Abstract
In this study, the ethyl acetate, acetone, and methanol extracts from the petals of the flowers of Peltophorum ferrugineum were evaluated for their phenolic content, antioxidant activity, and HPLC profile. The total phenolic contents of the ethyl acetate, acetone, and methanol extracts, as determined by the Folin-Ciocalteu method, were found to be 17.0 ± 0.8, 29.3 ± 0.4, and 18.6 ± 0.9%, respectively, as gallic acid equivalent. The antioxidant potentials of the extracts were found to be in the order of acetone > methanol > ethyl acetate as assayed through three different in vitro assay methods, such as phosphomolybdenum method, α, α-diphenyl-β-picrylhydrazyl method, and ferricyanide reduction method. HPLC analyses of the extracts showed the presence of gallic acid and catechin in ethyl acetate extract, ferulic acid, and catechin in methanol extract. In the present study, the flower of P. ferrigineum possessed a substantial amount of phenolics with marked antioxidant activity. Phenolics, such as gallic acid, catechin, and ferulic acid, were the antioxidant principles in the flowers.
INTRODUCTION
Antioxidants are often added to foods to prevent the radical chain reactions of oxidation by inhibiting the initiation and propagation step leading to the termination of the reaction and a delay in the oxidation process.[Citation1,Citation2] Synthetic antioxidants, such as butylated hydroxyanisole (BHA), butylated hydroxy toluene (BHT), and propyl gallate, have been used as food antioxidants. However, the use of these synthetic antioxidants is negatively perceived by consumers due to potential toxicity and their connotation as chemicals in food and is restricted by legislative rules.[Citation3,Citation4] Therefore, there is a considerable interest by the industry and a growing trend in consumer preferences for natural antioxidants over synthetic compounds and elimination of synthetic antioxidants in food applications. This has provided more impetus to explore natural sources of antioxidants. Natural antioxidants not only protect food lipids from oxidation, but may also provide health benefits associated with preventing damage due to oxidative stress.[Citation5] Recently, antioxidant phenolic extracts from the plants have been used in fruit juice and the manufacture of herby cheese.[Citation6,Citation7]
Many studies reported the antioxidant activity of crude extract, phenolic rich fractions, partially purified phenolic compounds, and purified compounds from the flowers of different plant species assayed through both in vitro and in vivo methods. Extractives from the flowers of Melastoma malabathricum,[Citation8] Punica granatum,[Citation9] Campsis grandiflora,[Citation10] Cassia auriculata,[Citation11] Filipendula hexapetala,[Citation12] Taraxacum officinale,[Citation13] and Anethum graveolens[Citation14] with various solvents, such as ethyl acetate, methanol, ethanol, and aqueous methanol, have shown antioxidant activity with in vitro and in vivo assays. Lutein from the flowers of marigold has shown antioxidant activity as assayed through some in vitro models, such as photochemiluminescence and β-carorotene-linoleic model systems.[Citation15] Li et al.[Citation16] have reported the antioxidant activity of gallic acid from the flowers of rose in senescent mice by assaying the levels of antioxidant enzymes, such as catalase and glutathione peroxidase, and the amount of malonaldehyde in various tissues.
Peltophorum ferrugineum belongs to the family of Leguminosae. Peltophorum ferrugineum is distributed in India and South East Asia, West Africa, and other tropical countries. Commonly this is known as Copperpod. The tree of Peltophorum ferrugineum grows up to 24 m high with a dense, spreading, and deep seated crown. Leaves are paripinnate with many leaflets. Inflorescences are in panicles of terminal spikes of yellow flowers. Pods are reddish-brown, relatively small, thin, flat, ovate, thinned on the margins, and indehiscent. The bark is used for dysentery, tooth powder, eye lotion, embrocating for pains, and sores and the bark gives yellow coloured dye. The foliage of P. ferrugineum is used as cattle feed.[Citation17]
The aim of the present study was to investigate the total phenolic content, antioxidant activity through some in vitro methods and identification of phenolics in the extracts from the flowers of P. ferrugineum.
MATERIALS AND METHODS
Materials
α, α-Diphenyl-β-picrylhydrazyl (DPPH), butylated hydroxyanisole (BHA), gallic acid, thiobarbituric acid, catechin, and ferulic acid were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Deoxyribose was obtained from Sisco Research Laboratories Pvt. Ltd. (Mumbai, India). All solvents and chemicals used were of analytical grade/HPLC grade and obtained from Merck (Mumbai, India) and Ranbaxy Fine Chemicals (Mumbai, India). Visible spectra measurements were done using a Spectronic 20 visible spectrophotometer (Spectronic Instruments Inc., Rochester, NY, USA). The flowers of Peltophorum ferrugineum were collected from the campus of Central Food Technological Research Institute (Mysore, India).
Extraction
The fresh flowers of P. ferrugineum were collected from the campus of Central Food Technological Research Institute (Mysore, India). The petals were separated and dried under shade. The dried petals were powdered in a mixer grinder and 50 g of powder was defatted with 250 ml of hexane in a Soxhlet extractor for 24 h at 50°C. The residue was successively extracted with ethyl acetate, acetone, and methanol. The extracts were filtered through Whatman No. 1 filter paper to obtain particle free extracts. The ethyl acetate, acetone, and methanol extracts were concentrated and dried under a vacuum at 45°C and the dried extracts were used for the present study.
Determination of Total Phenolics
The total phenolic content of ethyl acetate, acetone, and methanol extracts from the flowers of P. ferrugineum was determined as described by Singleton and Rossi.[Citation18] Results were expressed as gallic acid equivalent. A known quantity of each extract was dissolved (1 mg/ml) in a mixture of methanol and water (6:4 v/v). Samples (0.1 ml) were taken in test tubes and made up to the volume of 10 ml with distilled water. Then 0.5 ml of Folin-Ciocalteu reagent (1:1 with water) and 0.1 ml of sodium carbonate solution (7.5%) were subsequently added to each tube and mixed well by a vortex mixer. After standing for 30 min at room temperature, the absorbance was measured at 760 nm against the reagent blank using Spectronic 20 visible spectrophotometer. The amount of total phenolic content was calculated as gallic acid equivalents from the calibration curve. The estimation of phenolic content in the fractions was carried out in triplicate and the results were averaged.
Total Antioxidant Capacity
The total antioxidant capacity of ethyl acetate, acetone, and methanol extracts from the flowers of P. ferrugineum was evaluated by the method of Prieto et al.[Citation19] The sample extract solution (1.1 mg/ml) was prepared in a mixture of methanol and water (6:4 v/v) for each extract. An aliquot of 0.1 ml of sample extract solution was combined with 1 ml of reagent solution (0.6 M sulfuric acid, 28 mM sodium phosphate, and 4 mM ammonium molybdate). The tubes were capped and incubated in a boiling water bath at 95°C for 90 min. After the samples had cooled to room temperature, the absorbance of the aqueous solution of each was measured at 695 nm against a blank solution in a Spectronic 20 visible spectrophotometer. A typical blank solution contained 1 ml of reagent solution and the appropriate volume of the same solvent used for the sample and it was incubated under the same conditions as the sample. For samples of unknown composition, water-soluble antioxidant capacity was expressed as equivalents of ascorbic acid (mmole/g of extract).
Radical Scavenging Activity
Radical scavenging activity of the ethyl acetate, acetone, and methanol extracts from the flowers of P. ferrugineum was determined essentially as described by Blois.[Citation20] A known quantity of each extract was dissolved in a mixture of methanol and water (6:4 v/v). Different concentrations (20, 40, 60, 80, and 100 ppm) of extract and BHA (20, 40, 60, 80, and 100 ppm) were taken in different test tubes. The volume was adjusted to 100 μl by adding MeOH. An amount of 5.0 ml of 0.1 mM methanolic solution of DPPH was added to these tubes and well shaken. The tubes were allowed to stand at 27°C for 20 min. The control was prepared as above without any extract and MeOH was used for the baseline correction. The changes in the absorbance of the samples were measured at 517 nm. Radical scavenging activity was expressed as the inhibition percentage and was calculated using the following formula:
Determination of Reducing Power
The reducing power of ethyl acetate, acetone, and methanol extracts from the flowers of P. ferrugineum was determined essentially as described by Oyaizu.[Citation21] Extracts dissolved in methanol and water mixture (6:4 v/v) were mixed with 5 ml of sodium phosphate buffer (0.2 M, pH 6.6) and 2.5 ml of 1% potassium ferricyanide in test tubes with a final concentration of extract of 25, 50, and 100 ppm. The mixtures were incubated for 20 min at 50°C. At the end of the incubation, 2.5 ml of 10% trichloroacetic acid was added to the mixtures and centrifuged at 5000 rpm for 10 min. The upper layer (2.5 ml) was mixed with 2.5 ml of distilled water and 0.5 ml of 0.1% ferric chloride, and the absorbance was measured at 700 nm in a Spectronic 20 visible spectrophotometer against a blank solution prepared in a similar manner. The reducing power tests were run in triplicate. The increase in absorbance of the reaction mixture indicated the reducing power of the extracts.
HPLC Analysis
The HPLC analysis of ethyl acetate, acetone, and methanol extracts from the flowers of P. ferrugineum was conducted by using a high-performance liquid chromatographic system that consisted of a Hewlett-Packard HPLC model HP 1100 Series (Palo Alto, CA, USA) equipped with a quaternary HPLC pump, and fitted with a C18 analytical column (25 cm × 4.6 mm i.d., 5-micron particle size, Waters Corporation, MA, USA). The injection system (Rheodyne) used was a 20-μl sample loop. Detection was done by a HP 1100 Series variable wavelength detector at a wavelength of 280 nm. The mobile phase involved a gradient of water: acetic acid (100:1, v/v) as solvent A and methanol: acetonitrile: acetic acid (95:5:1, v/v/v) as solvent B at a constant flow rate of 1.5 ml/min. The gradient was comprised of an initial isocratic period of 2 min at 5% B followed by a linear increase to 25% B over 10 min, with further stepwise linear increases to 40% B at 20 min, 50% B at 30 min, and 100% B at 40 min, held for 5 min and returned to initial conditions over 10 min. At 280 nm UV, detection was routinely performed. All standards and samples were prepared with a concentration of 1 mg/ml of a mixture of methanol and water (6:4 v/v) and were filtered through a 0.45-μm Millipore filter and injected to HPLC.
RESULTS AND DISCUSSION
The yield of ethyl acetate, acetone, and methanol extracts from the petals of the flowers of P. ferrugineum was found to be 3.2, 2.7, and 19.1%, respectively. The total phenolic contents of the ethyl acetate, acetone, and methanol extracts as determined by the Folin-Ciocalteu method were found to be 17.0 ± 0.8, 29.3 ± 0.4, and 18.6 ± 0.9%, respectively, as gallic acid equivalent (). It has been demonstrated that plant tissues synthesize a wide variety of phenolic compounds.[Citation22] The majority of the antioxidant activity may be from compounds, such as flavonoids, isoflavones, flavones, anthocyanin, catechin, and other phenolics.[Citation23]
Table 1 Phenolic content (as gallic acid equivalent) of the extracts from the flowers of P. ferrugineu m
The antioxidant capacity of the extracts was measured spectrophotometrically through the phosphomolybdenum method, which is based on the reduction of Mo (IV) to Mo (V) by the sample analyte and the subsequent formation of green phosphate/Mo (V) compounds with a maximum absorption at 695 nm. At 100 ppm concentration, the antioxidant capacity of ethyl acetate, acetone, and methanol extracts was found to be 1316 ± 13, 2223 ± 39, and 1420 ± 11 μmoles/g of the extract as equivalent to ascorbic acid, respectively (). Prieto et al.[Citation19] have shown that reducing species like α-tocopherol, ascorbic acid, glutathione, and butylated hydroxytoluene (BHT) possessed the ability of reduction of Mo (IV) to Mo (V).
Table 2 Antioxidant capacity of the extracts from the flowers of P. ferrugineu m by phosphomolybdenum method
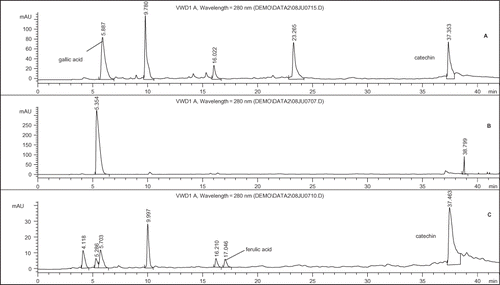
The reducing power of ethyl acetate, acetone, and methanol extracts from the flowers of P. ferrugineum was also evaluated by ferricyanide reduction method (). The increase in absorbance of the reaction mixture indicated the reducing power of the extracts. It was observed that with the increase of the concentration of extracts the absorbance increased. At 100 ppm concentration, ethyl acetate, acetone, and methanol extracts showed the absorbance of 0.422, 1.051, and 0.785, respectively. Tanaka et al.[Citation24] have reported that antioxidant activity is concomitant with the reducing power of the sample in analysis. The presence of reductones are associated with the reducing properties and reductones possess hydrogen donating capacity[Citation25] and the antioxidant action of reductones is based on the breaking of the free radical chain by donating a hydrogen atom.[Citation26]
Figure 1 Reducing power of the extracts from the flowers of P. ferrugineum at different concentrations.
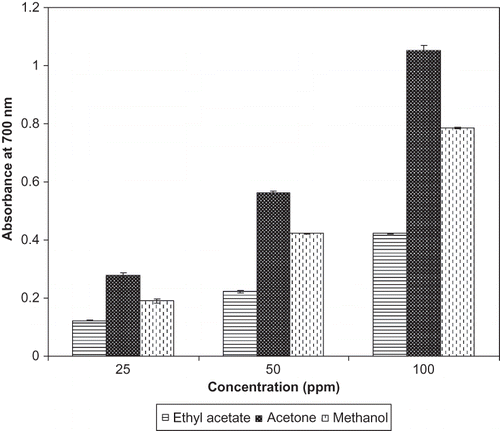
The free radical scavenging activity of ethyl acetate, acetone, and methanol extracts from the flowers of P. ferrugineum was tested through the DPPH method and the results are presented in the . The free radical scavenging activity of both the extracts and BHA was found to increase with the increase in concentration. The free radical scavenging activity of ethyl acetate, acetone, and methanol extracts and BHA was 44.16, 89.46, 75.03, and 91.58%, respectively, at 100 ppm concentration. In the present study, the degree of discolouration of α, α-diphenyl-β-picrylhydrazyl indicated the free radical scavenging potentials of the flower extracts. It has been reported that antioxidants, such as cysteine, glutathione, ascorbic acid, tocopherol, polyhydroxy aromatic compounds (hydroquinone, pyrogallol, etc.), and aromatic amines (p-phenylene diamine, p-aminophenol, etc.) reduce and decolourise α, α-diphenyl-β-picrylhydrazyl by their hydrogen donating ability.[Citation20] It appears that the extracts from the flowers of P. ferrugineum possess antioxidant activity because of their hydrogen donating capabilities, thereby neutralizing the free radical nature of DPPH either by transfer of one electron or hydrogen atom.[Citation27,Citation28] In the present study, flower extracts showed antioxidant activity to the extent of their respective phenolic content. Methanol extracts from the flowers of Filipendula hexapetala[Citation12] and Cassia auriculata,[Citation11] ethyl acetate and methanol extract from the flowers of Melastoma malabathricum L.,[Citation8] and aqueous methanolic extract from the Cassia siamea flowers showed antioxidant activity as assayed through various in vitro methods,[Citation29] which included DPPH radical scavenging and reducing power methods. The phenolic compounds are dominant antioxidants distributed widely in the plant kingdom that exhibit scavenging efficiency on free radicals and reactive oxygen species.[Citation30,Citation31] Several reports have conclusively shown a close relationship between total phenolic content and antioxidant activity.[Citation32–35]
Figure 2 Radical scavenging activity of the extracts from the flowers of P. ferrugineum at different concentrations by DPPH method.
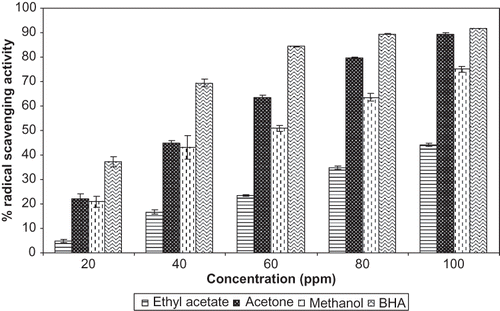
Ethyl acetate, acetone, and methanol extracts from the flowers of P. ferrugineum were analyzed by HPLC and the chromatograms were presented in . The HPLC chromatograms of the various extracts showed that the compounds are separated in the solvents used for extraction. The retention times for the standard phenolics, such as gallic acid, ferulic acid, and catechin, were found to be 5.8, 17.3, and 37.2 min, respectively. The identity of gallic acid and catechin peaks in HPLC chromatogram of ethyl acetate extract was confirmed by determining the relative retention times and by spiking with standard gallic acid and catechin. HPLC profile of acetone extract showed a major peak at the retention time of 5.3 min, which was not identified presently. Spiking of standard ferulic acid and catechin along with methanol extract showed the presence of ferulic acid and catechin in this extract. Phenolic antioxidants, such as gallic acid, catechin, and ferulic acid, are widely distributed in the plant kingdom. Both in vitro and in vivo antioxidant activity has been demonstrated with gallic acid from rose flowers.[Citation16] Catechin was found to be a major phenolic antioxidant in day lily flowers[Citation36] and catechin has enormous health benefits as an antioxidant.[Citation37] As reviewed by Srinivasan et al.,[Citation38] ferulic acid has a host of therapeutic potential through its antioxidant property.
CONCLUSION
The phenolic content and antioxidant activities of ethyl acetate, acetone, and methanol extracts from the petals of the flowers of P. ferrugineum were in the order of acetone > methanol > ethyl acetate as assayed through three different in vitro assay methods. HPLC analyses of the extracts showed the presence of gallic acid and catechin in ethyl acetate extract, ferulic acid, and catechin in methanol extract from the flower of P. ferrugineum. The presence of an unidentified but separate compound was found in the acetone extract. This is the first report on the antioxidant fractions and antioxidant phenolics from the flower of P. ferrugineum. Further studies are warranted for the identification of other individual phenolic compounds in these extracts and in vivo and toxicological studies are required for their possible use as nutraceutical and food biopreservative as antioxidants.
ACKNOWLEDGMENTS
The authors would like thank Dr. V. Prakash, Director and Dr. M. C. Varadaraj, Head, Human Resource Development, CFTRI, for their keen interest in this work.
REFERENCES
- Athukorala , Y. , Lee , K. , Shahidi , F. , Heu , M.S. , Kim , H. , Lee , J. and Jeon , Y. 2003 . Antioxidant efficacy of extracts of an edible red algae (Grateloupia filicina) in linoleic acid and fish oil . Journal of Food Lipids , 10 : 313 – 327 .
- Yu , L. , Scanlin , L. , Wilson , J. and Schidt , G. 2002 . Rosemary extracts as inhibitors of lipid oxidation and colour change in cooked turkey products during refrigerated storage . Journal of Food Science , 67 : 582 – 585 .
- Imaida , K. , Fukushima , S. , Shirui , T. , Ohtani , M. , Nakanishi , K. and Ito , N. 1983 . Promoting actions of butylated hydroxy anisole and butylated hydroxy toluene on 2-stage urinary bladder carcinogenesis and inhibition of γ-glutamyl transpeptidase-positive foci development in the liver of rats . Carcinogenesis , 4 : 895 – 899 .
- Jeong , S.M. , Kuo , S.Y.D. , Kim , R.S. , Jo , C. , Nam , K.C. , Ahn , D.U. and Lee , S.C. 2004 . Effect of heat treatment on the antioxidant activity of extracts from citrus peels . Journal of Agricultural Food Chemistry , 52 : 3389 – 3393 .
- Willet , W.C. 2002 . Balancing life-style and genomics research for disease prevention . Science , 296 : 695 – 698 .
- Arabsahahi-Delouee , S. and Urooj , A. 2007 . Application of phenolic extracts from selected plants in fruit juice . International Journal of Food Properties , 10 : 479 – 488 .
- Celik , S.E. , Ozyurek , M. , Altun , M. , Bektasoglu , B. , Guclu , K. , Berker , K.I. , Ozgokce , F. and Apak , R. 2008 . Antioxidant capacities of herbal plants used in the manufacture of van herby cheese: ‘Otlu peynir’ . International Journal of Food Properties , 11 : 747 – 761 .
- Susanti , D. , Sirat , H.M. , Ahmad , F. , Ali , R.M. , Aimi , N. and Kitajima , M. 2007 . Antioxidant and cytotoxic flavonoids from the flowers of Melastoma malabathricum L . Food Chemistry , 103 : 710 – 716 .
- Kaur , G. , Jabbar , Z. , Athar , M. and Alam , M.S. 2006 . Punica granatum (pomegranate) flower extract possesses potent antioxidant activity and abrogates Fe-NTA induced hepatotoxicity in mice . Food Chemical and Toxicology , 44 : 984 – 993 .
- Cui , X. , Kim , J. , Zhao , X. , Chen , B. , Lee , B. , Pyo , H. , Yun , Y. and Zhang , Y. 2006 . Antioxidative and acute anti-inflammatory effects of Campsis grandiflora flower . Journal of Ethnopharmacology , 103 : 223 – 228 .
- Kumaran , A. and Karunakaran , R.J. 2007 . Antioxidant activity of Cassia auriculata flowers . Fitoterapia , 78 : 46 – 47 .
- Maksimovic , Z. , Petrovic , S. , Pavlovic , M. , Kovacevic , N. and Kukic , J. 2007 . Antioxidant activity of Filipendula hexapetala flowers . Fitoterapia , 78 : 265 – 267 .
- Hu , C. and Kitts , D.D. 2005 . Dandelion (Taraxacum officinale) flower extract suppresses both reactive oxygen species and nitric oxide and prevents lipid oxidation in vitro . Phytomedicine , 12 : 588 – 597 .
- Shyu , Y. , Lin , J. , Chang , Y. , Chang , C. and Yang , D. 2009 . Evaluation of antioxidant ability of ethanolic extract from dill (Anethum graveolens L.) flower . Food Chemistry , 115 : 515 – 521 .
- Wang , M. , Tsao , R. , Zhang , S. , Dong , Z. , Yang , R. , Gong , J. and Pei , Y. 2006 . Antioxidant activity, mutagenicity/anti-mutagenicity, and clastogenicity/anti-clastogenicity of lutein from marigold flowers . Food Chemical and Toxicology , 44 : 1522 – 1529 .
- Li , L. , Ng , T.B. , Gao , W. , Li , W. , Fu , M. , Niu , S.M. , Zhao , L. , Chen , R.R. and Liu , F. 2005 . Antioxidant activity of gallic acid from rose flowers in senescence accelerated mice . Life Sciences , 77 : 230 – 240 .
- 1986 . The Useful Plants of India , 437 New Delhi, India : National Institute of Science Communication .
- Singleton , V.L. and Rossi , J.A. 1965 . Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents . American Journal of Enology and Viticulture , 16 : 144 – 158 .
- Prieto , P. , Pineda , M. and Aguilar , M. 1999 . Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E . Analytical Biochemistry , 269 : 337 – 341 .
- Blois , M.S. 1958 . Antioxidants determination by the use of a stable free radical . Nature , 4617 : 1199 – 1200 .
- Oyaizu , M. 1986 . Studies on products of browning reactions: Antioxidative activities of products of browning reaction prepared from glucosamine . Japanese Journal of Nutrition , 44 : 307 – 315 .
- Loliger , J. 1991 . “ The use of antioxidants in foods ” . In Free Radicals and Food Addititives , Edited by: Auroma , O.I. and Halliwell , B. 129 – 150 . London : Taylor and Francis .
- Kahkonen , M.P. , Hopia , A.I. and Vuorela , H.J. 1999 . Antioxidant activity of plant extracts containing phenolic compounds . Journal of Agricultural Food Chemistry , 47 : 3954 – 3962 .
- Tanaka , M. , Kuie , C.W. , Nagashima , Y. and Taguchi , T. 1988 . Application of antioxidative maillard reaction products from histidine and glucose to sardine products . Nippon Suisan Gakkaishi , 54 : 1409 – 1414 .
- Duh , P. 1998 . Antioxidant activity of Budrock (Arctium lappa Linn): Its scavenging effect on free radical and active oxygen . Journal of American Oil and Chemical Society , 75 : 455 – 461 .
- Gordon , M.F. 1990 . “ The mechanism of antioxidant action in vitro ” . In Food Antioxidants , Edited by: Hudson , B.J.F. 1 – 18 . London : Elsevier Applied Science .
- Sherwin , E.R. 1978 . Oxidation and antioxidants in fats and oil processing . Journal of American Oil and Chemical Society , 55 : 809 – 814 .
- Naik , G.H. , Priyadarshini , K.I. , Satav , J.G. , Banavalikar , M.M. , Sohoni , P.P. , Biyani , M.K. and Mohan , P.P. 2003 . Comparative antioxidant activity of individual herbal components in Ayurvedic medicine . Phytochemistry , 63 : 97 – 104 .
- Kaur , G. , Alam , M.S. , Jabbar , Z. , Javed , K. and Athar , M. 2006 . Evaluation of antioxidant activity of Cassia siamea flowers . Journal of Ethnopharmacology , 108 : 340 – 348 .
- Namiki , M. 1990 . Antioxidants/antimutagens in foods . Critical Reviews in Food Science and Nutrition , 29 : 273 – 300 .
- Prior , R.L. and Cao , G. 2000 . Antioxidant phytochemicals in fruits and vegetables . Diet and health implications. Horticulture Science , 35 : 588 – 592 .
- Deighton , N. , Brennan , R. , Finn , C. and Davies , H.V. 2000 . Antioxidant properties of domesticated and wild Rubus species . Journal of Science Food and Agriculture , 80 : 1307 – 1313 .
- Jimenez-Escrig , A. , Jimenez-Jimenez , I. , Pulido , R. and Saura-Calixto , F. 2001 . Antioxidant activity of fresh and processed edible seaweeds . Journal of the Science of Food and Agriculture , 81 : 530 – 534 .
- Nuutila , A.M. , Puuponen-Pimia , R. , Arani , M. and Oksman-Caldenly , K.M. 2003 . Composition of antioxidant activities of onion and garlic extracts by inhibition of lipid peroxiadation and radical-scavenging activity . Food Chemistry , 81 : 485 – 493 .
- Abdille , M.H. , Singh , R.P. , Jayaprakasha , G.K. and Jena , B.S. 2005 . Antioxidant activity of the extracts from Dillenia indica fruits . Food Chemistry , 90 : 891 – 896 .
- Fu , M. , He , Z. , Zhao , Y. , Yang , J. and Mao , L. 2009 . Antioxidant properties and involved compounds of daylily flowers in relation to maturity . Food Chemistry , 114 : 1192 – 1197 .
- Lotito , S.B. and Fraga , C.G. 1999 . (+)-Catechin as antioxidant: Mechanism preventing human plasma oxidation and activity in red wines . Bio Factors , 10 : 125 – 130 .
- Srinivasan , M. , Sudheer , A.R. and Menon , V.P. 2007 . Ferulic acid: Therapeutic potential through its antioxidant property . Journal of Clinical Biochemistry and Nutrition , 40 : 92 – 100 .