokyo Riko Co., Ltd., Tokyo, Japan) used in this study. The probe consisted of a constantan heater wire (0.1 mm in diameter, resistance of 14.8 Ω) with a small temperature coefficient of electrical resistance, and a T-type thermocouple (0.1-mm-diameter) in a stainless steel tube (1-mm-diameter and 100-mm-length). To avoid contact between the heater wire and thermocouple, the space inside the stainless steel tube was filled with silicone oil.
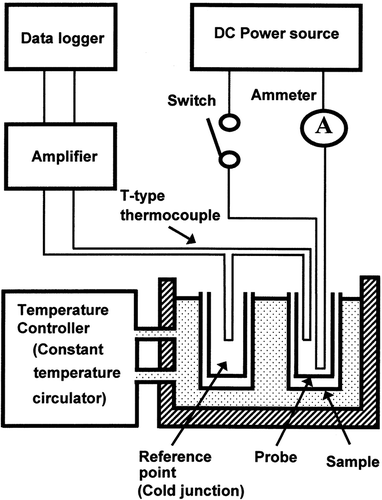
presents a diagram of the thermophysical property measurement apparatus. The apparatus consisted of three units: heating, temperature control, and recording. The heating unit consisted of the probe, a DC power source (Metronix Co., Ltd., model 526, Tokyo, Japan), a DC ammeter (Yokogawa Electric Co., Ltd., model 2012, Tokyo, Japan), and a switch. A circulating water bath (Tokyo Riko Co., Ltd., TC-100, Tokyo, Japan) with a temperature control accuracy of 0.004°C was used to hold the temperature constant throughout the tests. The temperature change in the heat source (probe), i.e., the change in the thermo-electromotive force, was amplified with an OP-amplifier (Tokyo Riko Co., Ltd., CA-25F, Tokyo, Japan) and recorded with a data logger (Advantest Co., Ltd., R7326B, Tokyo, Japan). Because of the high thermal conductivity requirement, the cylindrical sample container and reference point container were made of copper plate (0.3-mm thickness). These containers were 30 mm in diameter and 150 mm in height. The reference point container was filled with 1% agar gel.
Measurement of Thermophysical Properties
The thermophysical properties of the samples were measured with the modified transient heat-flow probe method at five different temperatures (10, 20, 30, 40, and 50°C) for each concentration of each sample. The measurement condition was selected in consideration of the treatment for cooling, vacuum evaporation, cream separation, and spray drying of milk. After the water temperature in the water bath reached a preset temperature, the probe was inserted into the center of the sample. The sample container was submerged in the water bath; thus, the temperatures of the sample and reference-point thermocouple (cold junction) were precisely controlled. When the change in thermo-electromotive force of the thermocouple in the sample was less than 0.4 μ V, it was assumed that the temperatures had reached the preset temperature, i.e., they were in thermal equilibrium. Approximately 90 min were necessary to achieve this state of equilibrium. After the thermal equilibrium state had been established, the probe heater wire was energized, and the temperatures of the probe increased with time. The probe temperature changes were amplified and recorded with the data logger. In this study, the input and output range of the amplifier were adjusted to 100 μ V and 10 m V, respectively, and the current applied to the heater wire in the probe was adjusted to 130 m A to raise the temperature of the probe to about 2°C. Current was supplied to the heater wire of the probe for 180 s. In this study, the measurements of thermophysical properties were replicated five times for each experimental condition.
Estimation Method of Thermophysical Properties
When the sample is held at constant temperature, constant heat is continuously supplied to the heater wire in the probe inserted in the sample, and the elapsed heating time is long enough, the temperature change of the probe is approximately expressed as follows[Citation17]:
EquationEquation (1) shows that when the elapsed heating time is long enough, the relationship between the probe temperature difference and the logarithm of elapsed time is approximately linear, and the slope and intercept of this straight line are A and B, respectively. Many researchers have measured the thermal conductivities of various foods by utilizing values of A. If the relationship between the probe temperature difference and the time is expressed by EquationEq. (1) with a high accuracy, then thermal conductivity and thermal diffusivity can be calculated from EquationEqs. (2) and Equation(3). The thermal conductivity and thermal diffusivity of the 1% agar gel were considered equal those of water, as the gel contained 99% water, and these parameters were determined by using EquationEqs. (2) and Equation(3) at temperatures of 10–50°C, and the estimated results were compared with values for water in the literature.[Citation1] In this case, the measured probe temperature changes in 30–180 s were fitted by a linear least squares method to EquationEq. (1), and the values of the parameters A and B were determined. Regression analysis of the measured data was carried out by the Microsoft Excel 2003. The two statistical parameters, i.e., the root mean squared error (RMSE) and the coefficient of determination (R2) were used to evaluate the accuracy of the fitting process. These values were calculated from the following equations by using the Microsoft Excel 2003:
The values of RMSE and R2 in EquationEq. (1) were about 1.0×10−3°C−1 and 0.9999 (−), respectively. The measured results agreed well with the calculated values. The thermal conductivity and thermal diffusivity of water were estimated from EquationEqs. (2) and Equation(3) using the values of parameters A and B. Because the length of the probe was 100 mm, the heat quantity per unit length of the probe and unit time in EquationEq. (2) (q) was (W/m). Where, q′ is the heat quantity of the probe per unit time (W), and
. As a result, the thermal conductivity of water estimated from EquationEq. (2) was 10–15% higher than the literature value. Under some conditions, the thermal diffusivity of water calculated from EquationEq. (3) was a negative value, or 50% smaller than the literature value. Therefore, even if the measured results agreed well with the values calculated from EquationEq. (1), it is not suitable for determining the thermal conductivity and diffusivity from EquationEqs. (2) and Equation(3). Although the length of the probe (length of the stainless pipe) was 100 mm, the heater wire was folded inside the stainless pipe, so the length of the heater wire was unknown. In addition, the values of A and B vary depending on the heat quantity of the probe, the heating time (supply time for the heat quantity), the range of the probe temperature change when fitted to EquationEq. (1) by the linear least squares method, and so on.
This study introduced two new correction coefficients (Pc and Ic) to easily and accurately determine the thermophysical properties. Pc is the correction coefficient for the length of the heater wire in the probe, i.e., heat quantity per unit length of the probe, and q is rearranged: . Ic is the correction coefficient for the method of supplying the heat quantity to the heater wire in the probe (current, voltage, supply time of heat quantity), the data range of the probe temperature change when fitted to EquationEq. (1), and the error of measured temperature. In this study, these coefficients were introduced, EquationEqs. (1)
Equation–Equation(3) were modified to following equations:
The correction coefficients (Pc and Ic) were determined using the measured results for the 1% agar gel at temperatures of 10–50°C. The method of supplying the heat quantity to the heater wire in the probe and the range of probe temperature changes when fitted to EquationEq. (6) were the same as those used for measuring the thermophysical properties of the sample. The values of the parameters F and G were determined by a linear least squares method. The known values (the heat quantity of the probe per unit time q 2 = I 3 × R, the outer diameter of the probe r, and the constant β), and the literature values[Citation1] of the thermal conductivity and thermal diffusivity of water were substituted into EquationEqs. (7) and Equation(8), and the values of the correction coefficients Pc and Ic were determined at each temperature. The values of the correction coefficients at temperatures from 10°C to 50°C ranged from 8.3 to 9.0 for Pc and from −0.39 to −0.35 for Ic. These correction coefficients had almost the same value regardless of temperature, remaining nearly constant from 10°C to 50°C. The values of Pc and Ic for the measurement conditions and apparatuses in this study were taken as the mean values of Pc and Ic in the temperature range of 10°C–50°C, i.e., Pc = 8.666, Ic = −0.3711.
To predict the thermophysical properties of the samples, the measured probe temperature changes in 30–180 s for samples were fitted by a linear least squares method to EquationEq. (6), and the values of the parameters F and G were determined. Using the values of F, G, Pc, and Ic, the thermal conductivity and thermal diffusivity of the sample were calculated from EquationEqs. (7) and Equation(8), respectively. The specific heat of the sample was estimated by substituting the thermal conductivity and thermal diffusivity calculated from EquationEqs. (7) and Equation(8) and the density of the sample under the same condition into the following equation for thermal diffusivity:
The values of the sample densities measured with a pycnometer were used in EquationEq. (9). The method for estimating thermophysical properties used in this study was calibrated at 20°C using 20%, 40%, and 60% sucrose solutions and 14%, and 23% sodium chloride solutions. As a result, the relative errors between the measured values and the literature values[Citation1,Citation18,Citation19] were 0–3% for thermal conductivity, 0–6% for thermal diffusivity, and 0–4% for specific heat. The measured values agreed well with the literature values. Therefore, EquationEqs. (6) Equation Equation–Equation(9) and the values of the two correction coefficients (Pc and Ic) were used to determine the thermophysical properties of the samples.
RESULTS AND DISCUSSION
Temperature Change of the Probe
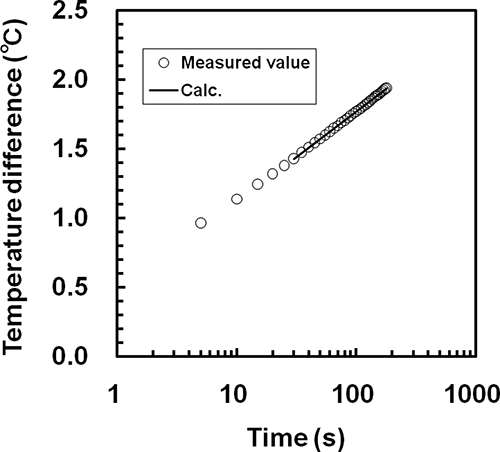
The measured data for the probe temperature changes over 30–180 s at each measurement condition were fitted by a linear least squares method to EquationEq. (6). A comparison between the measured results and the results calculated from EquationEq. (6) at a concentration of 10% and a temperature of 50°C for whole milk is shown in . The solid line and symbols in show the calculated values and the measured results, respectively. In this case, the values of parameters F and G, the RMSE and R2 in EquationEq. (6) were F = 0.2840, G = 0.4604, RMSE = 1.716 × 10−3°C, and R2 = 0.9999 (−). As shown in , the measured data for the temperature changes of the probe over 30–180 s agreed well with the calculated values. Similar results were obtained for all other measurement conditions. Therefore, the values of parameters F and G determined from the probe temperature changes over 30–180 s were used to predict the thermophysical properties of the samples.
Figure 3 Comparison of the observed temperature changes of the probe with the results calculated from EquationEq. (6) for whole milk at a concentration of 10% and a temperature of 50°C.
Thermal Conductivities of the Samples
The values of the thermal conductivity calculated from EquationEq. (7) were 0.43–0.61 W m−1°C−1 for whole milk, 0.47–0.62 W m−1°C−1 for skim milk, and 0.49–0.62 W m−1°C−1 for whey. The measured thermal conductivities of the samples were compared with the literature values.[Citation6,Citation12,Citation13] As a result, there were no significant errors between the measured thermal conductivities of the samples and the literature values under the same conditions. This result demonstrates that the method adopted in this study for estimating thermophysical properties was appropriate.
The thermal conductivities of liquid foods such as milk and fruit juice can be expressed as functions of temperature and concentration.[Citation6,Citation7,Citation20,Citation21] shows the relationships between thermal conductivity and temperature for whey, showing that the thermal conductivity of the whey increased linearly with increasing temperature. Results similar to were obtained for all samples. At the same temperature, the thermal conductivities of all samples decreased as the concentration increased, and a linear relationship existed between thermal conductivity and concentration. Therefore, we derived the following empirical equation to represent the relationship between thermal conductivity, concentration, and temperature:
Figure 4 Comparison of the measured thermal conductivity and the result calculated from EquationEq. (10) for whey.
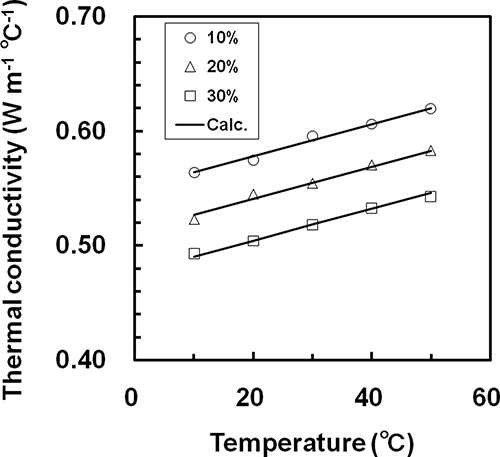
The measured data for each sample were fitted by a linear least squares method to EquationEq. (10). The solid lines in show the calculated results. For all samples, the calculated values agreed well with the measured data. The parameters, RMSE, and R2 of EquationEq. (10) for each sample are shown in . EquationEq. (10) accurately represented the relationships between thermal conductivity and both concentration and temperature for samples.
Table 2 The parameters, coefficient of determination (R2) and root means square (RMSE) of EquationEqs. (10), Equation(11), and (12) for each sample
Thermal Diffusivities of the Samples
The values of thermal diffusivity of the samples obtained from EquationEq. (8) were 1.1–1.6×10−7 m2 s−1. The thermal diffusivities of the orange juice as measured by the Dickerson method[Citation20] were 1.0×10−7−1.4×10−7 m2 s−1 at temperatures from 0.5–62°C and water content levels from 30–75% (total solid contents from 25–70%), and were written as a function of temperature and water content. Comparing the measured data to the literature values for water[Citation1] and orange juice,[Citation20] the measured values were almost the same as the reported values for these liquids. The thermal diffusivities of the samples decreased with increasing concentration. This tendency was also seen in a report by Telis-Romero et al.[Citation20] For each concentration, the thermal diffusivity of each sample was almost constant over temperature. The average values of the measured data at 10–50°C at each concentration and for each sample are plotted in . The thermal diffusivities of the samples decreased linearly with increasing concentration. The values of thermal diffusivity plotted in for each sample were fitted by a linear least squares method to the following equation:
Figure 5 Empirical relationships between the thermal diffusivity and the concentration for each sample.
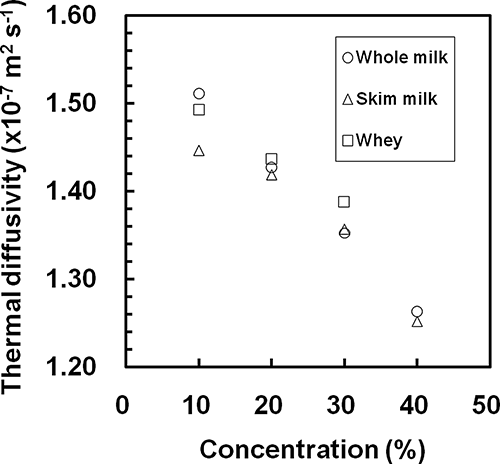
The values of the parameters are given in along with the RMSE and R2 of EquationEq. (11) for each sample. The RMSE and R2 values show that the measured data agreed well with the calculated values. Thus, the thermal diffusivity of each sample was represented by EquationEq. (11) as a function of concentration only within the measurement conditions in this study.
Specific Heats of the Samples
The specific heat of each sample was determined from EquationEq. (9) by substituting the thermal conductivity and thermal diffusivity calculated from EquationEqs. (7) and Equation(8) and the density of the sample under the same condition into EquationEq. (9). There were no significant errors between the measured specific heats of the samples and the literature values[Citation6] under the same conditions. shows the relationships between specific heat and temperature for whole milk. The symbols in show the measured results. The values of the specific heat of whole milk were 3.1–4.0 kJ kg−1°C−1 under the measured conditions, and increased linearly as the temperature rose. Relationships similar to were obtained for all samples. The specific heats of the samples decreased with increasing concentration for each temperature of each sample. Tansakul and Chaisawang[Citation22] reported that the specific heat of coconut milk measured with a DSC was related to temperature and concentration by the following equation:
Figure 6 Empirical relationships the between specific heat and the temperature at four different concentrations for whole milk.
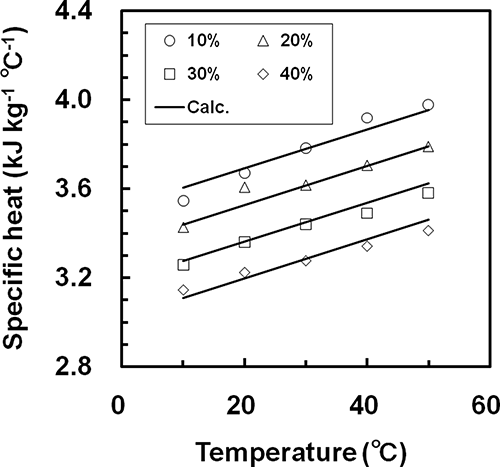
In this study, because both the temperature dependency and the concentration dependency were represented by a linear function, the measured specific heat data for each sample were fitted by a linear least squares method to EquationEq. (12). The solid lines in show the calculated results. As shown in , the calculations agreed well with the measured data. The values of the parameters, RMSE, and R2 of EquationEq. (12) for each sample are shown in . From , it is apparent that the relationships between specific heat and both concentration and temperature for each sample were accurately represented by EquationEq. (12).
In this study, two new correction coefficients were introduced, and the thermal conductivities, thermal diffusivities, and specific heats of samples were simultaneously determined using a modified transient heat-flow probe method, and these values were written as a function of temperature and concentration. The estimation method adopted in this study, expressed as EquationEqs. (6) Equation Equation–Equation(9), is suitable for predicting thermal conductivity, thermal diffusivity, and specific heat with accuracy sufficient for practical use. This estimation method can be easily determined the three kinds of thermophysical properties and applied to various foods. The empirical equations, i.e., EquationEqs. (10) Equation–Equation(12), will be useful in the design of equipment and in calculations for the thermal processing of milk.
CONCLUSION
A new method for the simultaneous determination of thermophysical properties using a modified version of current probe theory method was proposed. Two new correction coefficients were introduced, and using the values of these correction coefficients and an approximate solution of the heat conduction equation, the thermal conductivity and thermal diffusivity of three kinds of milk (whole milk, skim milk, and whey) were measured at various temperatures (10–50°C) and concentrations (10–40%), respectively. The specific heat of each sample was estimated from the definition of thermal diffusivity. The thermal conductivity and specific heat for each sample were represented by the empirical equations shown as a linear function of both concentration and temperature, respectively. The thermal diffusivity of each sample was represented as a linear function of concentration only within the measurement conditions in this study.
REFERENCES
- Singh , R.P. 1992 . “ Heating and Cooling Processes for Foods ” . In Handbook of Food Engineering , Edited by: Heldman , D.R. and Lund , D.B. 247 – 276 . New York : Marcel Dekker Inc .
- Sweat , V.E. 1994 . “ Thermal Properties of Foods ” . In Engineering Properties of Foods , 2nd , Edited by: Rao , M.A. and Rizvi , S.S.H. 99 – 138 . New York : Marcel Dekker Inc .
- Saravacos , G.D. and Maroulis , Z.B. 2001 . “ Thermal conductivity and diffusivity of foods ” . In Transport Properties of Foods , 269 – 358 . New York : Marcel Dekker Inc .
- Kocabiyik , H. , Kayisoglu , B. and Tezer , D. 2009 . Effect of moisture content on thermal properties of pumpkin seed . International Journal of Food Properties. , 12 ( 2 ) : 277 – 285 .
- Sweat , V.E. and Parmelee , C.E. 1979 . Measurement of thermal conductivity of dairy products and margarines . Journal of Food Process Engineering , 2 ( 3 ) : 187 – 194 .
- Ch.S , Reddy and Datta , A.K. 1994 . Thermophysical properties of concentrated reconstituted milk during processing . Journal of Food Engineering , 21 ( 1 ) : 31 – 40 .
- Constenla , D.T. , Lozano , J.E. and Crapiste , G.H. 1989 . Thermophysical properties of clarified apple juice as a function of concentration and temperature . Journal of Food Science , 54 ( 3 ) : 663 – 668 .
- Muramatsu , Y. , Tagawa , A. and Kasai , T. 2005 . Effective thermal conductivity of rice flour, whole and skim milk powder . Journal of Food Science , 70 ( 4 ) : 279 – 287 .
- Kasubuchi , T. 1977 . Twin transient-state cylindrical-probe method for the determination of the thermal conductivity of soil . Soil Science. , 124 ( 5 ) : 255 – 258 .
- Choi , Y. and Okos , M.R. 1983 . The thermal properties of tomato juice concentrates . Transactions of the ASAE , 26 ( 1 ) : 305 – 311 .
- Tagawa , A. and Murama , S. 1995 . Thermophysical properties of beans (Part 1). Prediction from results measured by transient heat flow method using probe . Journal of Japanese Society of Agricultural Machinery , 57 ( 4 ) : 27 – 35 .
- More , G.R. and Prasad , S. 1988 . Thermal conductivity of concentrated whole milk . Journal of Food Engineering , 10 ( 2 ) : 105 – 112 .
- Oguntunde , A.O. and Akintoye , O.A. 1991 . Measurement and comparison of density, specific heat and viscosity of cow's milk and soymilk . Journal of Food Engineering , 13 ( 3 ) : 221 – 230 .
- Mattar , H.L. , Minim , L.A. , Coimbra , J.S.R. , Minim , V.P.R. , Saraiva , S.H. and Telis-Romero , J. 2004 . Modeling thermal conductivity, specific heat, and density of milk: A neural network approach . International Journal of Food Properties , 7 ( 3 ) : 531 – 539 .
- Yoshitake , M. 1984 . “ Moisture Content of Foods—milk powder ” . In Methods of Food Analysis , 2nd , 73 Tokyo : Korin Co. Ltd . Japanese Society for Food Science and Technology
- Kagawa , Y. 2005 . Standard Table of Food Composition in Japan , 5th Revised and Enlarged , 220 Tokyo : Kagawa Nutrition University Publishing Division .
- Carslaw , H.S. and Jaeger , J.C. 1988 . “ The use of sources and sinks in cases of variable temperature ” . In Conduction of heat in solids , 255 – 281 . New York : Oxford Univ. Press .
- Hayashi , H. 1989 . “ Thermophysical properties of food ” . In Food physics , 84 – 125 . Tokyo : Yokendo Co. Ltd .
- The Japan Society of Mechanical Engineers . 1991 . “ Physical property ” . In JSME Data Book: Heat transfer , 4th , 307 – 365 . Tokyo : Maruzen Co. Ltd. .
- Telis-Romero , J. , Telis , V.R.N. , Gabas , A.L. and Yamashita , F. 1998 . Thermophysical properties of Brazilian orange juice as affected by temperature and water content . Journal of Food Engineering , 38 ( 1 ) : 27 – 40 .
- Zainal , B.S. , Abdul Rahman , R. , Ariff , A.B. , Saari , B.N. and Asbi , B.A. 2000 . Effects of temperature on the physical properties of pink guava juice at two different concentrations . Journal of Food Engineering , 43 ( 1 ) : 55 – 59 .
- Tansakul , A. and Chaisawang , P. 2006 . Thermophysical properties of coconut milk . Journal of Food Engineering , 73 ( 3 ) : 276 – 280 .