Abstract
A new innovative method was developed to measure the thermal-relaxation by maintaining isothermal condition in a Differential Scanning Calorimetry (DSC). The DSC thermal-relaxation characteristics of gelatin and date flesh were found to be related with the structural- and mechanical-glass transition measured by conventional DSC linear-heating and thermal-mechanical analysis (i.e, Differential Thermal Mechanical Analysis, DMTA). Initial slope of the thermal relaxation curve (i.e., heat flow versus relaxation time) was determined and it was plotted as a function of relaxation temperature (i.e., isothermal condition). The initial slope-temperature graph showed three thermal relaxation characteristics: limited mobile, freely mobile and restricted mobile regions. The region 1 showed limited molecular mobility with increasing temperature, while region 2 showed free mobility with increasing temperature sensitivity. The intersection of the regions 1 and 2 was related to the structural-glass transition measured by conventional linear-heating in a DSC. The region 3 showed restricted mobility due to enhanced interactions of the phases at higher temperature. This region 3 was related to the entangled flow region measured by thermal-mechanical analysis in a DMTA.
INTRODUCTION
Food materials could be in the amorphous, crystalline, or semi-crystalline states; or in a matrix containing combination of these states. Glasses are not crystalline with a regular structure, but retain the disordered structure similar to the liquid state. Physically it is a solid but more close to thermodynamic liquid. Formation of a glassy state results in a significant arrest of translational molecular motion, and chemical reactions become very slow. The glass transition greatly influences food stability, as the water in the concentrated phase becomes kinetically immobilized and therefore does not support or participate in reactions. The significant applications of the glass transition concept in the 1980s are emerged in food processing when Levine and Slade[Citation1] and Slade and Levine[Citation2] identified its major merits and their wide applications. It assists in identifying food's stability during storage as well as selecting a suitable condition of temperature and moisture content for processing. A complete review on this concept is provided by Rahman.[Citation3–5]
Measurement of glass transition is not straightforward, especially for non-homogeneous complex food materials. Differential Scanning Calorimetry (DSC) is the most common and widely used method for measuring glass transition of foods. Using this method a sample and a reference (generally an empty aluminum pan) are heated at a controlled rate. During a heating cycle, there is a stepwise decrease (i.e., a shift) in the heat capacity that indicates glass transition (onset, Tgi ; mid, Tgp ; end, Tge ) in the thermogram line. The onset of the shift in the thermogram is defined as the structural-glass transition (i.e., Tgi ).
Dynamic mechanical thermal analysis (DMTA) and oscillation analysis are also used frequently to study the mechanical or rheological characteristics over a wide range of temperature. These methods measure the effect of longitudinal and sinusoidal varying stress on dynamic moduli and considered to be more sensitive than DSC. Different structural behavior could be detected by this method and related with the structural-glass transition (i.e., linear-heating DSC, Tgi ). Weakness of this method are the necessity for solid samples, large and uniform enough to be clamped into the machine and difficulty in controlling moisture loss during the experiment.[Citation6,Citation7] Large sample causes more temperature lag between the sample and instrument. Even in pure synthetic polymers, glass transition can be difficult to identify and the use of combinations of instruments, such as DMTA and DSC may be necessary.[Citation8]
Spectroscopic techniques, such as nuclear magnetic resonance (NMR) and electron spin resonance (ESR) are also used since complementary information enabling the mobility of different components within the glass transition could be related with the structural-glass or mechanical-glass measured by DSC or DMTA. Cnossen et al.[Citation9] measured probe displacement of thermal mechanical analyzer (TMA) as a function of temperature and they defined the glass transition when they observed a change in slope of the displacement versus temperature plot. Similarly Boonyai et al.[Citation10] proposed another thermally controlled device, which can be used for static mechanical test. This device was attached to a texture analyzer using static compression technique (creep test). Changes in compression probe distance as a function of temperature were recorded. The glass transition was determined when the changes in the probe distance were observed. However, the low sensitivity of this method at low moisture content could be the main limitation of this method. The glass transition of a low water content sample is usually observed at higher temperature, thus a more lag between the sample and heating plate; and higher loss of moisture could reduce sensitivity. In addition, low displacement of the stiff sample could be another factor.[Citation11] Rahman et al.[Citation12] used glass transition of spaghetti by mechanical (DMTA), thermal (linear-heating DSC and modulated DSC), water diffusion and density methods for a comparison.
In many instances, tracing of glass transition (i.e., a shift in the thermogram line) is difficult to observe by DSC method using conventional linear-heating or modulated protocol. It is important to mention that for complex food systems, it is difficult to find a clear glass transition with thermal analysis since other changes interfere in the complexity of the structural glass. It has been identified in the literature that DSC could not detect easily the glass transition of high molecular weight biological polymers, such as starch and protein systems, especially low moisture. All biological materials did not show conventional DSC glass-rubber transition, and the transition could not be traced by conventional linear-heating DSC procedure. This is generally easy to detect for sugars or maltodextrins, but it becomes increasingly difficult to detect with increasing molecular weight and complexity of the material, as the transition becomes broader, and therefore, it can be spread over about 40°C. It is easier to detect the glass transition in materials which are amorphous, whereas in the case of partial crystalline or other forms of ordering (as exist in native proteins) can contribute to the broadening of the transition.[Citation6] DSC thermograms for chicken muscle showed very low specific heat change at the glass transition, thus the shift in the thermogram line was very difficult to detect.[Citation13] Acevedo et al.[Citation13] indicated that high amount of insoluble proteins and low amount of sugars made it difficult for their experimental determination. In the case of king fish muscle, Sablani et al.[Citation14] detected glass transition within 70–105°C (water content: 3.5 g/100 g sample) followed by melting. However, increasing water showed very little influence on the change in the glass transition temperature, i.e., plasticization effects of water on the protein was very difficult to trace.
In another study, Rahman et al.[Citation15] found similar complexity in the case of date-pits. They also did not trace the glass transition even though oil was separated from the date-pits and performed thermal analysis with more sensitive modulated DSC (MDSC). Sablani and Kasapis[Citation16] measured the glass-rubber transition of freeze-dried shark meat at moisture content 38.0 g/100 g sample using low-amplitude oscillatory shear rheometer. They did not observe expected shift in G′ and G′′ (or cross-over between G′ and G′′) when plotted as a function of temperature rather they observed a change in slope in the plot at −63.5°C . However, they proposed the change in slope in the G′ versus temperature plot as a glass-rubber transition. In addition they explained this characteristic as the formation of tightly packed aggregates of reduced molecular mobility. Green et al.[Citation17] concluded from their results that hydrated proteins indeed may be classed among glass-forming systems, but due to their special structural features and to the disposition of the bound water, great departures from the thermo or rheological simplicity was observed.
Godet et al.[Citation18] prepared highly crystalline amylose-fatty acid complexes with different amylose chain length and measured its characteristics by linear-heating DSC thermogram. They observed only melting endotherm without any trace of glass-rubber transition. Similar results (i.e., no trace of glass-rubber transition) were also observed for amylose-flavor complexes,[Citation19] maize starch-sugar extruded products,[Citation20] wheat, maize, soya, nuts, almonds, maize starch, amylose, amylopectin, and cellulose,[Citation21] and wheat starch.[Citation22] Original pectin was a crystalline polymer, and Iijima et al.[Citation23] also did not trace glass transition by linear-heating DSC method when pectin was heated from 0 to 180°C. However, once the crystalline sample was melted by heating up to 180°C and forming amorphous state. Amorphous pectin after melting did not crystallize by slow cooling or by annealing and then glass transition was observed at 37°C when heating second time from 0 to 180°C . In the case of pectin containing freezable water, glass transition was observed as Tg ′ (i.e., a shift in the thermogram before the ice melting endotherm).
Kim et al.[Citation24] did not trace the glass-rubber transition for un-milled potato starch, but ball milled sample showed a shift in the thermogram line indicating the formation of amorphous structure. They observed an endothermic peak much below the glass-rubber transition and suggested that this peak was related to the recovery of enthalpy relaxation or structure in the amorphous portion of the native starch, which had occurred during starch isolation and/or storage. They also proposed two possible interpretations: (1) impact of ball-mill would act as activation energy to rearrange the molecular arrangement toward equilibrium, in a way similar to heating for aging or annealing; and (2) de-polymerization may cause such acceleration. This is due to the nature of native starch at molecular and micro structural levels and small change in the heat capacity during the transition.[Citation25,Citation26] The glass transition is a kinetically time-dependent transition, thus the transition temperature depends on the heating rate used by linear-heating DSC to measure it. All foods do not transform into similar characteristics of glassy material, thus many instances it was difficult to trace by linear-heating DSC method.
No evidence of a glass-rubber transition was observed for cellulose by conventional linear-heating DSC method.[Citation27] However, different degree of water mobility was observed by 1H and 2H NMR. They observed three types of mobile water: region 1, immobile (0–0.027 g/g sample); region 2, moderately mobile (0.027–0.083 g/g sample); and region 3, highly mobile (>0.083 g/g sample). These data indicated that structural mobility (i.e., structural-glass transition measured by linear-heating DSC) and molecular mobility are not necessarily similar. This will have huge impact in determining food stability or deterioration during processing and its subsequent storage. Glass transitions could not be identified by the conventional linear scan protocol of DSC for re-hydrated freeze dried tuna muscle containing lower than 9.0 g/100 g sample, whereas more sensitive MicroDSC and modulated DSC techniques traced the glass transition.[Citation28] Both methods produced similar onset glass transition temperature, but modulated DSC in general provided broader onset and end. Orlien et al.[Citation28] concluded that MicroDSC results serve merely proof of a glassy state in tuna systems of low water content since this method provided the narrow and well-defined transition region.
Molecular, thermal and mechanical changes at the structural glass transition in very complex in the cases of biological materials. Thus different methods and procedures are being tested to see the molecular and structural changes in foods over wide range of temperature. Other methods are being used to characterize biological materials when DSC and DMTA methods are unable to trace the glass transition adequately. The main objective of this study was to develop an innovative method to measure thermal relaxation characteristics using isothermal condition in a DSC, and to relate free mobility characteristics with the structural-glass transition measured by linear-heating DSC and mechanical-glass transition measured by DMTA using conventional protocol.
MATERIALS AND METHODS
Tested Materials
Commercial bovine gelatin and date flesh were used in these experiments. Bovine gelatin was bought from Sigma, Louis, OM, USA (bovine powder: catalogue number: G 9382). The bovine samples (5 g) were placed in a glass jar with a beaker of saturated salt solutions (with a layer of crystal at the bottom) of lithium chloride (RH: 0.113), magnesium chloride (RH: 0.336) and serilium chloride (RH: 0.877). The bovine samples were then equilibrated for four weeks at room temperature (20°C). The equilibrated samples were placed in air tight glass bottles and stored at −20°C until used for DSC measurement. Commercial date of tamr stage of maturity was bought from the local supermarket and flesh was separated from pits and mashed into homogeneous mass for DSC measurement. For DMTA measurement, a rectangular slab of 1.0 cm × 1.0 cm were cut from the date flesh and placed it carefully on the round disk of the DMTA. The thickness of the flesh disk was around 4 mm.
Differential Scanning Calorimetry (DSC)
The transition temperature was measured by DSC (DSC Q10, TA Instruments, New Castle, DE, USA). Mechanical refrigerated cooling system was used to cool the sample up to −90°C. The instrument was calibrated for heat flow and temperature using distilled water (melting point, mp = 0°C; ΔHm = 334 J/g) and indium (mp = 156.5°C; ΔHm = 334 J/g). Aluminum pans of 30 μL, which could be sealed with lid, were used in all experiments with an empty sealed pan as reference. Nitrogen at a flow rate of 50 mL/min was used as a carrier gas.
Principles of the Conventional and Proposed Methods by DSC
In the conventional method, a sample and reference (an empty aluminum pan) are cooled to a specified temperature and then heated at a controlled heating rate up to a specified temperature. Conventional DSC provides a thermogram (heat flow versus temperature) during linear-heating and a shift in the thermogram line due to a decrease in specific heat is identified as glass transition. In the new proposed method, a sample was cooled to a specified temperature and heated to another specified temperature; kept the sample at that temperature isothermally and recorded the heat flow during thermal relaxation. The heat flow then recorded as a function of relaxation time (i.e., to develop heat flow curve). This procedure was performed at several temperatures above and below glass transition. Initial slope indicates the rate of heat flow due to the thermal relaxation during initial period. Initial slope of the heat flow curve was determined and plotted as a function of isothermal relaxation temperature (i.e., slope-temperature curve). The characteristics of a material could be identified based on the behavior of the slope-temperature curve.
Conventional Linear-heating DSC Procedure
Samples of 5–10 mg were placed in an aluminum pan and then sealed. The sealed pan with samples were cooled to −90°C at 5°C/min, and then kept for 10 min. It was then scanned from −90 to 120°C at a heating rate of 10°C/min. The thermogram was analyzed for the glass transition (onset, Tgi ; mid, Tgp ; end, Tge ) from a shift in the thermogram line. The onset of the shift in the thermogram was defined as the structural-glass transition (i.e., Tgi ).
Proposed Thermal-Relaxation DSC Procedure
Samples of 20–30 mg were placed in an aluminum pan and used a cylindrical rod to compress into a thin layer and cooled to 0°C for gelatin or to −90°C for date flesh. The cooled sample was then heated to different temperatures and kept at that temperature isothermally; and the heat flow as a function of time due to isothermal relaxation of the material was recorded. Initial slope of the thermal relaxation was determined considering initial linear part and plotted as a function of temperature and identified the characteristics of slope-temperature curve. The break or change in the slope-temperature line indicated characteristics changes in the sample.
Differential Mechanical Thermal Analysis (DMTA)
The Dynamic Mechanical Thermal Analyzer DMTA V (Rheometrics, Piscataway, NJ) in compression and parallel-plate geometry was used to determine the E′ (storage modulus), E′′ (loss modulus), and tan δ. The instrument was cooled using a cryogenic accessory with liquid nitrogen from −150°C to the furnace's upper limit of 600°C. The instrument was operated as a compressive in vertical orientation (i.e., drive shaft moved upward and downward direction). Sample was placed on the round stud attached with drive shaft and it was sandwiched with another fixed round stud fixed on the top. Initially linear viscoelastic region was determined at a 0.6% compression with a frequency range 0.1–100 Hz. To conduct temperature sweeps, samples were heated at a heating rate of 5°C/min from −40 to 40°C (for low temperature) or 40–230°C (for high temperature) keeping 0.6% compression at frequency 0.1 Hz. The transition when a shift or change in slope of E′ or G′ was defined as the mechanical-glass transition (onset, glass-leathery transition, Tri and end, onset of rubbery region Tru ), maximum peak of the loss factor (i.e., tan δ) was defined as loss-peak transition (Trp ), a cross over or matching point between E′ and E′′ or (G′ and G′′) was defined as the onset of liquid-like dominating transition (Tr ). In addition, DMTA can also detect sub-glass transitions, which are not normally detected by DSC and the frequency dependence of the transitions can be studied at the same time. Madeka and Kokini[Citation29,Citation30] also defined other structural characteristics from DMTA spectroscopy. These were: onset of entangled flow transition (Tre ) when E′ and E′′ started to decrease after glass transition shift, onset of reaction zone (Trz ) at minimum peak in the E′ and E′′ (and/or maximum separation between E′ and E′′) and onset of softening transition (Trs ) at the peak of E′ and E′′.
RESULTS AND DISCUSSION
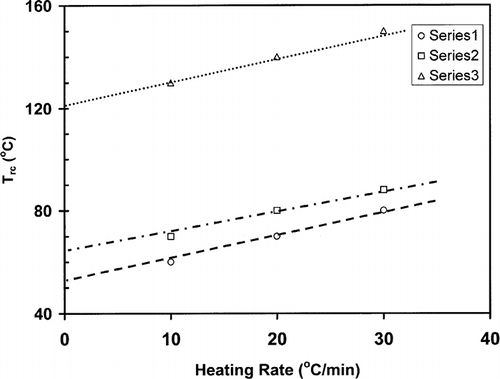
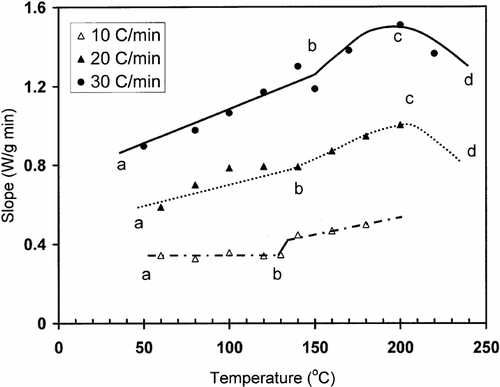
shows a typical heat flow curve for gelatin (moisture content: 20.9 g/100 g sample) as a function of time indicating the heating and relaxation cycles. Heat flow due to the thermal relaxation increased exponentially and reached to a nearly constant value. The slope of the initial relaxation curve (i.e. rate of relaxation heat flow) was determined to evaluate the thermal relaxation at constant temperature. shows the relaxation slope for bovine gelatin (moisture content: 20.9 g/100 g gelatin) as a function of heating rate to reach isothermal temperature used for relaxation. This figure shows the effect of heating rate on the slope-temperature curve. shows a change in slope of slope-temperature line except heating rate 2°C/min indicating that slowest heating rate did not caused significant detectable thermal stress in the molecule. Thus, it was important to use the optimum heating rate in order to increase the sensitivity of thermal relaxation. However, the change in slope shifted to higher temperature with increasing heating rate (). Similar results were also observed for bovine gelatin at moisture content 7.6 g/100 g sample (). The temperature at which the first change in slope was observed could be related to the structural glass-rubber transition. Over a wide temperature range, also shows three characteristic regions as limited mobility (region: ab), free mobility (region: bc), and restricted mobility (region: cd) (i.e., stationery or decline phase) when isothermal relaxation was extended up to 180°C . The point b was identified as the onset of free mobility temperature (Trc ) and point c as the onset of restricted mobility region (Trr ). In the case of moisture content 1.3 g/100 g sample, a clear change in slope was observed for 20°C /min, whereas at heating rate at 30°C/min the sensitivity was relatively reduced (). The plot of free mobility temperature (Tr c) as a function of heating rate in the shows an increasing trend. Rahman et al.[Citation31] reported the onset structural-glass transition of bovine gelatin as 61 ± 18°C (moisture content: 7.0 g/100 sample, and heating rate: 10°C/min) using conventional linear-heating DSC method. The value of 74°C (moisture content: 7.6 g/100 g sample, heating rate: 10°C/min) found by new method, which was comparable with the conventional linear-heating DSC method. shows that the extended line up to zero heating rate provides Trc as 60°C, which is very close to the conventional linear-heating DSC method. In the case of food materials, it was very common to observe increasing structural- and mechanical-glass transition with the increase of heating rate. Two types of behavior were usually observed: (1) glass transition increased exponentially and reached to a constant value at critical heating rate, and (2) glass transition increased linearly with the increase of heating rate.[Citation11] However the linearity depends on the range of heating rate used for the experiments.
Figure 1 A typical thermogram for bovine gelatin containing moisture 20.9 kg/100 kg sample (line ab: initial slope).
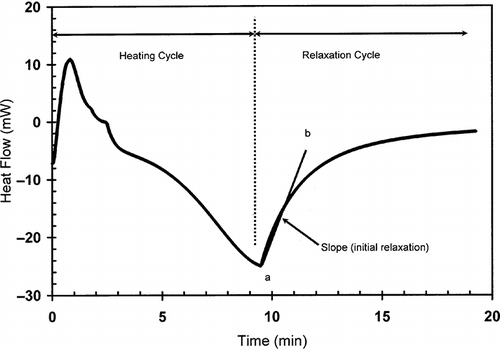
Figure 2 Slope (W/min) as a function of temperature for bovine gelatin at moisture 20.9 g/100 g sample.
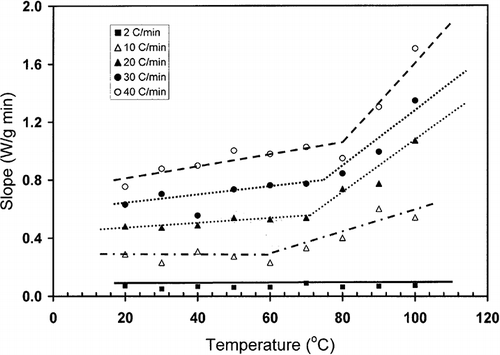
Figure 3 Slope (W/min) as a function of temperature for bovine gelatin at moisture content 7.6 g/100 g sample (ab: region 1; bc: region 2; cd: region 3; b: end of limited mobility, c: end of free mobility, Trc : onset of free mobility temperature, Trr : onset of restricted mobility temperature).
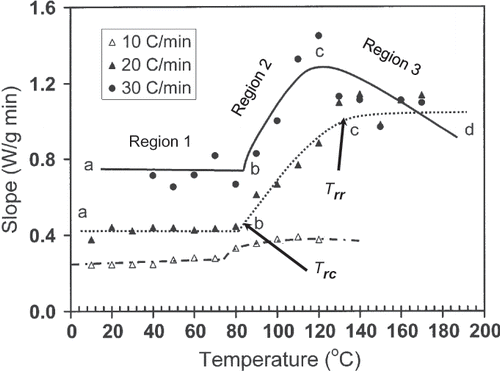
Figure 4 Slope (W/min) as a function of temperature for bovine gelatin at moisture content 1.3 g/100 g sample (ab: region 1; bc: region 2; cd: region 3).
shows a DSC thermogram of date flesh (moisture content: 27.0 g/100 g sample) measured by conventional linear-heating DSC procedure. The shift in the base line at A (marked as ab) indicated the glass transition and endothermic peak as sugar crystals melting (marked as B). The initial (Tgi ), mid (Tgp ), and final (Tge ) structural-glass transition temperatures are −50, −40 and −30°C, respectively. The slope-temperature plot in the for the proposed thermal-relaxation protocol indicates three regions for date flesh. At lower temperature from −80 to −40°C, the slope was relatively less steep (ab: limited mobility, region 1 ends at break 1) followed by a sharp rise in slope (bc: free mobility) and finally reached to a relatively constant region (region 2 ends at break 2). The onset of free mobile temperature (Trc ) and onset of restricted mobile (Trr ) temperature were found as −40 and 25°C, respectively. Similar to gelatin the onset of free mobile temperature of date flesh (Trc : −40°C) could be also related to the structural-glass transition temperature (Tgi : −50°C). In the case of date flesh there was an increasing tendency of increasing slope after softening as region 4 ().
Figure 6 DSC thermogram of date flesh containing moisture 15.0 g/100 g sample (heating rate: 10°C/min) (A: structural-glass transition; ab: shift in thermogram line at the structural-glass transition; and B: melting of sugar crystals).
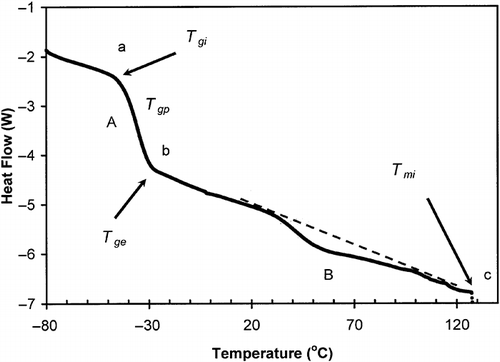
Figure 7 Slope (W/min) as a function of temperature for date flesh at moisture 15.0 g/100 g sample (heating rate: 10°C/min) (ab: region 1; bc: region 2; cd: region 3; Trc : onset of free mobility temperature; and Trr : onset of restricted mobility temperature).
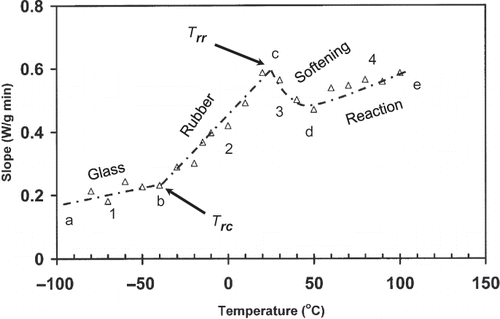
In the region 1 the thermal vibration of molecules was very low for more rigid, packed and minimal mobile matrix. In the case of second region the molecules were plasticized with temperature and resulting free mobility, thus relaxed faster than stiff or packed molecules in the region 1. In the region 3, molecules were interacting due to enough mobility. As a result interacting molecules may reduce or restricted the relaxation rate with further increase in temperature.
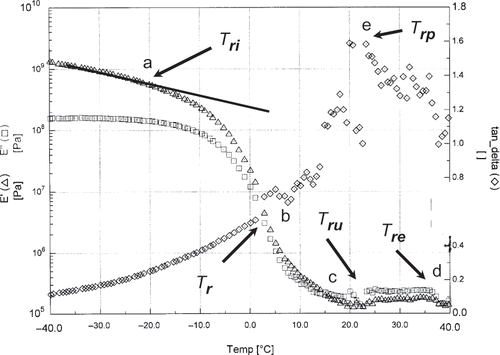
shows a typical plot of E′ and E′′ and tan δ as a function of temperature indicating three characteristic temperatures: mechanical-glass transition (Tri ) when a shift or change in slope of E′ starts (marked as a, i.e., −20°C), liquid-dominating temperature (Tr ) when a cross over or matching point between E′ and E′′ was observed (marked as b, i.e., 2°C, usually considered as the loosing of solid behavior), another transition as tan δ peak (Trp ) (i.e., 20°C, domination of liquid behavior over solid marked as e), onset temperature of rubbery state (Tru ) (marked as c, i.e., 20°C), and onset of entangled flow temperature (Tre ) (marked as d, i.e., 35°C). The mechanical-glass transition (Tri ) could be considered as glass-leather transition. In most cases the temperature at which the tan δ peak was observed was corresponded to almost complete plasticization of the material and its location depends on the degree of plasticization any material experiences.[Citation32] In the case of zein (moisture content: 15 g/100 g sample), Madeka and Kokini[Citation30] observed Tre above 70°C of Tgi (Tgi : 30°C, Tre : 100°C), whereas in the case of date flesh Tre was observed above 85°C of Tgi (Tgi : −50°C, Tre : 35°C).
Figure 8 DMTA mechanical spectra of fresh date flesh moisture content 15.0 g/100 sample at low temperature (Tri : onset of mechanical glass transition temperature; Tre : end of mechanical glass (i.e., onset of leathery) transition temperature; Tr : onset of liquid dominating region; Trp : peak of tanδ; and Tre : onset of rubbery flow).
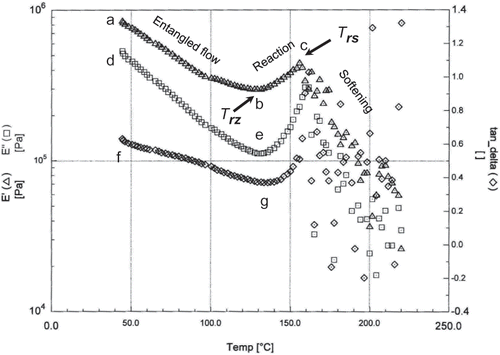
shows mechanical spectra at higher temperature range (40–230°C). It indicates three characteristics as entangled polymer flow region (marked as ab), reaction zone (marked as bc) and softening zone (marked as above c). A decrease in the magnitude of E′ and E′′ was observed as the temperature increased from a to b (i.e., Trz to Trs ). This region was characterized as the entangled polymer flow.[Citation30] At the distance between points of b, e, and g, the separation between E′ and E′′ was observed maximum. As the temperature exceeded from b or e, a sharp increase in E′ and E′′ was observed. This was due to cross-linking reaction occurring once the ability of the molecules in the date flesh was high enough. In this case molecules gain enough mobility to come together and caused cross-link formation. Above point c, the matrix appeared to have reached a maximum cross-link density. The maximum in E′′ would suggest melting of the cross-linked structure allowed to have increased mobility necessary for softening. The point b is defined as the onset of reaction zone, and point c is defined as the onset of softening zone. In the case of date flesh the reaction temperature (Trz ) and softening temperature (Trs ) were 130 and 160°C, respectively. The DSC thermogram with linear-heating showed onset (Tmi ) and peak (Tmp ) of solids-melting temperatures for date flesh (moisture content: 24.6 g/100 g sample) as 163 and 180°C.[Citation33] However completely dried date flesh showed 175 and 191°C, respectively.[Citation33] Therefore, the melting process by linear-heating DSC method could be related with the thermal-mechanical reaction and softening temperature. However the onset melting by linear-heating DSC (Tmi : 163°C) showed similar to the softening temperature by thermal-mechanical method (Trs : 160°C). In the case of zein (moisture content: 15.0 g/100 g sample), Madeka and Kokini (29) found Tre , Trz and Trs as 100, 122, and 160°C, respectively. They also estimated an average number of cross-links equal to 0.75. The reaction temperature, by thermal-mechanical method provided additional information for reacting molecules in the matrix, could not be determined by the thermal analysis.
Figure 9 DMTA mechanical spectra of fresh date flesh at moisture 15.0 g/100 g sample at high temperature (Trz : onset temperature of reaction zone; and Trs : onset of softening temperature).
Combining thermal-relaxation, linear-heating, and mechanical-thermal analyses could explore different structural characteristics of gelatin and date flesh. The new proposed thermal-relaxation method could have potential to identify molecular mobility (i.e., molecular vibration), and it could be related with the structural- and mechanical-glass transition. The results of the three methods indicated that detailed structural characteristics of complex biological materials could be explored. In addition, the proposed new method could be used when commonly used linear-heating DSC and mechanical-thermal DMTA methods fail to determine the glass-rubber transition. However, the proposed method needs at least 6 samples to run (before and above break 1) to determine the free mobility transition during thermal relaxation. The conventional linear-heating DSC and mechanical-thermal methods need one sample to run in order to identify the structural characteristics. In the future work, the relaxation curve could be analyzed based on the theoretical based model instead of simple linear relation and different model parameters could be explored to explain other structural characteristics of complex biological materials. In addition, other complex biological materials need to be used in order to test the validity and applications of the proposed thermal-relaxation DSC method.
CONCLUSION
A new innovative method was developed to measure thermal relaxation of gelatin and date flesh by maintaining isothermal condition in a DSC. Thermal relaxation showed three characteristic behaviors as limited mobility, free mobility and restricted mobility. The onset of free mobile temperature (Trc ) was related to the structural-glass (Tgi ) and mechanical-glass (Tri ) transition temperatures. The proposed thermal relaxation method could have high potential to measure glass transition when most commonly used conventional linear-heating DSC and DMTA methods are failed. The mechanical spectra measured by DMTA at higher temperature indicated three characteristics as entangled flow region, reaction zone, and softening zone. The relationships of these characteristics with thermal transition measured by DSC were further discussed.
ACKNOWLEDGMENTS
The authors would like to acknowledge the support of Sultan Qaboos University towards this research in the area of food structure and stability. They are grateful to all members in the research group for their continued support and encouragement. Special thanks to Dr. Amanat Ali for checking the clarity of this paper.
REFERENCES
- Levine , H. and Slade , L. 1986 . A polymer physico-chemical approach to the study of commercial starch hydrolysis products (SHPs) . Carbohydrate Polymer , 6 : 213 – 244 .
- Slade , L. and Levine , H. 1988 . Non-equilibrium behavior of small carbohydrate-water systems . Pure Applied Chemistry , 60 : 1841 – 1864 .
- Rahman , M. 2006 . S State diagram of foods: Its potential use in food processing and product stability . Trends in Food Science and Technology , 17 : 129 – 141 .
- Rahman , M.S. 2009 . Food Stability beyond water activity and glass transition: Macro-micro region concept in the state diagram . International Journal of Food Properties , 12 ( 4 ) : 726 – 740 .
- Rahman , M.S. 2010 . Food stability determination by macro-micro region concept in the state diagram and by defining a critical temperature . Journal of Food Engineering , 99 ( 4 ) : 402 – 416 .
- Kalichevsky-Dong , M.T. 2000 . “ The glass transition and microbial stability ” . In The Stability and Shelf Life of Food , Edited by: Kilcast , D. and Subramaniam , P. 25 – 53 . Cambridge, , England : Woodhead Publishing . CRC Press: Boca Raton, FL
- Hashimoto , T. , Hagiwara , T. , Suzuki , T. and Takai , R. 2003 . Study on the glass transition of Katsuobushi (boiled and dried bonito fish stick) by differential scanning calorimetry and dynamic mechanical analysis . Fisheries Science , 69 : 1290 – 1297 .
- Vodovotz , Y. and Chinachoti , P. 1996 . Thermal transitions in gelatinination wheat starch at different moisture contents by dynamic mechanical analysis . Journal of Food Science , 61 ( 5 ) : 932 – 941 .
- Cnossen , A. G. , Siebenmorgen , T. J. , Yang , W. and Bautista , R. C. 2001 . An application of glass transition temperature to explain rice kernel fissure occurrence during the drying process . Drying Technology , 19 ( 8 ) : 1661 – 1882 .
- Boonyai , P. , Bhandar , B. and Howes , T. 2005 . Measurement of glass-rubber transition temperature of skim milk powder by static mechanical test . Drying Technology , 23 : 1499 – 1514 .
- Rahman , M.S. , Senadeera , W. , Al-Alawi , A. , Truong , T. , Bhandari , B. and Al-Saidi , G. 2010 . Thermal transition properties of spaghetti measured by differential scanning calorimetry (DSC) and thermal mechanical compression test (TMCT) . Food and Bioprocess Technology , in press
- Rahman , M.S. , Al-Marhubi , I.M. and Al-Mahrouqi , A. 2007 . Measurement of glass transition temperature by mechanical (DMTA), thermal (DSC and MDSC), water diffusion and density methods: A comparison study . Chemical Physics Letters , 440 : 372 – 377 .
- Acevedo , N. , Schebor , C. and Buera , M. P. 2006 . Water-solids interactions, matrix structural properties and the rate of non-enzymatic browning . Journal of Food Engineering , 77 : 1108 – 1115 .
- Sablani , S.S. , Rahman , M.S. , Al-Busaidi , S. , Guizani , N. , Al-Habsi , N. , Al-Belushi , R. and Soussi , B. 2007 . Thermal transitions of king fish whole muscle, fat and fat-free muscle by differential scanning calorimetry . Thermochimica Acta , 462 : 56 – 63 .
- Rahman , M.S. , Kasapis , S. , Al-Kharusi , N.S.Z. , Al-Marhubi , I.M. and Khan , A.J. 2007 . Composition characterization and thermal transition of date pits powders . Journal of Food Engineering , 80 ( 1 ) : 1 – 10 .
- Sablani , S.S. and Kasapis , S. 2006 . Glass transition and water activity of freeze-dried shark . Drying Technology , 24 : 1003 – 1009 .
- Green , J.L. , Fan , J. and Angell , C.A. 1994 . The protein class analogy some insights from homopeptide comparisons . Journal of Physical Chemistry , 98 : 13780 – 13790 .
- Godet , M.C. , Bizot , H. and Buleon , A. 1995 . Crystallization of amylase-fatty acid complexes prepared with different amylase chain lengths . Carbohydrate Polymers , 27 : 47 – 52 .
- Nuessli , J. , Sigg , B. , Conde-Petit , B. and Escher , F. 1997 . Characterization of amylose-flavor complexes by DSC and X-ray diffraction . Food Hydrocolloids , 11 ( 1 ) : 27 – 34 .
- Fan , J. , Mitchell , J.R. and Blanshard , J.M.V. 1996 . The effect of sugars on the extrusion of maize grits: II. Starch conversion . International Journal of Food Science and Technology , 31 : 67 – 76 .
- Raemy , A. and Loliger , J. 1982 . Thermal behavior of cereals studied by heat flow calorimetry . Cereal Chemistry , 59 ( 3 ) : 189 – 191 .
- Jang , J.K. and Pyun , Y.R. 1996 . Effect of moisture content on the melting of wheat starch . Starch , 48 ( 2 ) : 48 – 51 .
- Iijima , M. , Nakamura , K. , Hatakeyama , T. and Hatakeyama , H. 2000 . Phase transition of pectin with sorbed water . Carbohydrate Polymers , 41 : 101 – 106 .
- Kim , Y.J. , Suzuki , T. , Hagiwara , T. , Yamaji , I. and Takai , R. 2001 . Enthalpy relaxation and glass to rubber transition of amorphous potato starch formed by ball-milling . Carbohydrate Polymers , 46 : 1 – 6 .
- Biliaderis , C.G. , Page , C.M. , Maurice , T.J. and Juliano , B.O. 1986 . Thermal characterization of rice starches: a polymer approach to phase transitions of granular starch . Journal of Agricultural and Food Chemistry , 34 : 6 – 14 .
- Zelenak , K.J. and Hoseney , R.C. 1987 . The glass transition of starch . Cereal Chemistry , 64 ( 2 ) : 121 – 124 .
- Vittadini , E. , Dickinson , L. C. and Chinachoti , P. 2001 . 1H and 2H NMR mobility in cellulose . Carbohydrate Polymers , 46 : 49 – 57 .
- Orlien , V. , Risbo , J. , Andersen , M.L. and Skibsted , L.H. 2003 . The question of high- or low-temperature glass transition in frozen fish. Construction of the supplemented state diagram for tuna muscle by differential scanning calorimetry . Journal of Agricultural Food Chemistry , 51 : 211 – 217 .
- Madeka , H. and Kokini , J.L. 1994 . Changes in rheological properties of gliadin as a function of temperature and moisture: Development of a state diagram . Journal of Food Engineering , 22 : 241 – 252 .
- Madeka , H. and Kokini , J.L. 1996 . Effect of glass transition and cross-linking on rheological properties of zein: development of a preliminary state diagram . Cereal Chemistry , 73 ( 4 ) : 433 – 438 .
- Rahman , M.S. , Al-Saidi , G.S. and Guizani , N. 2008 . Thermal characterization of gelatin extracted from yellowfin tuna skin and commercial mammalian gelatin . Food Chemistry , 108 : 472 – 481 .
- Peleg , M. 1995 . A note on the tan δ (T) peak as a glass transition indicator in biosolids . Rheology Acta , 34 : 215 – 220 .
- Rahman , M.S. 2004 . State diagram of date flesh using differential scanning calorimetry (DSC) . International Journal of Food Properties , 7 ( 3 ) : 407 – 28 .