Abstract
Polyphenols were extracted from olive cake and then concentrated at 50, 75, and 100°C by the evaporation of extract. Antioxidant activity of total polyphenols in olive cake extract decreased while the concentration of total polyphenols increased during the evaporation of extract at 100°C, but concentration of polyphenols slightly increased while antioxidant activity did not change at 75°C. These results showed that antioxidant activity is not related and estimated with the amount of total polyphenols. Reaction rate for the loss of polyphenols is found zero order at 50 and 75°C with r2 of 0.99 and at 100°C with r2 of 0.85. Low r2 value at 100°C requires the consideration of a different kinetic model to predict the losses of polyhenols at a high temperature of 100°C.
INTRODUCTION
There are several types of stress, and one of them affecting human health is oxidative stress in which reactive oxygen species (ROS) may damage DNA, proteins, and lipids. Antioxidants scavenge ROS and reduce cell proliferation in cancer lines.[Citation1,Citation2] Therefore, antioxidants have recently attracted a considerable interest. Consumption of antioxidants is also suggested for the preventation of cardiocirculatory diseases. Both natural and synthetic antioxidants are commercially available but synthetic antioxidants have an unnatural molecular structure that may turn out to be hazardous. Polyphenols are natural antioxidants present in olive that are consumed as table olive or olive oil. When oil is extracted from the olive, olive cake is left as a waste product. Although polyphenols of olive oil have been studied,[Citation3,Citation4] there are limited studies about polyphenols of olive cake. Polyphenols of olive are consumed via the olive, but they can also be added to food products after being extracted from olive cake as if they can be extracted from other sources.[Citation5–8] Polyphenols can be extracted from olive cake by solvent, and then extracted polyphenols in solvent require concentration. Extracted polyphenols can be concentrated by a thermal process like evaporation and drying, but these processes may affect the amount and activity of polyphenols in the extracts. Even if there are studies about effect of thermal treatments on polyphenols encapsulated in cocoa beans, pearl millet, and oils,[Citation9–11 there is no study showing the effects of a thermal treatment on the polyphenols in water extract of olive cake.
Polyphenols may function in several ways, but their best beneficial property is to be antioxidant. Total polyphenol content of antioxidative agents is measured to estimate antioxidant activity in addition to determine polyphenol content. Polyphenol concentration decreases during a thermal treatment of oil including polyphenol.[Citation3] Amati et al.[Citation4] found both the reduction of antioxidant activity and amount of total polyphenols during a thermal treatment of polyphenols in olive oil at a constant volume. Since both antioxidant activity and amount of total polyphenols decreased, it seems that antioxidant activity of polyphenols depends on amount of a total polyphenol that underwent a thermal treatment. Presence or absence of this dependency can be understood by a thermal treatment of polyphenols at decreasing volume by the evaporation and concentration of polyphenols, and then measuring antioxidant activity of polyphenols. Dependency of antioxidant activity on total polyphenol content determines correctness of antioxidant activity estimation with total polyphenol content.
Modeling a thermal process like evaporation and drying requires knowledge of a kinetic behavior of polyphenol losses, which is not only necessary to model evaporation or drying but is also necessary to predict losses of polyphenols incorporated into food products like snacks undergoing thermal process in the extruder[Citation12] to determine residence time being affected with process parameters.[Citation13,Citation14] A kinetic of polyphenol loss of olive was examined in a limited study in which Campanella et al.[Citation3] used ‘Model fitting and MacCallum method’ for a kinetic analysis of polyphenol losses in olive oil that underwent a thermal treatment at 98°C, but checking reaction order as a zero, first and second order for kinetic of polyphenol loss was not reported. The objectives of this study were to determine polyphenols of olive cake extract; presence of dependancy of antioxidant activity on a total amount of polyphenol; and kinetic parameters like reaction order, rate constant, and activation energy of polyphenol losses in olive cake extract during the evaporation of extract.
MATERIALS AND METHODS
Materials
Olive mill solid waste (OMSW) was received from a three-phase olive oil processing mill in Canakkale, Turkey. OMSW samples were soon transported to our laboratory and conserved in a closed container at 4°C. Folin-Ciocalteu's phenol reagent and caffeic acid were obtained from Fluka (Buchs, Switzerland) while methanol and sodium carbonate anhydrous were obtained from Riedel-de Haen (Seelze, Germany). A chemical agent used for the antioxidant activity test, 2,2 diphenyl-1-picrylhydrazyl (DPPH), was purchased from Fluka (Buchs, Switzerland).
Extraction of Polyphenols from Olive Cake
OMSW was used as olive cake, and polyphenols of olive cake (2.37 g) were extracted with distilled water (112.5 ml) in a shaking water bath (Nüve ST 402, Ankara, Turkey) at ambient temperature (22 ± 2°C). After shaking for 10 min, it was left for 50 min of standing. Upon filtration with filter paper (MN 640 we, Macherey-Nagel, Düren, Germany), coarse particles were separated and the extract phase was obtained. After confirming the presence of polyphenol in the extract phase by a chemical analysis, the extract phase was used for analysis in subsequent experiments.
Thermal Treatment of Polyphenol Extract by Evaporation
Polyphenol extracts were evaporated with a thermal treatment at three different temperatures (50, 75, 100°C) during 105 min. Polyphenol extracts in a graduated cylinder were heated to the defined temperatures described above with a heating element (Ikamag RCT, Staufen, Germany), and extracts were evaporated at that stable temperature for 105 min by stirring. Evaporated samples were labeled as concentrate. The losses in volume of samples during evaporation were measured by the graduated cylinder.
Determination of Total Polyphenols
The total polyphenol content of olive cake extracts was determined by using the Folin-Ciocalteu method.[Citation15,Citation16] Olive cake extract of 0.8 ml was mixed with 0.8 ml methanol, 0.2 ml Folin-Ciocalteu's phenol reagent, and 0.4 ml saturated sodium carbonate solution. Then the volume of mixture was increased to 10 ml with distilled water. After 1 h storage at dark, the mixtures were centrifuged (Elektromag M 4812 P laboratory centrifuge, Istanbul, Turkey) at 1500 rpm for 15 min, and absorbance was measured at 725 nm using a UV/vis spectrophotometer (Lambda 35; PerkinElmer, Shelton, CT, USA). The concentration of total polyphenol was calculated from the calibration curve (r 2 = 0.9969) obtained with a standard solution of caffeic acid, and a total polyphenol content was expressed as caffeic acid equivalents (mg CAE/ml sample). The total solid and polyphenol content of olive cake extract is presented in .
Table 1 Total solid and total polyphenols of olive cake and extract of olive cake
Antioxidant Activity with DPPH Radical Scavenging Method
DPPH radical scavenging activity of olive cake extracts and concentrates were evaluated according to the free radical method[Citation17] with a minor modification. The concentrate of 2.6 ml was added to 3.9 ml of 6 × 10−5 M DPPH solution in methanol. After the samples were well shaken and kept in the dark, the absorbance of samples were measured at 517 nm when the reaction reached a plateau. Methanol was used for zero absorbance in the spectrophotometer and absorbance of the DPPH radical solution without antioxidant was measured daily and used as a control. This solution was kept in the dark at 4°C for subsequent usage of it. All spectrometric data were acquired with the UV/vis spectrophotometer (Lambda 35; PerkinElmer, Shelton, CT, USA) and percentage scavenging of DPPH radical was calculated according to the following equation:[Citation18]
Ac and As is the absorbance of control and sample as the reaction reached a plateau.
Kinetic of Polyphenol Loss
Actual reaction mechanisms and kinetics in food systems are so complex. Therefore, reactions in foods have been reported to generally follow either pseudo zero or first order. Kinetics of poyphenol losses is represented by:
For a first-order reaction, the integration of EquationEq. (2) gives:
For a second order reaction, the integration of EquationEq. (2) gives:
Statistical Analysis
The experiments were arranged by completely randomized design. One-way ANOVA (MS Excel) was used to determine p values and the effect of treatment (time) is accepted to be significant as p < 0.05. While the effect of treatment was analyzed by one-way Anova, the effect of temperature on a related measurement was analyzed by a paired comparison because the effect of temperature was analyzed through a whole process of evaporation, and temperature effect was accepted significant as p < 0.05. On the other hand, kinetic data was analyzed by regression analysis.
RESULTS AND DISCUSSION
Effect of Evaporation on Total Polyphenol Content
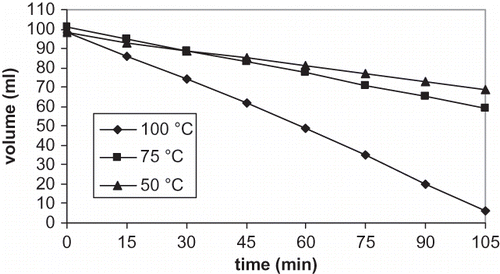
Polyphenols were detected in olive cake extracts, and then extract phases were evaporated so volume of an original extract decreased during a thermal treatment as shown in (p < 0.0005). Evaporation may affect polyphenol concentration by the changes in volume of olive cake extract and a thermal loss of polyphenols in olive cake extract. The amount of polyphenol in samples that underwent evaporation was presented as mg polyphenol per ml of an original extract that was evaporated. Presenting polyphenols of samples in this way eliminated the effect of evaporation on concentration of total polyphenols by the changes in volume during evaporation, and provided the understanding of effect of evaporation temperature on the losses of polyphenols.
The effect of evaporation at 50, 75, and 100°C on concentration of total polyphenols (mg polyphenol per ml original extract) was shown in . There was a decrease in total polyphenols per ml original extract during evaporation (p < 0.0005), but disappearance rate was dependent on temperature. The concentration of total polyphenols as a function of evaporation time does not appear significantly different for temperatures of 50 and 75°C (p < 0.2). A total polyphenol content decreased 23% at the end of 105 min, and the decrease of total polyphenols was smooth up to 105 min at 50 and 75°C. On the other hand, evaporation at 100°C temperature and 105 min decreased total polyphenol content of 65% (p < 0.0005), which is higher polyphenol reduction than polyphenol reduction with evaporation at 50 and 75°C (p < 0.0005). Total polyphenol content decreased smoothly up to 75 min during evaporation and then a sharp decrease was observed up to 105 min at 100°C.
Figure 2 Total polyphenol content of olive cake extracts during evaporation at different temperatures.
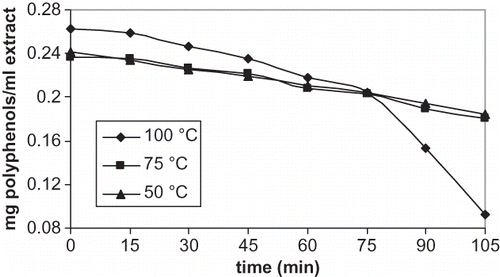
Brenes et al.[Citation11] determined a significant loss of specific polyphenols in olive oil during frying at 180°C, and a minor loss of specific polyphenols with microwave heating for 10 min and boiling with water in a pressure cooker for 30 min. A low percentage of total polyphenols losses in olive cake extract during evaporation in our study showed a similar trend of losses of specific polyphenols in olive oil reported by Brenes et al.[Citation11] when thermal treatment time is less than 75 min. Amati et al.[Citation4] and Campanella et al.[Citation3] also found that total polyphenol content of olive oil decreased slightly up to 20 min but decreased sharply after 20 min at 98°C, and there was a 60% reduction at the end of 60 min at a constant volume. Lower or similar reduction of total polyphenols in this study rather than the reduction of a total polyphenols in the studies of Amati et al.[Citation4] and Campanella et al.[Citation3] shows that there is no evaporation of polyphenols in olive cake extract during the evaporation of extract in the study.
Effect of Evaporation on Total Antioxidant Activity
Phenolic compounds of olive are strong freee radical scavengers. Olive oil fractions are scavenged by a stable radical, such as DPHH.[Citation19,Citation20] Reaction occurs between DPHH and antioxidant (AH) as
The radical (A) may not react further or rereact with another radical to form a stable molecule. Antioxidant activity of olive cake extract may change during the evaporation of olive cake extract, so the effect of evaporation temperature on antioxidant activity was tested by DPHH and was presented as % scavenging of DPPH radical. The volume of extract decreased with time (), and a 3-ml sample was collected at 15 min from concentrate of polyphenol extract during evaporation. Total polyphenols in the concentrate of polyphenol extract were presented as mg polyphenol per ml concentrate to interpret the effect of evaporation on antioxidant activity. The amount of total polyphenol per ml of concentrate did not significantly change with time at 50°C (p < 0.5) as shown in . The increase in amount of total polyphenol with time due to concentration should be supressed with decrease of a total polyphenol with time due to a thermal loss of polyphenols () so that the amount of a total polyphenol per ml of concentrate did not significantly change.
Figure 3 DPPH radical scavenging activity and polyphenol content of concentrates of olive cake extract evaporated at (a) 50°C, (b) 75°C, and (c) 100°C.
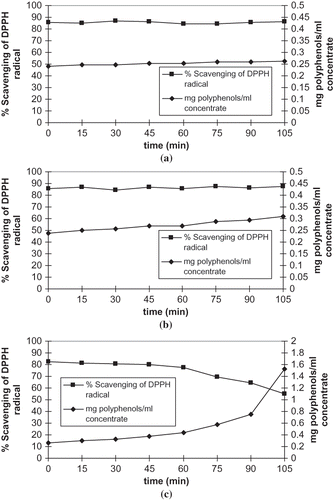
Percentage scavenging of DPPH did not significantly change at 50°C as it did not change at 75°C (p < 0.5) as shown in . On the other hand, there was a slight increase in mg polyphenols per ml concentrate at 75°C (p < 0.005) because % reduction of volume was higher () than % reduction of polyphenol amount during evaporation (). Since polyphenol content of concentrates increased with time, % scavenging of DPPH radical is expected to increase with time but it did not.
The evaporation of olive cake extract at 100 °C resulted in a different effect on % scavenging of DPPH radical. Percentage scavenging of DPPH did not change up to 60 min of evaporation, but it decreased from 60 to 105 min at 100°C as shown in (p < 0.000005). During evaporation, the volume of concentrate decreased, but mg polyphenol per ml concentrate increased (p < 0.005) from 60 to 105 min at 100°C due to the reason explained above. The trend in % scavenging of DPPH radical up to 60 min at an evaporation of 100°C is similar to the trend during an evaporation of 75°C. The amount of polyphenol per ml concentrate increased sharply from 60 to 105 min during an evaporation at 100°C, but % scavenging of DPPH radical decreased sharply. Since the amount of polyphenol per ml of concentrate increased, % scavenging of DPPH radical is expected to increase, but it decreased at 100°C in contrast to being constant at 50 and 75°C.
Valavanidis et al.[Citation19] examined antioxidant capacity by IC 50 values, which is the amount of sample required to lower initial DPPH concentration 50% and found that IC 50 values of several oils increased upon heating samples at 160 and 190°C for 2 h. On the other hand, Roy et al.[Citation21] examined the effect of thermal treatments on antioxidant activity of several vegetables and determined that thermal treatments at 75 and 100°C decreased both phenolic content and anti-DPPH radical activity. Amati et al.[Citation4] also found that both total polyphenol amount and antioxidant activity decreased in olive oil that underwent a thermal treatment at 98°C. It may seem that antioxidant activity depends on amount of total polyphenols that underwent a thermal process, but our study showed that antioxidant activity of polyphenol decreased even if the total polyphenol amount increased (). This result shows that total polyphenol activity does not depend on the amount of a total polyphenol that underwent a thermal treatment. Study of Amati et al.[Citation4] shows higher percentage reduction of antioxidant activity of polyphenols than percentage reduction of the total polyphenol amount. If antioxidant activity depends on the total polyphenol amount, there must be similar percentage reduction of antioxidant activity and amount of a total polyphenol that underwent a thermal treatment. Results of Amati et al.[Citation4] support our finding. Since total polyphenol activity did not depend on amount of a total polyphenol that underwent a thermal treatment (), there is an additional factor affecting antioxidant activity of total polyphenols that underwent a thermal process.
There are several polyphenols, like hydroxytyrosal, tyrosal, and oleuropein, in olive, and Carrasco-Pancorbo et al.[Citation20] detected a higher amount of hydroxytyrosol than tyrosol in olive oil. Not only the amount of these polyphenols but also antioxidant activity of these polyphenols varies. Hydroxytyrosol, oleuropein, and tyrosol have decreasing antioxidant activity, respectively.[Citation19,Citation22] Thermal stabilities of polyphenols also depend on the type of polyphenol.[Citation20] Nissiotis and Tasioula-Margari[Citation23] found that hydroxytyrosol derivatives are first antioxidants that are lost, and tyrosal is the most stable during a thermal treatment. Hydroxytyrosol with greater antioxidant capacity has lower thermal stability, but tyrosol with lower antioxidant activity has greater thermal stability. When a thermal treatment reduces an amount of hydroxytyrosol with greater antioxidant activity more than an amount of tyrosol with lower antioxidant activity, percentage reduction of total antioxidant activity may be higher than percentage reduction of a total amount of polyphenols. Lost polyphenols like hydroxytyrosol have higher antioxidant activity than remaining polyphenols like tyrosol. On the other hand, a total amount of polyphenols may increase with concentration during evaporation due to the concentration of thermally stable polyphenols like tyrosol, while total antioxidant activity may decrease due to the loss of thermally unstable polyphenols like hydroxytyrosol with high antioxidant activity in the case of the concentration of polyphenols. There are several polyphenols, and measured antioxidant activity is total antioxidant activity of total polyphenols. Presence of several polyphenols with varying amounts and a different effect of thermal treatments on antioxidant activity and a thermal stability of polyphenols[Citation19,Citation20,Citation22,Citation23] cause independency of total antioxidant activity on the amount of total polyphenols.
Kinetic Models
Total polyphenol amounts were fitted into a zero-order (concentration against time), a first order (Ln concentration against time), and a second order (1/concentration against time) equations. The examination of correlation coefficient (r 2) shows that total polyphenols losses followed a zero-order reaction kinetics during a thermal treatment at 50 and 75°C (r 2 = 0.99). A zero-order kinetic is also found for the losses of polyphenols at 100°C, but r 2 value was 0.85, which is lower than r 2 values of thermal treatments at 50 and 75°C. The reactions in food are characterized as a zero or first order. Kyi et al.[Citation24] determined enzymatic reactions of polyphenols in cocoa beans as a first order, but Franzen et al.[Citation25] reported that nonenzymatic reactions follow a zero order. Since loss of polyphenols was not an enzymatic reaction, zero order reaction is expected during loss of polyphenols with evaporation.
Rate constants (k) of total polyphenol losses at varying temperatures were calculated from corresponding zero order rate equations and then presented in with r 2 value of 0.99 for 50 and 75°C and r 2 value of 0.85 for 100°C. The results in show that polyphenol loss rate increases with increasing temperature. It should be noted that k is approximately three times greater at 100°C than at 50°C. Since polyphenol loss rates were very low at the low temperatures, it can be concluded that polyphenols were less sensitive to a temperature of 50 and 70°C than a temperature of 100°C. Activation energy (Ea) was calculated from the slope of the graph obtained by plotting ln k versus 1/T (temperature) according to Arrhenius equation. Activation energy was calculated as 19.9 kJ/mol with r 2 value of 0.8 from corresponding a zero order rate constants and corresponding temperatures.
Table 2 Zero order rate constants (k) for losses of polyphenol in olive cake extract evaporated at different temperatures
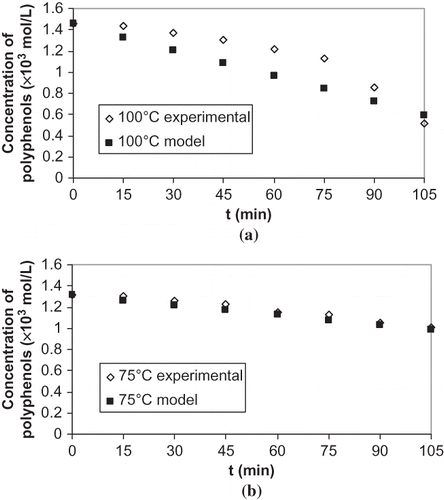
In view of limited kinetic data for the loss of polyphenols, Campanella et al.[Citation3] determined rate constant as 0.07 × 10−3 s−1 and activation energy as 36 kJ/mol by ‘Model-fitting’ method for the loss of polyphenols in olive oil that underwent thermal treatments at 98, 120, 140, 160, and 180°C. Rate constants and activation energy obtained for total polyphenols in the present study are different in comparison to rate constants and activation energy reported by Campanella et al.[Citation3] In this study, unit of rate constant is different and thermal treatments at temperatures between 50 and 100°C were used to calculate activation energy in a contrast to temperatures used between 100 and 180°C in the study of Campanella et al.[Citation3] Kinetics of a thermal loss of polyphenols extracted with water was examined in our study rather than kinetics of thermal losses of polyphenols in oils studied by Campanella et al.[Citation3] A different composition of polyphenol of olive cake extract and polyphenols of olive oil, different temperature range used to determine activation energy, and different methods used to determine rate constants in the study of Campanella et al.[Citation3] can be reasons for different results. Experimental losses were compared with theoretical estimations obtained by a zero-order kinetics (). Estimations were close to the experimental values at evaporation temperature of 50 and 75°C, but there was 20% difference between estimations and experimental values at 100°C.
CONCLUSIONS
Polyphenols were detected in olive cake extract, so olive cake can be used as a source of polyphenol as natural antioxidant. A decrease of total polyphenols concentration at 50 and 75°C was similar but lower than at 100°C during the evaporation of polyphenols in extract of olive cake, so the polyphenols in olive cake extract should be concentrated at 75°C because the losses of polyphenols were lower at 75°C than 100°C, and polyphenol concentration is similar at 50 and 75°C. Antioxidant activity of total polyphenols in olive cake extract decreased while the concentration of total polyphenols increased during the concentration of polyphenols by evaporation of olive cake extract at 100°C, but concentration of polyphenols slightly increased while antioxidant activity did not change at 75°C. These results showed that antioxidant activity is not related and estimated with the amount of total polyphenols. Not only the effect of evaporation temperature on polyphenol content but also the effect of evaporation temperature on antioxidant activity of polyphenol is a critical factor for the determination of evaporation temperature. The examination of correlation coefficient (r 2) shows that total polyphenols losses followed zero-order reaction kinetics during a thermal treatment at 50 and 75°C, but r 2 value for a thermal treatment at 100°C was lower than r 2 values of a thermal treatment at 50 and 75°C. The estimations of polyphenols were also close to experimental values at evaporation temperatures of 50 and 75°C, but there was a 20% difference between estimations and experimental values at 100°C. So different kinetic models should be examined to predict the losses of polyhenols at a high temperature of 100°C.
REFERENCES
- Kampa , M. , Hatzoglou , A. , Notas , G. , Damianaki , A. , Gemetzi , E.C. and Kouroumalis , E. 2000 . Wine antioxidant polyphenols inhibit the proliferation of human prostate cancer cell lines . Nutrition and Cancer , 37 : 223 – 233 .
- Meyer , F. , Galan , P. , Douville , P. , Bairati , I. , Kegle , P. and Bertrais , S. 2005 . Antioxidant, vitamin, and mineral and prostate cancer preventation in the SU.VI.MAX trial . International Journal of Cancer , 116 : 182 – 186 .
- Campanella , L. , Nuccilli , A. , Tomassetti , M. and Vecchio , S. 2008 . Biosensor analysis for the kinetic study of polyphenols deterioration during the forced thermal oxidation of extra-virgin olive oil . Talanta , 74 : 1287 – 1298 .
- Amati , L. , Campanella , L. , Dragone , R. , Nuccilli , A. , Tomassetti , M. and Vecchio , S. 2008 . New investigation of the isothermal oxidation of extra virgin olive oil . Journal of Agricultural and Food Chemistry , 56 : 8287 – 8295 .
- Rahman , M.S. 2007 . Allicin and other functional active components in garlic: Health benefits and bioavailability . International Journal of Food Properties , 10 ( 2 ) : 245 – 268 .
- Rababah , T.M. , Ereifej , K.I. , Al-Mahasneh , M.A. , Ismaeal , K. , Hidar , A.G. and Yang , W. 2008 . Total phenolics, antioxidant activities, and anthocyanins of different grape seed cultivars grown in Jordan . International Journal of Food Properties , 11 ( 2 ) : 472 – 479 .
- Arabshahi-Delouee , S. and Urooj , A. 2007 . Application of phenolic extracts from selected plants in fruit juice . International Journal of Food Properties , 10 ( 3 ) : 479 – 488 .
- Gungor , N. and Sengul , M. 2008 . Antioxidant activity, total phenolic content and selected physicochemical properties of white mulberry (Morus alba L.) fruits . International Journal of Food Properties , 11 ( 1 ) : 44 – 52 .
- Daud , W.R.W. , Talib , M.Z.M. and Kyi , T.M. 2007 . Drying with chemical reaction in cocoa beans . Drying Technology , 25 : 867 – 875 .
- Archana , S.S. and Kawatra , A. 1998 . Reduction of polyphenol and phytic acid content of pearl millet grains by malting and blanching . Plant Foods for Human Nutrition , 53 : 93 – 98 .
- Brenes , M. , Garcia , A. , Dobarganes , M.C. , Velasco , J. and Romero , C. 2002 . Influence of thermal treatments simulating cooking processes on the polyphenol content in virgin olive oil . Journal of Agricultural Food Chemistry , 50 : 5962 – 5967 .
- Altan , A. , McCarthy , K.L. and Maskan , M. 2009 . Effect of extrusion process on antioxidant activity, total phenolics and beta-glucan content of extrudates developed from barley-fruit and vegetable by-products . International Journal of Food Science and Technology , 44 : 1263 – 1271 .
- Seker , M. , Sadikoglu , H. and Hanna , M.A. 2004 . Properties of cross-linked starch produced in a single screw extruder with and without a mixing element . Journal of Food Process Engineering , 27 : 47 – 63 .
- Seker , M. 2005 . Residence time distributions of starch with high moisture content in a single-screw extruder . Journal of Food Engineering , 67 : 317 – 324 .
- Singleton , V.L. , Orthofer , R. and Lamuela-Raventos , R.M. 1999 . Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent . Methods in Enzymology , 229 : 152 – 178 .
- Bubba , M.D. , Checchini , L. , Pifferi , C. , Zanieri , L. and Lepri , L. 2004 . Olive mill wastewater treatment by a pilot-scale subsurface horizontal flow (SSF-h) constructed wetland . Annali di Chimica , 94 : 875 – 887 .
- Brand-Williams , W. , Cuvelier , M.E. and Berset , C. 1995 . Use of a free radical method to evaluate antioxidant activity . Lebensmittel-Wissenschaft und Technologie , 28 : 25 – 30 .
- Yen , G. C. and Duh , P.D. 1994 . Scavenging effect of methanolic extracts of peanut hulls on free-radical and active-oxygen species . Journal of Agriculture and Food Chemistry , 42 : 629 – 632 .
- Valavanidis , A. , Nisiotou , C. , Papageorgıou , Y. , Kremli , I. , Satravelas , N. , Zinieris , N. and Zygalaki , H. 2004 . Comparison of the radical scavenging potential of polar and lipidic fractions of olive oil and other vegetable oils under normal conditions and after thermal treatments . Journal of Agricultural Food Chemistry , 52 : 2358 – 2365 .
- Carrasco-Pancorbo , A. , Cerretani , L. , Bendini , A. , Segura-Carretero , A. , Lercker , G. and Fernandez-Gutierrez , A. 2007 . Evaluation of the influence of thermal oxidation on the phenolic composition and on the antioxidant activity of extra-virgin olive oils . Journal of Agricultural Food Chemistry , 55 : 4771 – 4780 .
- Roy , M.K. , Takenaka , M. , Isobe , S. and Tsushida , T. 2007 . Antioxidant potential, anti-proliferative activities, and phenolic content in water soluble fractions of some commonly consumed vegetables: Effect of thermal treatments . Food Chemistry , 103 : 106 – 114 .
- Chimi , H. , Cillard , J. , Cillard , P. and Rahmani , M. 1991 . Peroxyl and hydroxyl radical scavenging activity of some natural phenolic antioxidants . Journal of the American Oil Chemists’ Society , 68 : 307 – 311 .
- Nissiotis , M. and Tasioula-Margari , M. 2002 . Changes in antioxidant concentration of virgin olive oil during thermal oxidation . Food Chemistry , 77 : 371 – 376 .
- Kyi , T.M. , Daud , W.R.W. , Mohammead , A.B. , Samsudin , M.W. , Kadhum , A.A.H. and Talib , M.Z.M. 2005 . The kinetics of polyphenol degradation during the drying of Malaysian cocoa beans . International Journal of Food Science and Technology , 40 : 323 – 331 .
- Franzen , K. , Singh , R.K. and Okos , M.R. 1990 . Kinetics of nonenzymatic browning in dried skim milk . Journal of Food Engineering , 11 : 225 – 239 .