Abstract
This study evaluates the antioxidant potential of selected plant species by assessing three parameters: antioxidant inhibition of hydrogen peroxide induced hemolysis, free radical scavenging activity and presence of superoxide dismutase activity. Protection against hemolysis was assessed by the rabbit erythrocyte hemolysis assay while free radical scavenging activity was estimated spectophotometrically using 2,2–diphenyl-1-picrylhydrazil as the scavenger. Superoxide dismutase was assessed by measuring the inhibition by superoxide dismutase on the reaction of xanthine oxide generated superoxide radical with a tetrazolium salt. The best protection against hydrogen peroxide induced hemolysis was by extracts of lemon grass (Cymbopogon citratus) leaves. Hempedu bumi (Andrographis paniculata), garlic (Allium sativum) and turmeric (Curcuma domestica) also showed significant anti-hemolytic effect. Good free radical scavenging activity was shown by raw and fermented bean (Phaseolus vulgaris), hempedu bumi (Andrographis paniculata) and ulam raja (Cosmos caudatus). Ulam raja (Cosmos caudatus), lemon grass, hempedu bumi and turmeric showed significant superoxide dismutase activity. The antioxidant potential of Andrographis paniculata was good as evident by the three parameters assayed. However, neem (Azadirachta indica) has limited antioxidant potential. Plants with good antioxidant capabilities include lemon grass and garlic.
INTRODUCTION
Hydrogen peroxide, superoxide, hydroxyl, peroxyl, peroxynitrite, nitric oxide, and the alkoxyl radicals are some of the endogenous free radicals, which are generated in normal and pathological cell metabolism. These oxidants can cause extensive damages to body cells by nucleic acid and protein oxidation as well as lipid peroxidation.Citation[1] The oxidative damage can have a strong relationship with diseases such as cancers, atherosclerosis, Alzheimer's disease and Parkinson's disease.Citation[2,Citation3]
Although natural antioxidant enzymes are responsible for removing free radicals, any interruption in their function, caused by environmental stresses or a disease, can potentially increase the risk of oxidative damage in cells. Furthermore, an increase in the concentration of the oxidizing species can cause oxidative stress in the body and natural antioxidants may not adequately neutralize free radicals. Hence, consuming a diet high in antioxidants is helpful in order to reduce the harmful effects of oxidative stress.Citation[4]
Components with antioxidant activity present in plants, such as ascorbic acid (vitamin C), tocopherols (vitamin E), carotenoids and several phenolic compounds, caused vegetables were proposed as good sources of antioxidants by some researchers.[Citation5–8] According to these studies some plants show a high antioxidant activity and therefore can be used in foods and pharmaceutical preparations as suitable sources of free radical scavenging compounds.
There are various model systems to determine the antioxidant potential of different samples. However, due to the complex composition of antioxidant compounds, applications of different methods of evaluation for the antioxidant capacity are based on different features of the antioxidant activities.Citation[9] In the present study, three different in vitro assay methods were used to evaluate the antioxidative activity of selected raw plant and fermented extracts.
The first group of plants examined in this study included five local (Malaysian) plants. Four of them, Oenanthe javanica, Cosmos caudatus, Centella asiatica, and Ocimum basilicum, indigenously called ULAMs, are commonly consumed raw as vegetables in Malaysia. The fifth is a local Malaysian plant, Euodia redlevi, whose leaves are eaten raw. The second group contained plants, which are known and reported as good sources of antioxidant compounds. These were Andrographis paniculata,Citation[10,Citation11] Azadirachta indica,Citation[12,Citation13] Carica papaya,Citation[14,Citation15] Cymbopogon citratus,Citation[16,Citation17] Curcuma domestica,Citation[18,Citation19] and Allium sativum.Citation[20,Citation21]
There were two main reasons to evaluate the antioxidant activity of the second group of selected plants. Firstly, based on our knowledge, none of the previous studies utilized all three methods of assessing antioxidative activity, which were used in this research. The current results could then be readily compared with previous reports. Secondly, these plants could be a convenient reference point to evaluate the first group of plants for their antioxidant potential. Although there are some studies on antioxidant activity of the ULAMs, they use different plant species, utilize different extraction methods and/or antioxidant evaluation assays.[Citation22–28
MATERIAL AND METHODS
Chemicals
Hydrogen peroxide (H2O2) was obtained from UNI-CHEM® (Belgrade). 2,2 –diphenyl-1-picrylhydrazil (DPPH*), tert-butylated hydroxytoluene (BHT), and ascorbic acid (vitamin C) were purchased from Sigma-Aldrich (St. Louis) while other chemicals were of the highest analytical grade available.
Sample Preparation and Extraction
a) Plant Samples
The botanically identified plant species were purchased from Malaysian Agriculture, Horticulture and Agrotourism (MAHA) Exhibition, August 2008, Selangor, Malaysia. The scientific and common names of plants and the part used are shown in . The selected part of plants (10–20 g) were cleaned with water and air-dried at room temperature in the dark until its water content was below 10%. Fine homogenous powders were prepared by grinding 3 g of each sample using an electrical blender (Super Blender National®, Tokyo). The fine plant powder was soaked in 95% ethanol for 72 hours in the dark. Then it was filtered and the filtrate was evaporated to dryness using a rotary evaporator at temperature below 40°C. A stock solution (100 mg of ethanol extract /ml of 5% (v/v) Tween-80 in PBS) was prepared from the plants and kept at 4°C until required for experiments. Prior to experimentation, this stock solution was diluted 10 fold in PBS. Neither PBS nor Tween-80 (at a concentration of 0.5%) had any effect on our antioxidant assays.
Table 1 Selected plants and the parts used in this study
b) Fermented Samples
Dried raw bean and lactobacillus fermented bean () were ground using the electrical blender and ethanolic extracts of them were prepared using the same procedure as above.
Table 2 Dried raw bean and fermented bean used in the study
Assay Methods
Rabbit Erythrocytes Hemolysis
a) Blood Sampling
Healthy normal New Zealand white rabbits were selected for blood sampling. The fur on the ear proximal to the marginal vein was removed, and the skin was sterilized with 95% ethanol. The ear was dilated by rapid and gentle massage. A needle (26G × 1/2” TERUMO®, Belgium) was carefully inserted into the vein and blood slowly withdrawn using a 5 ml disposable syringe. The collected blood was quickly aspirated into Silicone Coated Blood Collection Tube (Vacutainer®, Becton Dickinson, Franklin Lakes) to avoid the formation of blood clots.
b) Preparation of Erythrocyte Suspension
Rabbit erythrocytes were isolated by centrifugation of collected blood at 1000x g at 4°C for 20 min. After removal of the buffy coat and plasma, the cells were washed three times with isotonic phosphate buffer (IPB), pH 7.4. The final suspension of erythrocytes was prepared by adding an equal volume of IPB to the washed erythrocytes.
c) Erythrocytes Pretreatment Procedure
Before induction of oxidative stress by hydrogen peroxide (H2O2), 500 μl of erythrocyte suspension was pretreated with 1 ml of sample (equivalent to 10 mg of ethanolic extract) for 40 minutes. 500 μl of erythrocyte suspension was pretreated with vitamin C (1 ml of a 10 mg/ml solution in PBS) as the positive control.
d) Oxidative Stress Induction and Hemolysis Assay
Oxidative stress was induced in the pretreated erythrocyte suspension by using H2O2. The volume of pretreated erythrocyte suspension adjusted to 9 ml by adding IPB, then 1 ml of 10 mM H2O2 was mixed up and the suspension was gently shaken. Oxidative stress was induced for a non-pretreated erythrocyte suspension as well to be represented as the negative control. The released hemoglobin into the supernatant of the mixture was measured in a spectrophotometer at 540 nm after 150 min inoculation of induced samples at 37°C. Erythrocyte hemolysis in pure water was considered complete erythrocytes hemolysis (100%) while hemolysis of the pretreated erythrocytes was expressed as a percentage of this value.
Free Radical Scavenging Activity
The free radical scavenging capacities of samples were measured by using 2,2 –diphenyl-1-picrylhydrazil (DPPH*). Briefly, 50 μl of each sample (10 mg of ethanolic extract/ml of PBS) was added to 950 μl of 90 μM DPPH* solution and made to the final volume of 4 ml with 95% ethanol. The mixtures were vigorously shaken and incubated for 2 hours at room temperature in the dark before their absorbance were measured at 515 nm. Tert-butylated hydroxytoluene (BHT) at a concentration of 10 mg/ml of PBS was used as the positive control. The capability of samples to scavenge DPPH* was calculated using the following equation:
Superoxide Dismutase (SOD) Activity Assay
SOD activity was determined using the SOD Assay Kit-WST (Dojindo Molecular Technologies, Gaithersburg). The samples (20 μl of a stock solution of 10 mg of ethanolic extract/ml of PBS) and reaction mixtures from the kit were mixed according to the technical manual. The absorbance of the mixtures was determined at 450 nm using a microplate reader after 20 min incubation at 37°C to measure the inhibition activity of SOD (inhibition rate expressed as% inhibition) on the reaction of xanthine oxide generated superoxide with a tetrazolim salt. Ascorbic acid (10 mg/ml in PBS) was used as the positive control in this experiment.
Experimental Design and Statistical analysis
For all assays, the experiments were arranged in a completely randomized design (CRD) with three replicates. The data were analyzed using one-way analysis of variance (ANOVA) (p < 0.05) and means separated by Duncan's multiple range tests using the Statistical Analysis System (SAS) program (SAS Institute Inc., Cary).
RESULTS
Hemolysis Assay
Hemolysis is expressed as percentage values based on erythrocytes hemolysis in pure water (100%). shows the results of the rabbit erythrocytes hemolysis assay. A final concentration of 1 mM H2O2 resulted in a mean of 58.9% hemolysis of rabbit erythrocytes. Percentage of hemolysis in all samples pre-treated by extracts, were significantly less than non-pretreated erythrocytes sample (negative control). The highest reduction of hemolysis was observed in erythrocytes pretreated with lemon grass (Cymbopogon citratus) extract (mean hemolysis of 18%). It had significantly higher anti-hemolytic effect compared to the other samples. The next lowest hemolysis percentages after lemon grass pretreated erythrocytes were achieved by hempedu bumi (19.45%), garlic (20.77%), and turmeric (21.45%) pretreatment respectively, although there is no significant difference between these three values. Pretreatment of erythrocytes using fermented extract strongly prevented hemolysis and this effect is not significantly different compared to pretreatment using either hempedu bumi, garlic and turmeric.
Figure 1 Hemolysis of rabbit erythrocytes is expressed as percentage values. The inhibition of hemolysis given in the vertical axis is attributed to pretreatment with 10 mg of ethanolic extract from each of the samples. Vitamin C (10 mg) was used as the positive control and while non-pretreated erythrocytes were used as the negative control. Changes between the samples were analyzed by one-way ANOVA followed by Duncan's Multiple Comparison Test. Sample represented with different letters are significantly different (p < 0.05). Each determination was performed in triplicates.
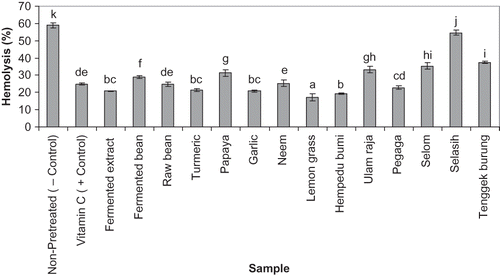
The best reduction of hemolysis by pretreatment among the ULAMs samples was by Pegaga and its effect was significantly different from that of the other ULAMs. The highest hemolysis (mean of 54.54%) was observed in samples pretreated with Selasih (Ocimum Basilicum) although it was significantly lower than non-pretreated samples (mean of 58.9%). Among the samples used, only the fermented extract, turmeric, garlic, lemongrass, Hempedu bumi and Pegaga extracts significantly reduced hemolysis when compared to the positive control (ascorbic acid).
DPPH Radical Scavenging Activity
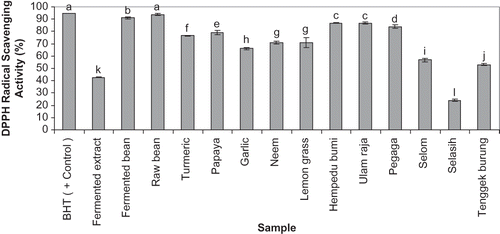
The reaction of antioxidant with the stable free radical DPPH causes its decoloration. This decoloration can be monitored spectrophotometrically. shows the free radical scavenging potential of the samples. All samples had significantly lower free radical scavenging effects compared with the positive control (mean of 94.86%) except raw bean extract (93.69%). Except for Selasih and the fermented extract, the other samples had scavenging effects greater than 50% at the concentration of 10 mg/ml. Among the plant extracts, hempedu bumi (86.87%) and ulam raja (86.85%) had the strongest DPPH* neutralization activity respectively. Selasih (24.09%) significantly showed the lowest free radical scavenging capacity in this study. The results also show that fermented bean significantly has a lower radical scavenging activity than row bean.
Figure 2 Neutralization of DPPH radical of plant and fermented extracts samples (50 μl of a stock solution of 10 mg ethanolic extract per ml of PBS) in the free radical scavenging activity assay. BHT (10 mg/ml) was applied as the positive control. Determinations were performed in triplicates. The mean changes between the samples were analyzed by one-way ANOVA followed by Duncan's Multiple Comparison Test. Samples represented with different letters are significantly different (p < 0.05).
Superoxide Dismutase (SOD) Activity
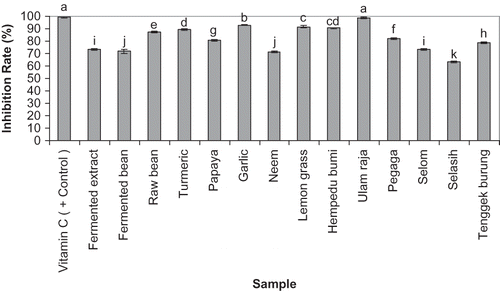
shows the results obtained from our enzyme based antioxidant activity assay. Ulam raja (98.56%) showed the highest SOD activity among the samples examined and it was not significantly different from the inhibition rate of ascorbic acid (99.20%) at a concentration of 10 mg/ml as the positive control. Garlic (92.98%), lemongrass (91.54%), Hempedu bumi (90.41%), and turmeric (89.60%) respectively had the highest SOD activities after ulam raja but all of them significantly were different from the positive control. The SOD activity was significantly higher for the raw bean extract (87.22%) compare with fermented bean extract (71.75%). For fermented extract (73.20%) inhibition rate was not significantly different with Selom extract (73.51%). Not only between ULAMs, also among all experimented samples, Selasih (63.61%) obtained the lowest inhibition rate and it was significantly different with all other samples SOD activity. None of samples in this study could achieve a higher inhibition rate than the positive control (ascorbic acid).
Figure 3 SOD activities of the examined samples (20 μl of a stock solution of 10 mg/ml) are presented as inhibition rate. Ascorbic acid (20 μl of a stock solution of 10 mg/ml) was used as the positive control. Determinations were performed in triplicates. The mean changes between the samples were analyzed by one-way ANOVA followed by Duncan's Multiple Comparison Test. Samples represented with different letters are significantly different (p < 0.05).
DISCUSSION
To avoid using high temperature during the sample preparation process, which may damage some of the antioxidant compounds, ethanol extraction was carried out without allowing temperature to increase beyond 40°C. Plant extracts contain a variety of phytocompounds which are dependent on the solvent used for plant extraction and extraction conditions would therefore have an effect on antioxidant activity evaluation. Aqueous extracts of Eclipta prostrate leaf have been reported to have lower antioxidant activity compare with ethanolic leaf extracts.Citation[29] Núñez et al.Citation[30] proposed that ethanol should be used as a solvent for extraction since it offered the best yield with grape and apple pomaces, pine sawdust, and almond hulls compared with methanol and water.
Vitamin C and tert-butylated hydroxytoluene (BHT) which are established antioxidants were used as positive controls in our experimental model systems. In the first group of plants, ulam raja (Cosmos caudatus) and pegaga (Centella asiatica) in all three assays obtained the highest antioxidant activity ranks when compared for all three assays. Although, the highest reduction of erythrocyte hemolysis observed on pegaga treatment, ulam raja was an effective inhibitor of erythrocyte hemolysis in this group of plants. On the other two assays, DPPH radical scavenging and SOD assays, ulam raja significantly showed the highest antioxidant potential compare to other plants in this group. Pegaga was the second highest of the ULAMs, after ulam raja, which caused the highest antioxidant activity in free radical scavenging and SOD experiments. All three applied assays showed the lowest antioxidant value for selasih compared to the other plants in this group. The moderately high antioxidant activity of the ethanolic extract of pegaga in our study was in agreement with the report by Huda-Faujan et al.Citation[28] who utilized water extracts of this plant in their study. The extremely high antioxidant activity of ulam raja in this study is also in agreement with a previous report by Shui et al.Citation[26]
The results from the erythrocyte hemolysis and SOD assays indicated that lemon grass, hempedu bumi and garlic had the most active antioxidants of the second group of plants. However, the DPPH radical scavenging experiment results were not in agreement with the two other assays in regards to the antioxidant capacity of garlic and lemon grass. All three assays are in agreement that the antioxidant activity of hempedu bumi is one of the highest among the plants in this group. Although there is no agreement between three different antioxidant evaluation assays to point to the plant with the lowest antioxidant activity, neem in all three experiment shows a low antioxidant potential.
All three assays are in agreement that the antioxidant capacity of raw bean extract significantly is higher than the fermented one. Therefore, presumably the fermentation process itself reduced the antioxidant potential of the bean. As shown by the erythrocyte hemolysis assay as well as the SOD assay, our fermented extract has a high antioxidant potential. The fermented extract has an antioxidant activity significantly higher than vitamin C, which was used as the positive control. In addition, the SOD assay results show that there is no significant difference between antioxidant capacity of fermented extract and neem extract.
CONCLUSION
The study has shown that ulam raja (Cosmos caudatus) has the highest antioxidant capacity based on SOD results followed by garlic. This result may justify that the popularity of the plant as an indigenously salad in the local cuisine. It is also evident from this study that fermentation did not enhance but reduced the bioavailability of antioxidants in comparison to the raw unfermented form. So the illusion in oriental practice that fermentation may enhance antioxidant activity may not have strong scientific basis. It is also evident that the different bioassays for antioxidant activity are necessary to draw reliably conclusive results.
ACKNOWLEDGMENT
The authors wish to thank University of Malaya for the provision of facilities to conduct the research and provision of grant number FS257/2008C.
REFERENCES
- Frei , B. 1994 . Natural Antioxidants in Human Health and Disease , 588 San Diego : Academic Press .
- Ames , B.N. , Shigenaga , M.K and Hagen , T.M. 1993 . Oxidants, antioxidant and the degenerative diseases of aging . Proceedings of the National Academy of Sciences , 90 : 7915 – 7922 .
- Giasson , B.I. , Ischiropoulos , H. , Lee , V.M. and Trojanowski , J.Q. 2002 . The relationship between oxidative/nitrative stress and pathological inclusions in Alzheimer's and Parkinson's diseases . Free Radical Biology and Medicine , 32 : 1264 – 1275 .
- Packer , L. , Hiramatsu , M. and Yoshikawa , T. 1999 . Antioxidant Food Supplements in Human Health , San Diego : Academic Press .
- Sellapan , S. , Akoh , C.C. and Krewer , G. 2002 . Phenolic Compounds and Antioxidant Capacity of Georgia-Grown Blueberries and Blackberries . Journal of Agricultural and Food Chemistry , 50 : 2432 – 2438 .
- Teow , C. C. , Truong , V. D. , McFeeters , R. F. , Thompson , R. L. , Pecota , K. V. and Yencho , G. C. 2007 . Antioxidant activities, phenolic and β-carotene contents of sweet potato genotypes with varying flesh colours . Food Chemistry , 103 : 829 – 838 .
- Gungor , N. and Sengul , M. 2008 . Antioxidant activity, total phenolic content and selected physiochemical properties of white mulberry (Morus alba L.) fruits . International Journal of Food Properties , 11 : 44 – 52 .
- Mandic , A. I. , Dilas , S. M. , Cetkovic , G. S. , Anadanovi c-Brunet , J. M. C and Tumbas , V. T. 2008 . Polyphenolic composition and antioxidant activities of grape seed extract . International Journal of Food Properties , 11 : 713 – 726 .
- Wichtl , M. 2001 . Herbal Drugs and Phytopharmaceuticals , 704 Boca Raton, , New York : CRC Press .
- Sheeja , K. , Shihab , P. K. and Kuttan , G. 2006 . Antioxidant and Anti-Inflammatory Activities of the Plant Andrographis Paniculata . Nees. Immunopharmacology and Immunotoxicology , 28 : 129 – 140 .
- Verma , N. and Vinayak , M. 2007 . Antioxidant action of Andrographis paniculata on lymphoma . Molecular Biology Reports , 35 ( 4 ) : 535 – 540 .
- Sithisarn , P. , Supabphol , R. and Gritsanapan , W. 1998 . Antioxidant activity of Siamese neem tree (VP1209) . Journal of Ethnopharmacology , 99 : 109 – 112 .
- Shaharuddin , N.A. , Kawamura , F. , Sulaiman , O. and Hashim , R. Evaluation on antioxidant activity, antifungal activity and total phenolic of selected commercial Malaysian Timbers , Penang, , Malaysia : International Conference on Environmental Research and Technology 28–30th May 2008 .
- Ayoola , G.A. , Coker , H.A.B. , Adesegun , S.A. , Adepoju-Bello , A.A. , Obaweya , K. , Ezennia , E.C. and Atangbayila , T.O. 2008 . Phytochemical screening and antioxidant activities of some selected medicinal plants used for malaria therapy in Southwestern Nigeria . Tropical Journal of Pharmaceutical Research , 7 : 1019 – 1024 .
- Mahattanatawee , K. , Manthey , J. A. , Luzio , G. , Talcott , S. T. , Goodner , K. and Baldwin , E. A. 2006 . Total antioxidant activity and fiber content of select Florida-grown tropical fruits . Journal of Agricultural and Food Chemistry , 54 : 7355 – 7363 .
- Lim , P.L. and Mohamed , S. 1999 . Antioxidative and antimycotic effects of turmeric, lemon-grass, betel leaves, clove, black pepper leaves and Garcinia atriviridis on butter cakes . Journal of the Science of Food and Agriculture , 79 : 1817 – 1822 .
- Ojo , O.O. , Kabutu , F.R. , Bello , M. and Babayo , U. 2006 . Inhibition of paracetamol-induced oxidative stress in rats by extracts of lemongrass (Cymbropogon citratus) and green tea (Camellia sinensis) in rats . African Journal of Biotechnology , 5 : 1227 – 1232 .
- Tilak , J.C. , Banerjee , M. , Mohan , H. and Devasagayam , T.P. 2004 . Antioxidant availability of turmeric in relation to its medicinal and culinary uses . Phytotherapy Research , 18 ( 10 ) : 798 – 804 .
- Jayaprakasha , G.K. , Rao , L.J. and Sakariah , K.K. 2006 . Antioxidant activities of curcumin, demethoxycurcumin and bisdemethoxycurcumin . Food Chemistry , 98 : 720 – 724 .
- Kempaiah , R.K. and Srinivasan , K. 2004 . Influence of dietary curcumin, capsaicin and garlic on the antioxidant status of red blood cells and the liver in high-fat-fed rats . Annals of Nutrition and Metabolism , 48 : 314 – 320 .
- Gorinstein , S. , Leontowicz , H. , Leontowicz , M. , Drzewiecki , J. , Najman , K. , Katrich , E. , Barasch , D. , Yamamoto , K. and Trakhtenberg , S. 2006 . Raw and boiled garlic enhances plasma antioxidant activity and improves plasma lipid metabolism in cholesterol-fed rats . Life Sciences , 78 : 655 – 663 .
- Jayamalar , P. and Suhaila , M. 1998 . Antioxidative activities of Malaysian plants . Malaysian Applied Biology , 27 : 56 – 58 .
- Zin , Z.M. , Abdul Hamid , A. and Osman , A. 2002 . Antioxidative activity of extracts from mengkudu (Morinda citrifolia L.) root, fruit and leaf . Food Chemistry , 78 : 227 – 231 .
- Zainol , M.K. , Abdul-Hamid , A. , Yusof , S. and Muse , R. 2003 . Antioxidative activity and total phenolic compounds of leaf, root and petiole of four accessions of Centella asiatica (L.) Urban . Food Chemistry , 81 : 575 – 581 .
- Noriham , A. , Babji , A.S. and Aminah , A. 2004 . Determination of antioxidative activities of selected Malaysian plant extracts . ASEAN Food Journal , 13 : 193 – 199 .
- Shui , G. , Leong , L. P. and Wong , S. P. 2005 . Rapid screening and characterisation of antioxidants of Cosmos caudatus using liquid chromatography coupled with mass spectrometry . Journal of Chromatography B , 827 : 127 – 138 .
- Abas , F. , Lajis , N. H. , Israf , D. A. , Khozirah , S. and Kalsom , Y. U. 2006 . Antioxidant and Nitric Oxide inhibition activities of selected Malay traditional vegetables . Food Chemistry , 95 : 566 – 573 .
- Huda-Faujan , N. , Noriham , A. , Norrakiah , A.S. and Babji , A.S. 2007 . Antioxidative activities of water extracts of some Malaysian herbs . ASEAN Food Journal , 14 : 61 – 68 .
- Karthikumar , S. , Vigneswari , K. and Jegatheesan , K. 2007 . Screening of antibacterial and antioxidant activities of leaves of Eclipta prostrate (L) . Scientific Research and Essay , 2 ( 4 ) : 101 – 104 .
- Núñez , M.J. , Sineiro , J. and Pinelo , M. 2003 . Profit out of residues from agricultural and forestal industries: optimization of extraction yields. Electronic . Journal of Environmental, Agricultural and Food Chemistry , 2 : 215 – 218 .