Abstract
Colour compounds of rosé wines can be very sensitive to storage conditions because of the low levels of naturally-occurring protective compounds. This article deals with the effects of storing rosé wines under different temperature and light conditions, with regard to their colour characteristics and anthocyanin profile. Samples were taken seven times altogether over a period of 582 days. At the end of the storage period, the resulting differences in the wines were studied by μLC/MS. It was found that storing rosé wine at room temperature caused a reduction of 50% in the levels of anthocyanins compared to storing them at 3°C. Increasing the storage temperature to 45°C caused the total decomposition of all anthocyanins. Furthermore, during the maturation of the wine, a loss of sugar moiety, hydration of aglycone, and the formation of the corresponding chalcone or diketone were observed. Levels of pyranoanthocyanins in the wines were studied as well. Vitisins A and B, hydroxyphenyl-pyranomalvidin-3-glucoside, dihydroxyphenyl-pyranomalvidin-3-glucoside, and methoxyhydroxyphenyl-pyranomalvidin-3-glucoside were identified. The highest levels of both chalcones and pyranoanthocyanins were found in samples stored at room temperature. The effect of irradiation on the pigments also appeared to be significant but less important than the effect of high temperature.
INTRODUCTION
Colour is an important sensory indicator of wine quality, and it is the first property of a drink to be assessed by any potential consumer. The anthocyanin pigments are responsible for the colour of rosé wines. Anthocyanins in beverages are sensitive to changes in pH, oxidation, increased temperatures, and also light. These reactions are responsible for the changes in colour hue and colour intensity during the processing and storage of food.[Citation1–3The levels of anthocyanins in wine depend above all on the variety, the degree of maturity of the grapes,[Citation4] the method of vinification,[Citation5–7 the various wine additives employed, and also on the degree of ageing.[Citation8–10 In young red and rosé wines, anthocyanins occur as monomers, and during the period of ageing, they pass through numerous condensation, polymeration, and oxidation reactions,[Citation11,Citation12] which result in changes to the anthocyanin profile and, consequently, to the colour of the wine. Detectable changes in colour take place and are manifested, above all, as a reduction in colour intensity and the occurrence of yellowish-brown hues. The course of these reactions is significantly influenced by temperature and light.[Citation12–14 Citation Citation14
Glass bottles, especially those with clear glass, are not ideal for protecting their contents against the negative effects of long-wave radiation.[Citation14,Citation15] This means that the wine is at risk from the effects of photo-oxidation. Such a reaction is often supported and potentiated by the higher temperatures that are, for example, common on the shelves of some supermarkets. Sometimes one could even imagine a Maillard reaction taking place, in the course of which the original colour compounds are changed into brownish fragments and/or polymers.[Citation16] In wine, anthocyanins, as the most important colour substances, and also some other, initially colourless, compounds related to polyphenols participate in these changes. In general, controlled oxidation processes and reactions with tannins are important for wine colour stabilization. However, if both processes take place too intensively, a negative effect on the anthocyanin profile and colour is observed.[Citation16,Citation17] Colour stability can also be significantly influenced by temperature. If rosé wines are aged under conditions with increased temperatures, their colour usually changes to orange because of an increase in levels of yellow pigments.[Citation18] This phenomenon is quite often used for the artificial ageing of wines, e.g., to adjust the rosé colour to resemble the hue of onion skins.
MATERIALS AND METHODS
Samples and Storage Conditions
Rosé wine, vintage 2006, was made from grapes of the grapevine (Vitis vinifera L.) varieties Blaufränkisch and St.Laurent in a 1:1 ratio. Both were produced in the wine growing region of Velké Pavlovice (South Moravia). The grapes were processed together, and the sugar content in the subsequent juice was 21.2° of must density (NM, i.e., kg of sugar per 100 l of juice). Rosé wine was produced by a short maceration of the must on the skins (8 h) and only the free-run juice (i.e., without pressing) was used. This juice was settled by simple sedimentation for 10 h and then racked. Then the juice was inoculated with a pure culture of Saccharomyces cerevisiae yeasts (Oenoferm rosé, Erbslöh Geisenheim) and fermented at a temperature of 15°C. After the end of fermentation, the rosé wine was racked off the yeast and treated with bentonite (100 g.hl−1), sulphured three times with a dose of 20 mg.l−1 of sulphur dioxide, and stored in a glass demijohn. Prior to storage, the results of a chemical analysis were as follows: 12.3% vol. alcohol, 1.8 g.l−1 residual sugar, 5.4 g.l−1 titrable acids, and 18 mg.l−1 free SO2. The initial colour parameters are presented in .
Table 1 Initial colour parameters of the rosé wine studied (colour intensity, colour hue, total anthocyanins content, total polyphenols content, tri-chromatic parameters L*, a*, b*, and chemical age)
Wine was drawn into transparent bottles with a volume of 0.35 l and stoppered with corks of standard quality (Colmated Cork Janosa). There were three replications (to prevent influence of oxygen, which goes through the stopper). Bottles were stored in a vertical position to simulate the conditions in retail stores as far as possible. Information about the storage conditions of bottles is presented in . The code numbers for the samples are composed of a number, which represents the temperature, and a letter, which indicates the lighting conditions. The letter T means the wines were stored in darkness without any light at all, because most wines in practice are stored in darkness. Z means the wines were stored under a cool fluorescent lamp, which is most common in supermarkets. D means the samples were stored in daylight (light during the day alternating with darkness during the night, to reflect the conditions to be found in small shops). K represents an alternative fluorescent lamp used to simulate non-stop daylight, and UV refers to those samples irradiated with UV beams. The wines were analysed by spectrophotometric and chromatographic methods at regular intervals ().
Table 2 Code numbers used for the different treatments of the rosé wines
Table 3 Observations on the colour parameters (total anthocyanins content, total polyphenols content, chemical age) of rosé wines stored under different conditions for 582 days
Spectrophotometric methods
Concentrations of total polyphenols were determined by spectrophotometry (Helois β/Unicam, Spectronic Unicam, Cambridge, UK) with the Folin-Ciocalteu agent calculated as ]gallic acid.[Citation19,Citation20] Total anthocyanins, values of colour intensity, colour hue, and the index of “chemical age” of wine were estimated by spectrophotometry (Helois β/Unicam) using the method described by Somers and Evans.[Citation13] Colour coordinates CIE L*, a*, b* were measured by a tri-chromatic method using a Chroma-Meter CT 210/Minolta (EU no. 2676/90; methodological OIV manuals—1990; Minolta, Osaka, Japan).
μLC/MS method
Analyses of individual anthocyanins were performed using μLC/MS methods (microliquid chromatograph CapLC XE and Q-T of Premier Mass Spectrometer [both; Waters, Milford, MA, USA]). Chromatographic separation was performed using a Gemini C-18 micro-column (150 × 0.3 mm; Phenomenex, Torrance, CA, USA). Gradient elution was used for the chromatography, with mobile phase A using 0.12% (v/v) of trifluoroacetic acid and 5% (v/v) of acetonitrile in water, and mobile phase B using 0.12% (v/v) of trifluoroacetic acid in acetonitrile. The gradient profile was 0–5 min 10% B, 5–10 min 10–20% B, 10–30 min 20–30% B, 30–40 min 30–50% B, 40–50 min 50–70% B, 50–54 min 70–100% B, 54–59 min 100% B. The flow rate of the mobile phase was 5 μL/min. Electrospray (ESI+, Z-spray) was used as the ion source. Other parameters of the mass spectrometer were as follows: spray voltage +3.2 kV, temperature of ion source 120°C, desolvation temperature 150°C, flow rate of nebulizing gas 30 L.h−1, and flow rate of desolvation gas 300 L.h−1. Identification of anthocyanins was based on an interpretation of MS and MS2 spectra, accurate mass measurement, and a comparison of retention times. Determination of the levels of compounds under investigation was based on a calibration curve method using standard solutions of malvidin-3-glucoside (differences in response factors were neglected). The calibration dependence was created at four concentration levels ranging from 10–100 mg/L. Each level was the mean of three repetitions. Linear regression was used for evaluation (R 2 = 0.99).
Reagents, Solvents, and Sample Preparation
Standards: Water, methanol, acetonitrile, and trifluoroacetic acid were purchased from Fluka (Buchs, Switzerland). The standard for malvidin-3-glucoside was provided by Carl-Roth (Karlsruhe, Germany). Prior to μLC/MS, samples were purified and pre-concentrated using SPE columns (Strata SDB-L, 200 mg/3 mL, Phenomenex, Torrance, CA, USA). The procedure for sample purification was based on the previously described procedure[Citation21] and samples were enriched six times. After purification samples were stored in a freezer at −80°C. Before carrying out the analysis, samples were dissolved in an appropriate amount of mobile phase A, briefly sonicated and centrifuged.
Statistical Methods
Data were evaluated using principal components analysis (Unistat programme).
RESULTS AND DISCUSSION
During the 2006 vintage, rosé wine was produced from the varieties Frankovka (Blaufränkisch) and Saint Laurent, which are traditional red grapevine varieties of Vitis vinifera L. in South Moravia, Czech Republic. The levels of anthocyanins in fresh grapes ranged from 0.86 to 1.78 g kg−1.[Citation22] After bottling, the wine was stored under conditions described in and the following parameters were measured a total of seven times over a period of 582 days: colour hue and intensity, levels of polyphenols, levels of total anthocyanins, L*, a*, and b* values and the chemical age of the wine.
Wine stored at 45°C was markedly different from the other treatments in nearly all parameters; this was obviously due to the fact that at such temperatures very intensive chemical reactions take place, which result in the formation of brownish products. The parameter chemical age was evaluated according to the method published by Somers and Evans.[Citation13] It is used as an indicator of the importance of polymer anthocyanins for the development of wine colour and its intensification in the course of the ageing of red wines. Colour intensity was expressed as the sum of the absorbance values of red and/or rosé wine compared to distilled water in a ten-millilitre cuvette at λ = 420, 520, and 620 nm. Hues of rosé and red wines were expressed as a share of absorbance values at λ = 420 and 520 nm with an identical optical bandwidth, compared to distilled water. In the course of storage, a significant decrease in the levels of anthocyanins was observed under all types of storage conditions. Minimal changes were found in wine stored at a temperature of 3°C. On the other hand, high degradation activity was recorded in wines stored at 45°C. At such temperatures, the levels of anthocyanins dropped to less than 10% of the initial value within 30 days of the commencement of storage.
There are several mechanisms for the degradation of anthocyanins. Each of them is based on the transformation of anthocyanidin into colourless chalkon (when the heterocyclic ring of the carbinol base breaks).[Citation23] Changes in the concentrations of all colour forms (hues), expressed as anthocyanins, as well as concentrations of all monomer anthocyanins (measured by various methods), showed a very similar pattern, with a tendency to decrease. The ionised monomer anthocyanins were the most sensitive components. In many cases, however, this was not the direct result of a decrease in concentration resulting from the degradation of anthocyanins, but rather because of a shift in their absorption balance, caused by their reactions with either other phenolic structures, acetaldehyde, or sulphur dioxide.Citation[13] The decrease of anthocyanins is shown in . An increase in values for chemical age documented a gradual increase in polymer and co-polymer concentrations (or increase in “polymer and co-polymer hues”), and naturally came at the expense of monomer anthocyanins. However, in spite of this, it was possible to conclude that a certain reduction of colour intensity occurred in the experimental wines in the course of ageing because the colour intensity of all stored samples decreased by more than 50%. At the same time, a marked increase in the brick colour was observed, associated with a continuously increasing value of the measured hue. Gradually predominating brick tones were directly associated with changes in wine absorbance at a wavelength of 420 nm.
Figure 1 Changes in levels of anthocyanins during the storage of rosé wine for 582 days (measured by the method from Somers and Evans[Citation13]) (45T—darkness, 45°C; 3Z—fluorescent lamp, 3°C; 3T—darkness, 3°C; 25Z—fluorescent lamp, 25°C; 25UV—UV lamp, 25°C; 25T—darkness, 25°C; 25K—nonstop daylight, 25°C; 25D—daylight, 25°C).
![Figure 1 Changes in levels of anthocyanins during the storage of rosé wine for 582 days (measured by the method from Somers and Evans[Citation13]) (45T—darkness, 45°C; 3Z—fluorescent lamp, 3°C; 3T—darkness, 3°C; 25Z—fluorescent lamp, 25°C; 25UV—UV lamp, 25°C; 25T—darkness, 25°C; 25K—nonstop daylight, 25°C; 25D—daylight, 25°C).](/cms/asset/c67f9c55-5d61-49fb-8262-b348c6a8be76/ljfp_a_511751_o_f0001g.gif)
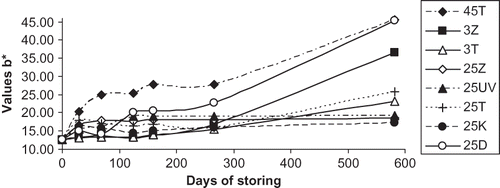
A longer period of ageing was associated with a decrease in the values of the parameter L* in all stored samples of wine, to greater or lesser extents (). These results suggest that the wines lost brightness and became darker. This could be explained by the loss of native anthocyanins with the formation of their degradation products, which are mostly dark and brown, and condensation with other polyphenols. The results correspond with trends described by Van Buren et al.,[Citation24] who studied the effects of light and temperature on the parameter L* in the variety Pinot noir. In the course of storage, values of the parameter b* increased, and this corroborates the fact that the stored wine acquires yellow hues, as shown in . Among the wine samples in this study, this phenomenon was most obvious in wine stored either in darkness at 45°C or in daylight at 25°C (the latter storage conditions are the most common in practice, and are, therefore, the most significant for the storage of rosé wine).
Figure 2 Changes in the colour parameter CIE L* during the storage of rosé wine for 582 days (45T—darkness, 45°C; 3Z—fluorescent lamp, 3°C; 3T—darkness, 3°C; 25Z—fluorescent lamp, 25°C; 25UV—UV lamp, 25°C; 25T—darkness, 25°C; 25K—nonstop daylight, 25°C; 25D—daylight, 25°C).
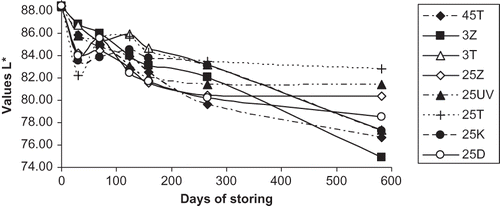
Figure 3 Changes in the colour parameter CIE b* during the storage of rosé wine (45T—darkness, 45°C; 3Z—fluorescent lamp, 3°C; 3T—darkness, 3°C; 25Z—fluorescent lamp, 25°C; 25UV—UV lamp, 25°C; 25T—darkness, 25°C; 25K—nonstop daylight, 25°C; 25D—daylight, 25°C).
The data (i.e., total anthocyanins, total polyphenols, colour intensity, hue, CIE parameters L*, a*, and b*) were examined using principal components analysis. The first two of them (depicted as axes in ) explain 75.4% of the total variability of the original variables and classify the samples of rosé wine under study into several markedly different colour groups (A, B, C, D, E, and F). The CIE colour parameter b* correlated positively with the major component, as did the colour hue, which was also positively correlated with the second major component. On the other hand, the content of total anthocyanins was negatively correlated with Component 1.
Figure 4 Principal components analysis of the colour parameters of stored rosé wines (A—Original sample; B—storage in darkness at 3°C; C—storage under different light conditions at 3°C; D—storage for 69 days (main part of samples); E—storage at 45°C; F—storage for 582 days).
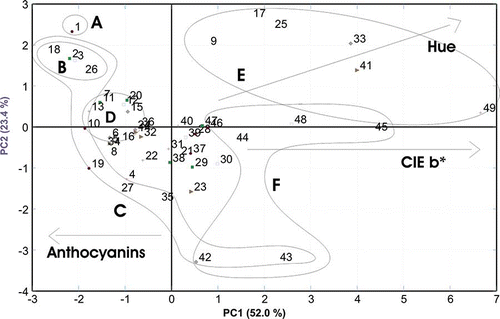
Using Component 1, samples of rosé wine stored at 3°C (Group B) differed from those stored at 45°C (Group E). This, however, did not apply to those samples that were also stored at 3°C, but for a period of as much as 582 days (sample nos. 42 and 43). All samples that were stored at 3°C were in Group C. Similarly, samples stored for a long period of 582 days (Group F) were also separated. From the viewpoint of Component 1 values of these samples were positive. When using Component 2, sample nos. 42 and 43 were also separated. Parameters of wine stored in darkness at 3°C were most similar to those of a non-stored (showed the smallest changes over time) control sample. Although the symptoms of ageing (expressed as changes in the colour parameters of the stored wine) showed the lowest dynamics under these storage conditions, they were not able to prevent the occurrence of significant changes in colour hues of stored rosé wine samples. This fact was documented also by the measured trend of the lowest loss of anthocyanins, as shown in , and/or the lowest value of chemical age (). The data corroborated the fact that the temperature and the time of storage were more important factors influencing the quality of wine (and above all its colour hues) than the light conditions.
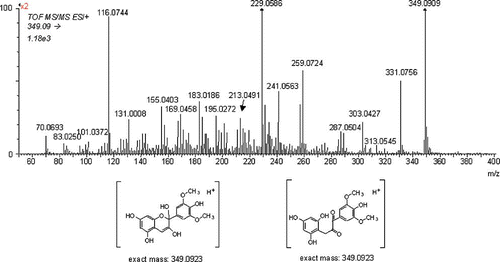
Selected samples were analyzed using μLC/DAD/ESI-MS2. shows the peak of the main native anthocyanin malvidin-3-glucoside (Mv-3-Gl, m/z 493.1331, with its exact mass determined with an accuracy of −3.0 ppm in the sample stored at 25°C in darkness. The upper chromatogram obtained using UV/VIS detector (λ = 520 nm) confirms that this compound is the predominant pigment in this sample (the difference in the retention time of the main peak obtained using the DAD detector and the peak of Mv-3-Gl corresponds with the time shift of both detectors connected in series). The bottom chromatogram illustrates the process of anthocyanin degradation. A relatively broad peak eluting before the main anthocyanin can be seen in the reconstructed chromatogram of m/z 349.09 (, trace 3). This compound corresponds with the loss of sugar moiety, hydration of aglycone, and/or formation of the corresponding chalcone or diketone, respectively, which is in accordance with already published results.[Citation25] The identity of the proposed structure is supported by the MS/MS spectra (, upper spectrum). The loss of water and formation of malvidin (or its isomer; 349 – 331 = 18) and the consequent cascade of losses of water, carbon monoxide, and cleavage of methyl groups can be seen in the spectrum. Exact mass measurement performed using direct infusion in independent experiments provided ions at m/z 349.0916 (accuracy −2 ppm).
Figure 5 μLC/MS analysis of malvidin-3-glucoside and the hydrated form of malvidin or the related chalcone (sample of rosé wine stored for 582 days at 25°C in darkness).
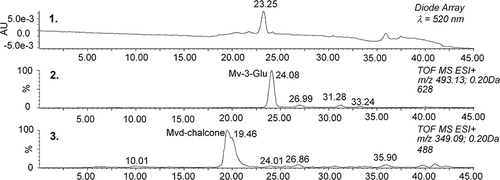
Figure 6 MS/MS spectra of the hydrated form of malvidin or the related chalcone (sample of rosé wine stored for 582 days at 25°C in darkness).
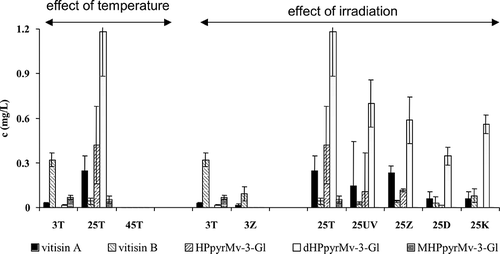
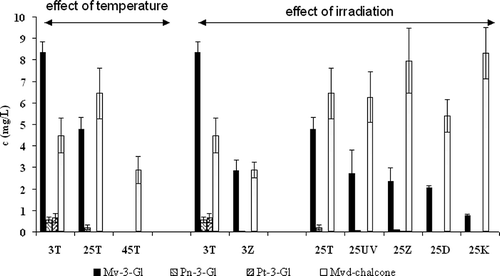
reflects the effect of different storage conditions on the levels of anthocyanins. It can be seen that ageing the rosé wine at room temperature causes a dramatic decrease in the levels of Mv-3-Gl (by half, compared to those stored at 3°C). The decrease of peonidin-3-glucoside and petunidin-3-glucoside (Pn-3-Gl and Pt-3-Gl) exhibits a similar trend. On the other hand, the increase in the levels of the hydrated forms (chalcone and diketone) with increasing temperature is significant. Further increase of the storage temperature, up to 45°C, causes the decomposition of both the anthocyanins and their hydrated forms (the native anthocyanins disappeared completely). Our experiments also confirmed that all of the types of radiation studied caused a substantial decrease in the levels of native anthocyanins at both the studied temperatures (42.9–83.8% compared to storage in darkness). This experiment confirms that high temperature has a more destructive effect than any of the tested radiations. No direct connection between irradiation and the formation of hydrated forms, or chalcon, was proven.
Figure 7 Effect of different storage conditions on anthocyanins and their degradation (45T—darkness, 45°C; 3Z—fluorescent lamp, 3°C; 3T—darkness, 3°C; 25Z—fluorescent lamp, 25°C; 25UV—UV lamp, 25°C; 25T—darkness, 25°C; 25K—nonstop daylight, 25°C; 25D—daylight, 25°C) (Mv-3-Gl–Malvidin-3-glukoside; Pn-3-Gl–Peonidin-3-glukoside; Pt-3-Gl–Petunidin-3-glukoside; Mvd-chalcone–Malvidin chalcone).
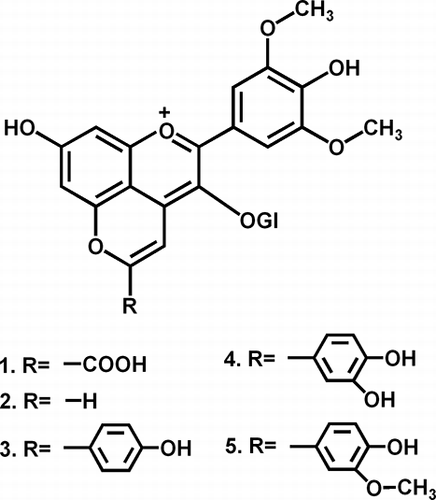
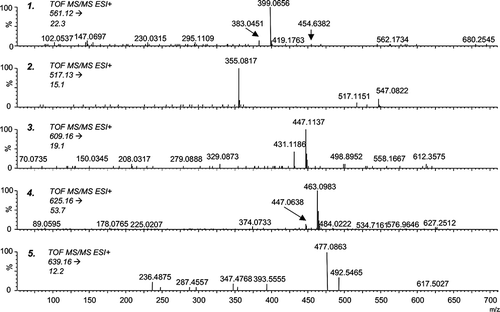
It is well known that during wine maturation more complex pigments are formed. Perhaps the most frequently mentioned in the literature are vitisins A and B (, structures 1, 2),[Citation26,Citation27] belonging to the wide group of pyranoanthocyanins.[Citation25] Regardless of the uncertainties about their exact structure and the way they are formed, the number of derivatives that have been identified continues to increase and their influence on the colour of wine is significant. Rosé and pale red wines, especially that contain relatively low levels of tannins (which are known to protect anthocyanins), form complex pigments easily.[Citation28,Citation29] shows the structures of pyranoderivatives found and monitored throughout this study (structure 3, hydroxyphenyl-pyranomalvidin-3-glucoside, HPpyrMv-3-Gl; structure 4, dihydroxyphenyl-pyranomalvidin-3-glucoside, dHPpyrMv-3-Gl; structure 5, methoxyhydroxyphenyl-pyranomalvidin-3-glucoside, MHPpyrMv-3-Gl). Their chromatographic separation and fragmentation patterns can be seen in and . The loss of dehydrated hexose can be easily detected. Many polyphenolic structures with the same nominal mass, or even isomers, can be taken into consideration. Peonidin-3-glucoside, petunidin-3(6-coumaroyl)glucoside, or theoretically even methylquercetin-3-rutinoside, can be considered besides the dHPpyrMv-3-Gl, for instance. However, thanks to being able to accurately determine mass, the study of the fragmentation pattern and the evaluation of retention times (polarity) excludes these alternatives. In the case of the ions at m/z 609.16 and 639.16, the situation is similar.
Figure 9 μLC/MS analysis of pyranoanthocyanins (sample of rosé wine stored for 582 days at 25°C in darkness).
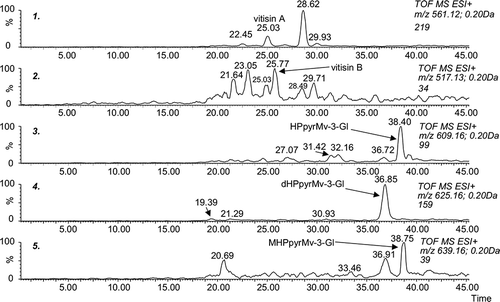
Figure 10 MS/MS spectra of identified pyranoanthocyanins (sample of rosé wine stored for 582 days at 25°C in darkness).
The effect of different storage conditions on the levels of pyranoanthocyanins can be traced in . Generally, storage at room temperature leads to a substantially higher yield of pyranoanthocyanins compared to cold storage (except vitisin B). Storage at 45°C caused the complete disappearance of these pigments. The effects of lighting are less pronounced and more dependent on the structure of the pyranoanthocyanins. The relative decrease in the levels of vitisin B in the irradiated samples with respect to the non-irradiated one is comparable with the decrease of Mv-3-Gl when stored at 3°C. When those differences are studied in samples stored at room temperature the a relative decrease of vitisin B and dHPpyrMv-3-Gl is significantly smaller than the decrease of Mv-3-Gl. This trend is not distinct in the case of the other pyranoderivatives.
CONCLUSIONS
This study demonstrates the significant effect of storage conditions on the quality of rosé wine; the most important factors are temperature and length of storage. The type of radiation was shown to be insignificant. The most pronounced changes were observed during the ageing of wine at 45°C, and these mostly occur in the first few months of storage. The anthocyanin levels were shown to be the most sensitive components of the wine, and these compounds quickly degraded when the wines were stored under unfavourable conditions. μLC/MS proved that higher storage temperatures cause a significant decrease in particular native anthocyanins. During maturation, the formation of hydrated forms of anthocyanins (chalcones and diketones) was found to be important in influencing the quantity and quality of anthocyanins. Temperature appeared to be a critical parameter also for the formation of pyranoanthocyanins, since, although these condensation processes are enhanced at room temperature, ageing the wine at 45°C caused a significant decrease in the level of pyranoanthocyanins. Long-term irradiation of samples at 3 and 25°C caused considerable decomposition of individual pigments as well (as measured by μLC/MS).
ACKNOWLEDGMENTS
The authors gratefully acknowledge financial support by the Grant Agency of Czech Republic (project No. P206/10/0625). This work was also supported by Operational Program Research and Development for Innovations-European Social Fund (Project CZ.1.05/2.1.00/03.0058) of the Ministry of Education Youth and Sports of the Czech Republic.
REFERENCES
- Jyothi , A.N. , Moorthy , S.N. and Eswariamma , C.S. 2005 . Anthocyanins in sweet potato leaves-varietal screening, growth phase studies and stability in a model system . International Journal of Food Properties , 8 ( 2 ) : 221 – 232 .
- Fulcrand , H. , Duenas , M. , Salas , E. , Salas , E. and Cheynier , V. 2006 . Phenolic reactions during winemaking and aging . American Journal of Enology and Viticulture , 57 ( 3 ) : 289 – 297 .
- Jackaman , L.R. , Yada , Y.R. , Tung , A.M. and Speers , A.R. 1987 . Anthocyanins as food colorants . Journal of Food and Biochemistry , 11 : 201 – 247 .
- Peréz-Magarino , S. and Gonzáles-San José , M.L. 2006 . Polyphenols and colour variability of red wines made from grapes harvested at different ripeness grade . Food Chemistry , 96 : 197 – 208 .
- Morel-Salmi , C. , Bouquet , J.-M. , Bes , M. and Cheynier , V. 2006 . Effect of flash release treatment of on phenolic extraction and wine composition . Journal of Agricultural and Food Chemistry , 54 : 4270 – 4276 .
- Álvarez , I. , Alexandre , J.L. , García , M.J. and Lizama , V. 2006 . Impact of prefermentation maceration on the phenolic and volatile compounds in Monastrell red wines . Analytica Chimica Acta , 563 : 109 – 115 .
- Pérez-Lamela , C. , García-Falcón , M.S. , Simal-Gándara , J. and Orriols-Fernández , I. 2007 . Influence of grape variety, vine system and enological treatments on the colour stability of young red wines . Food Chemistry , 101 : 601 – 606 .
- Castillo-Sánchez , J.J. , Mejuto , J.C. , Garrido , J. and García-Falcón , S. 2006 . Influence of wine-making protocol and fining agents on the evolution of the anthocyanin content, color and general organoleptic duality Vinhao wines . Food Chemistry , 97 : 130 – 136 .
- Alcade-Eon , C. , Escribano-Bailón , M.T. , Santos-Buelga , C. and Rivas-Gonzalo , J.C. 2006 . Changes in the detailed pigment composition of red wine during maturity and ageing. A comprehensive study . Analytica Chimica Acta , 563 : 238 – 254 .
- Rival , E.G.-P. , Alcade-Eon , C. , Santos-Buelga , C. , Rivas-Gonzalo , J.C. and Escribano-Bailón , M.T. 2006 . Behaviour and characterisation of the colour during red winemaking and maturation . Analytica Chimica Acta , 563 : 215 – 222 .
- Sims , C. and Morrison , J. 1984 . The effect of pH, sulphur dioxide, storage time and temperature on the color and stability of red muscadine grape wine . American Journal of Enology and Viticulture , 35 : 35 – 39 .
- Brouillard , R. , Chassaing , A. and Fougerousse , A. 2003 . Why are grape/fresch wine anthocyanins so simple and why is it red wine color last so long? . Phytochemistry , 64 : 1179 – 1186 .
- Somers , T.C. and Evans , M.E. 1977 . Spectral evaluation of young red wines: Anthocyanin equilibria, total phenolics, free and molecular SO2 “ chemical age” . Journal of the Science of Food and Agriculture , 28 : 279 – 287 .
- Revilla , E. , López , J.F. and Ryan , J-M. 2005 . Anthocyanin pattern of Tempranillo wines during ageing in oak barells and storage in stainless-steel tanks . European Food and Research Technology , 220 : 592 – 596 .
- Selli , S. and Canbas , A. 2004 . Ünal, Ü . Effect of bottle colour and storage conditions on browning of orange wine. Molecular Nutrition and Food Research , 46 : 64 – 67 .
- Gómez , E. , Martinéz , A. , Laencina , J. and Gómez , E. 1995 . Prevention of oxidative browning during wine storage . Food Research International , 28 : 213 – 127 .
- Jordao , A.M. , Ricardo-da-Silva , J.M. and Laureano , O. 2006 . Effect of oak constituents and oxygen on the evolution of malvidin-3-glucoside and (+) catechin in model wine . American Journal of Enology and Viticulture , 57 : 377 – 381 .
- Adams , J.B. and Brown , H.M. 2007 . Discoloration in raw and processed fruits and vegetables . Critical Reviews in Food Science and Nutrition , 47 : 319 – 333 .
- Singleton , V.L. and Rossi , J.A. 1965 . Colorimetry of total phenolic with phosphomolybdic-phosphotungstic acid reagents . American Journal of Enology and Viticulture , 16 : 144 – 158 .
- Zocklein , B.W. , Fugelsang , K.C. , Gump , B.H. and Nury , F.S. 1990 . Production Wine Analysis , New York : Van Nostrand Reinhold .
- Bednář , P. , Papoušková , B. , Müller , L. , Barták , P. , Stávek , J. , Pavloušek , P. and Lemr , K. 2005 . Utilization of capillary electrophoresis/mass spectrometry (CE/MSn) for the study of anthocyanin dyes . Journal of Separation Sciences , 28 : 1291 – 1299 .
- Balík , J. and Kumšta , M. 2008 . Evaluation of colour content in grapes originating from south Moravia . Czech Journal of Food Sciences , 26 : S18 – S24 .
- Adams , J.B. 1973 . Thermal degradation of anthocyanins with particular reference to 3-glucosides of cyanidin. 1. Acidified aqueous-solution at 100 degrees C . Journal of Science of Food and Agriculture , 24 : 747 – 762 .
- Van Buren , J.P. , Bertino , J.J. and Robinson , W.B. 1968 . The stability of wine anthocyanins on exposure to heat and light . American Journal of Enology and Viticulture , 19 : 147
- Castaneda-Ovando , A. , Pacheo-Hernández , M. , Páez-Hernández , M.E. , Rodríguez , J.A. and Galán-Vidal , C.A. 2009 . Chemical studies of anthocyanins: A review . Food Chemistry , 113 : 859 – 871 .
- Bakker , J. and Timberlake , C.F. 1985 . The distribution of anthocyanins in grape skin extracts of port wine cultivars as determined by high performance liquid chromatography . Journal of Science of Food and Agriculture , 36 : 1315 – 1324 . 1325 – 1333 .
- Bakker , J. and Timberlake , C.F. 1997 . Isolation, identification and characterization of new color-stable anthocyanins occuring in some red wines . Journal of Agricultural and Food Chemistry , 45 : 35 – 43 .
- Abril , M. , Negueruela , A.I. , Pérez , C. , Juan , T. and Estopanán , E. 2005 . Preliminary study of resveratrol content in Aragón red and rosé wines . Food Chemistry , 92 : 729 – 736 .
- Cliff , M.A. , King , C.M. and Schlosser , J. 2007 . Anthocyanin, phenolic composition, colour measurement and sensory analysis of BC commercial red wines . Food Researche International , 40 : 92 – 100 .