Abstract
Soybean protein hydrolysates were prepared by hydrolyzing soybean protein isolates with a protease alcalase to a degree of hydrolysis of 16.6%, and then modified by alcalase-catalyzed plastein reaction to reveal the impact of plastein reaction on the ACE-inhibitory activity of the modified product in vitro. The suitable conditions of plastein reaction of soybean protein hydrolysates were selected based on the results of response surface methodology with the decreased amount of the free amino groups of the modified product as response. When reaction temperature was fixed at 30°C, the selected conditions were as follows: concentration of soybean protein hydrolysates of 45% (w/w), addition level of alcalase of 275 U/g peptides, and reaction time of 3 to 4 h. Soybean protein hydrolysates and eight modified products were evaluated for their ACE-inhibitory activities in vitro. The assay results highlighted that plastein reaction improved the ACE-inhibitory activity of the modified product. The IC50 of the modified products ranged from 0.64 to 1.11 mg/mL, while that of soybean protein hydrolysates was 1.45 mg/mL. The decreased amount of the free amino groups of the modified product showed influence on the ACE-inhibitory activity in vitro. Analysis results from size exclusion chromatography confirmed that some plasteins with higher molecular weights were formed in the modified product. Our results showed that alcalase-catalyzed plastein reaction could be applied as a potential approach to enhance the ACE-inhibitory activity of soybean protein hydrolysates in vitro.
INTRODUCTION
Hypertension is a common disease among adults and often leads to serious health problems. Hence, numerous researchers have been focusing their research on the prevention and treatment of hypertension.Citation[1] Angiotensin-I-converting enzyme (ACE, EC 3.4.15.1) plays a key physiological role in the control of blood pressure via the renin-angiotensin system.Citation[2] Potent synthetic ACE inhibitors, such as captopril, enalapril, alacepil, lisinopril, and ramipril, are widely used in clinical medicine to treat hypertension and heart disorders in humans.Citation[3] These synthetic drugs, however, have some side effects, including coughing, taste disturbances, and skin rashes.Citation[4] Food-derived ACE inhibitors, like bioactive peptides, could be used as potential alternatives to synthetic drugs and have attracted the attention of many researchers. A variety of ACE-inhibitory peptides have been isolated from food proteins, including soybean proteins, corn gluten, and milk proteins.Citation[5 – Citation8]
As one of the most cultivated plants in the world, soybean is an important protein source and has various potential health effects.Citation[9] Soybean-protein-derived ACE-inhibitory peptides are receiving more and more attention since they have high ACE-inhibitory activity in vitro. Rho et al.Citation[10] fractionated ACE-inhibitory peptides from fermented soybean extract into four individual fractions with an ultrafiltration system, and found that the IC50 values of these fractions were 1.53, 3.5, 3.5, and 1.12 mg proteins/mL, respectively. Farzamirad and AlukoCitation[11] hydrolyzed soybean protein isolates with pepsin and pancreatin followed by separating the hydrolysates on a SP-Sepharose column to obtain ACE-inhibitory fractions, and found that three of the five fractions inhibited ACE activity with the IC50 values of 1.09, 0.42, and 0.25 mg/mL, respectively. Chiang et al.Citation[12] employed five proteolytic enzymes to hydrolyze soybean protein isolates, and their results indicated that hydrolysis of soybean protein isolates for 0.5–6 h with alcalase produced the highest ACE-inhibitory activity in vitro. The optimal conditions to obtain soybean protein hydrolysates with the highest ACE-inhibitory activity were E/S of 1% (w/w), hydrolysis temperature of 50°C, pH 9.0, and hydrolysis time of 6 h. Under these conditions, the IC50 value of the hydrolysates they prepared was 0.67 mg /mL.
Plastein reaction enables peptide fragments of protein hydrolysates to join enzymatically through peptide bonds.Citation[13] A mixture of higher-molecular, protein-like substances formed from lower-molecular peptides during the plastein reaction is called plastein.Citation[14] Although the plastein reaction has been observed for more than 100 years, the precise reaction mechanism remains at the stage of an intellectual curiosity, and there is continuing argument about it.Citation[15] The mechanism of plastein reaction seems to involve condensation, transpeptidation, and physical aggregation.Citation[16 – Citation18] A recent study suggested that several pathways might be present in the plastein reaction simultaneously.Citation[19] The synthesis of plasteins by plastein reaction could be of considerable interest to food chemists, since it can add essential amino acids (e.g., L-tryptophan or L-methionine) to some proteins that are deficient in them,Citation[20,Citation21] reduce the content of some amino acids (e.g., L-phenylalanine) for dietetic applications,Citation[22] or improve functional properties (e.g., emulsification, viscosity, or foaming) of food proteins.Citation[23] If condensation and transpeptidation do exist in the plastein reaction of protein hydrolysates, some new peptides with different sequences from raw protein might be generated, which might lead to the alteration in their bioactivity, including ACE-inhibitory activity. Based on such consideration, plastein reaction might be applied as an approach to modify the biological activity of protein hydrolysates. In a recent study, plastein reaction was used to modify the antioxidant activity of the hydrolysates from squid hepatopancreas with alcalase.Citation[24] Our previous study showed that when plastein reaction was applied to modify casein hydrolysates, the resulting product had better ACE-inhibitory activity or radical scavenging activity in vitro.Citation[25,Citation26] Whether plastein reaction might impact the ACE-inhibitory activity of soybean protein hydrolysates is unknown.
In our presented work, soybean protein hydrolysates were prepared by hydrolyzing soybean protein isolates with a protease alcalase, and were modified by alcalase-catalyzed plastein reaction. In order to obtain higher condensation extent during plastein reaction, some reaction conditions were studied by response surface methodology with the decreased amount of free amino groups of the modified product as response. The ACE-inhibitory activities of soybean protein hydrolysates and some modified products prepared were measured in vitro and compared to reveal the impact of plastein reaction on the ACE-inhibitory activity of the modified product. The peptide profiles of some modified products were analyzed with size exclusion chromatography and were compared to that of soybean protein hydrolysates to show the impact of plastein reaction on the peptide distributions of the modified product.
MATERIALS AND METHODS
Materials
Alcalase with activity of 110,000 U/g was purchased from Pangbo Biochem. Inc. (Nanning, China). N-(3-[2-furyl]acryloyl)-L-phenylalanylglycylglycine (FAPGG) and rabbit lung acetone powder were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Other reagents used were chemicals of analytical grade. Highly purified water was prepared with Milli-Q PLUS (Millipore Corporation, New York, NY, USA) and used for the preparation of all buffers and solutions.
Soybean protein isolates (SPI) used were prepared from defatted soybean flour (a by-product of Harbin High-Tech Protein Co., Ltd., Harbin, China) by extracting the proteins with alkaline water (1:10 w/v, flour/water ratio) for 2 h at ambient temperature. The pH of suspension was periodically adjusted to 8.5 with 1 mol/L NaOH, and then centrifuged at 4000× g for 20 min at 4°C with a low-temperature centrifuge (LG-21M, Shanghai Institute of Centrifuges and Equipments, Shanghai, China). The supernatant obtained was brought to pH 4.5 with 2 mol/L HCl. An isoelectric precipitate was collected by centrifugation at 4000× g for 20 min at 4°C with the centrifuge. The precipitate was washed with distilled water three times, adjusted to pH 7.0 with 1 mol/L NaOH, then lyophilized and stored at −20°C for later use.
Preparation of Soybean Protein Hydrolysates
Prepared SPI was dissolved in 100 mL of water to give an original protein concentration of 10% (w/v). The solution was adjusted to pH 8.5 with 1 mol/L NaOH. Some alcalase (addition level 550 U/g proteins) was premixed with water and added to SPI solution while stirring. The hydrolysis reaction was carried out at 55°C with agitation, and SPI was hydrolyzed for 0.5, 1, 2, 3, 4, 5, 6, and 7 h, respectively. After the hydrolysis reaction finished, the solutions were heated to 100°C for 15 min to inactivate alcalase, and then were cooled and centrifuged at 11000× g for 30 min. The supernatant was lyophilized and stored at −20°C.
Modification of Soybean Protein Hydrolysates by Plastein Reaction
Soybean protein hydrolysates were dissolved in purified water to give a solution of pH 7.0 and an original substrate concentration as described in . Based on previous work resultsCitation[25] and the results from single factor experiments (not present here), reaction temperature was fixed at 30°C. Other condition parameters studied are listed in . After the reaction, the solutions were heated to 100°C for 15 min to inactivate alcalase, and were then stored at −20°C until analysis. The decrease in the amount of free amino groups of the modified product prepared was calculated by subtracting the content of free amino groups of the modified product after plastein reaction from that of soybean protein hydrolysates before plastein reaction, and expressed as μmol-NH2/g peptides.
Table 1 Range of values for the response surface methodology
The impacts of E/S ratio (X 1), substrate concentration (X 2), and reaction time (X 3) on plastein reaction of soybean protein hydrolysates were studied. Design Expert 7.0 software (Stat-Ease Inc., Minneapolis, MN, USA) was used to generate a three-factor, five-level central composite design combination consisting of 20 runs, and to analyze the data. The decreased amount of free amino groups of the modified product was used as response to reflect the extent of plastein reaction.
Chemical Analysis and Evaluation of ACE-Inhibitory Activity In Vitro
Alcalase activity and protein or peptide content
The activity of alcalase was spectrophotometric assayed by the method described by Sarath et al.Citation[27] Nitrogen contents of soybean protein isolates, soybean protein hydrolysates, and the modified product were all determined by the Kjeldahl procedure according to FIL-IDF 20B:1993,Citation[28] and multiplied by 5.71 to give the value for protein content or peptide content.
Content of Free Amino Groups and Degree of Hydrolysis
The content of free amino groups of soybean protein hydrolysates or the modified product on peptide basis was measured by o-phthaldialdehyde (OPA) assay used by Church et al.Citation[29] and Spellman et al.Citation[30] The final solution of OPA was made by combining the following reagents and diluting them to 100 mL with boric-borate buffer: boric-borate buffer of pH 9.5 consisting of 0.4 mol/L boric acid and 0.3 mol/L NaOH, 5 mL 400 g/L SDS, 80 mg of OPA (dissolved in 1 mL of ethanol), and 400 μL of β-mercaptoethanol. The reagent was prepared daily and protected from light. The assay was carried out by the addition of 3.0 mL soybean protein hydrolysates (or standard L-leucine or the modified product) solution to 3.0 mL of the OPA reagent. The solution was mixed well and incubated for 5 min at ambient temperature. The absorbance of the solution was measured at 340 nm in a 3-mL quartz cuvette against water in a UV spectrophotometer (UV-2401PC, Shimadzu, Japan), and reading was taken after 5 min. All analyses were carried out at least in triplicate. L-Leucine solution (12 to 36 μg/mL) was used as standard.
The degree of hydrolysis (DH) of soybean protein hydrolysates was determined by assaying the content of free amino groups of soybean protein hydrolysates by OPA method, and calculated by using EquationEq. (1) given by Adler-Nissen:Citation[31]
where h is the number of broken peptide bonds per unit weight and htot is the total number of bonds per unit weight, which equals 7.8 meq/g proteins for soybean proteins.Citation[31]
Assay of Size Exclusion Chromatography
The procedure described by Chu and NgCitation[32] was applied with some modifications. Soybean protein hydrolysates or three modified products were dissolved in 0.1 mol/L Na2HPO4-0.1 mol/L NaOH buffer (pH 12) to give a final peptide concentration of 2.25 mg/mL. Half a milliliter of the samples was applied to a 10 × 300 mm Amersham Pharmacia Superdex-75 10/300 GL column (GE Healthcare, Amersham Biosciences, Uppsala, Sweden), and analyzed in an AKTA Explorer 100 (GE Healthcare). The assay was operated with a fixed pressure of 1.80 MPa, an elution rate of 0.5 mL/min in a temperature-controllable cabinet with a fixed temperature of 4°C, and the elution was monitored with an UV-detector at 280 or 204 nm to show the peptide distribution of soybean protein hydrolysates and the modified products analyzed. Bovine serum albumin (66.2 kDa), cytochrome c (12.4 kDa), insulin (5.7 kDa), oxidized L-glutathione (0.6 kDa), and L-tyrosine (0.2 kDa) were used as standards.
Assay of ACE-Inhibitory Activity In Vitro
The ACE-inhibitory activity in vitro of soybean protein hydrolysates or the modified product was measured by a spectrophotometric method of Murray et al.Citation[33] with FAPGG as substrate and the extract of rabbit lung acetone powder as the ACE source. The reaction mixture contained 100 μL of soybean protein hydrolysates (or the modified product) dissolved in deionized water in a concentration range from 0 to 4.5 mg/mL, 500 μL of 1.6 mmol/L FAPGG in 100 mmol/L sodium borate buffer (pH 8.3) with 300 mmol/L NaCl, and 300 μL 10× diluted rabbit lung acetone extract in 100 mmol/L sodium borate buffer (pH 8.3) containing 5% (v/v) glycerol. The ACE extract was added to initiate the reaction. The reaction was terminated by the addition of 100 μL of 100 mmol/L EDTA solution after 30 min incubation at 37°C, and then the mixture was diluted with 3.0 mL water. EDTA solution was added immediately before the ACE extract in zero-time control assays. The decrease in absorbance at 340 nm was determined in triplicate over a 30 min incubation period, and was taken as a measure of the ACE activity. A control sample containing 100 μL of deionized water instead of soybean protein hydrolysates (or the modified product) was assayed in quadruplicate. The ACE inhibition (%) was calculated using EquationEq. (2):
where ΔAI and ΔAC were the decrease in absorbance at 340 nm of the sample of soybean protein hydrolysates (or the modified product) and of the control sample, respectively. The concentration of soybean protein hydrolysates (or the modified product) needed to inhibit ACE by 50% (IC50) under these conditions was determined by assaying different diluted solutions of soybean protein hydrolysates (or the modified product) and plotting the ACE inhibition percentage as a function of peptide concentration.Citation[34] Captopril, a synthetic ACE inhibitor, was used as a positive control with IC50 value of 5.2 nmol/L.
Statistical Analysis
All experiments were carried out at least in triplicate. The data obtained from plastein reaction were analyzed according to response surface methodology. In the analysis, one-way analysis of variance (ANOVA) with Duncan's multiple range tests was employed to test the significance (set at P < 0.05) of E/S ratio (U/g peptides), substrate concentration (%), and reaction time (h) on the decrease amount of free amino groups of the modified product. Design Expert 7.0 (Stat-Ease Inc., Minneapolis, MN, USA), SPSS 13.0 for Windows (SPSS Inc., Chicago, IL, USA), and MS Excel 2003 (Microsoft Corporation, Redmond, WA, USA) software were used to analyze and report data.
RESULTS AND DISCUSSION
Preparation of Soybean Protein Hydrolysates
Alcalase was selected in this work to hydrolyze soybean protein isolates to prepare soybean protein hydrolysates. The ACE-inhibitory activities in vitro of the hydrolysates prepared over a 7-h period of hydrolysis were evaluated at a peptide concentration of 1 mg/mL. The evaluation results, together with the degree of hydrolysis (DH) of the hydrolysates, are shown in As the hydrolysis reaction progressed from 0.5 to 4 h, the DH of soybean protein hydrolysates increased from 7.9 to 16.6%, and the ACE-inhibitory activity in vitro of the hydrolysates increased from 14.3 to 39.9%. Thereafter, the DH of soybean protein hydrolysates increased slowly to 19.6% while the ACE-inhibitory activity in vitro of the hydrolysates showed a decreased trend. The ACE-inhibitory activity in vitroof soybean protein hydrolysates with DH of 16.6% appeared to be the highest (IC50 value 1.45 mg/mL). Based on this fact, it could be deduced that if soybean protein hydrolysates with DH 16.6% were hydrolyzed or resynthesized by alcalase to a higher or lower DH during plastein reaction, the ACE-inhibitory activity in vitro of the modified product would be decreased, viz. lower than that of soybean protein hydrolysates. To show the impact of alcalase-catalyzed plastein reaction on the ACE-inhibitory activity in vitro of soybean protein hydrolysates, soybean protein hydrolysates of DH 16.6% were thereby chosen as the substrate of plastein reaction.
Figure 1 ACE-inhibitory activity in vitro and degree of hydrolysis of soybean protein hydrolysates prepared with different hydrolysis times. The concentration of soybean protein hydrolysates for ACE-inhibitory activity assay was 1 mg/mL on a peptide basis. The bars were for the ACE-inhibitory activity of the hydrolysates and the solid line was for degree of hydrolysis. Different capital letters A to E (or lowercase letters a to g) above the columns (or below the line) indicate that one-way ANOVA of means obtained are significantly different (P < 0.05).
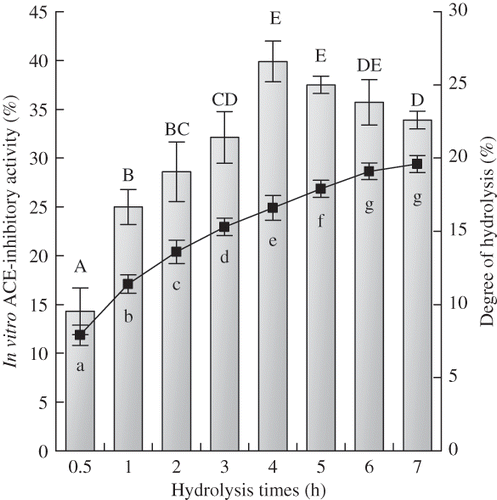
Lee et al.Citation[9] prepared soybean protein hydrolysates with DH in the range of 6 to 15% by four proteolytic enzymes, including bromelain, papain, neutrase, and flavourzyme, and found that the hydrolysates with DH 15% had the highest ACE-inhibitory activity in vitro, no matter what enzyme was used. Marambe et al.Citation[35] hydrolyzed flaxseed proteins with flavourzyme at E/S 1.5 for12 h to obtain protein hydrolysates with a DH of 11.94%, which exhibited IC50 of 0.07 mg/mL. Kuba et al.Citation[36] hydrolyzed β-conglycinin and glycinin for different times (0–10 h) with an acid proteinase from Monascus purpureus, and their results showed that the DH and ACE-inhibitory activity of the prepared hydrolysates in vitro increased with increasing proteolysis time. After 10 h of incubation, the IC50 values of the hydrolysates prepared from β-conglycinin and glycinin (DH 70 to 80%) were to be 0.126 and 0.148 mg/mL, respectively. Tovar-Pérez et al.Citation[37] had employed alcalase to hydrolyze globulins from amaranth grain, and found that after 15 h hydrolysis globulin hydrolysates (DH 13%) showed the highest ACE-inhibitory activity in vitro (IC50 was 0.6 mg/mL). Other studies also found that the peptides produced during the initial stages of hydrolysis had a greater ACE-inhibitory activity than peptides produced during the later stages of hydrolysis.Citation[38,Citation39] Soybean protein hydrolysates prepared in this study showed the highest ACE-inhibitory activity in vitro when their DH was 16.6%, which shows similarity to these results mentioned above except for Kuba's result.
The Suitable Conditions of Plastein Reaction for Soybean Protein Hydrolysates
Potential hydrolysis and peptide joint of soybean protein hydrolysates might happen simultaneously during plastein reaction. If the most susceptible peptide bonds had already been cleaved by alcalase during hydrolysis of soybean protein isolates, the hydrolysates would have more opportunity to form protein-like plasteins when being catalyzed by alcalase. Alcalase was, therefore, used in this work to hydrolyze soybean protein isolates, and especially to modify soybean protein hydrolysates.
The matrix of the central composite design was applied in the study to obtain some suitable conditions of plastein reaction for soybean protein hydrolysates, including E/S ratio, substrate concentration, and reaction time. Reaction temperature was fixed at 30°C. It was reported that if reaction temperature was too high, although the initial rate of plastein reaction was very rapid, the reaction might be soon stopped and the overall result was much lower than at low temperature for a long reaction time.Citation[40] And the previous results from single factor experiments showed 30°C to be a suitable temperature (data were not given in this article). The selection on the variables and their levels were based on the previous results from single factor experiments (data were not given in this article). The experimental design consisted of three independent variables at five different levels (), and 20 runs were carried out. The final results are given as response surfaces shown in , which indicate the impacts of E/S ratio (X 1), substrate concentration (X 2), and reaction time (X 3) on the decrease amount of free amino groups of the modified product. Response results are analyzed using Design Expert 7.0, and given in to represent the linear, quadratic, and cross-product effects of the X 1, X 2, and X 3 on the response, respectively.
Table 2 ANOVA response for linear, quadratic, and interactive effect of variables used in the model.Footnote a
Figure 2 Response surface graphs for the decreased amount of free amino groups of the modified products of plastein reaction as a function of: (a) E/S ratio and substrate concentration (reaction time at the central of its level), (b) substrate concentration and reaction time (E/S ratio at the central of its level), and (c) E/S ratio and reaction time (substrate concentration at the central of its level). (Color figure available online.)
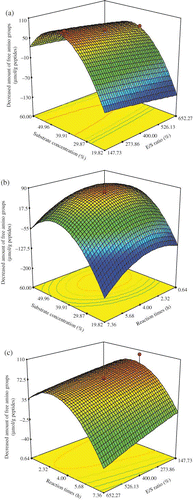
The data presented in showed that when the E/S ratio enhanced in plastein reaction of soybean protein hydrolysates, the decrease amount of free amino groups of the modified product increased insignificantly, which was confirmed by the analysis results listed in that the E/S ratio (X 1) was not a significant factor (P > 0.05). E/S ratio of 275 U/g peptides was selected, which equals half of +1 level of the E/S ratio (e.g., half of 550 U/g peptides). Substrate concentration (X 2) had a prominent effect (P < 0.0001) on the decrease amount of free amino groups. At a fixed level of E/S ratio or reaction time, the decrease amount of free amino groups of the modified product increased first and then decreased with the increasing of substrate concentration ( and ). The highest level of the decrease amount of free amino groups of the modified product was observed at around 45% (w/w) of substrate concentration. Reaction time (X 3) also had a prominent effect (P < 0.01) on the decrease amount of free amino groups of the modified product. As reaction time increased, the decrease amount of free amino groups of the modified product increased first and then decreased at fixed substrate concentration or E/S ratio (see and ). If the plastein reaction progressed about 3 to 4 h, the highest level of the decrease amount of free amino groups of the modified product was obtained. Based on these results, the suitable conditions of plastein reaction for soybean protein hydrolysates were selected as: reaction temperature 30°C, substrate concentration 45% (w/w), E/S ratio 275 U/g peptides, and reaction time of 3 to 4 h.
Plastein reaction undertook usually at a higher substrate concentration ranging from 30 to 50% by weight.Citation[16,Citation41] Sukan and AndrewsCitation[40] also reported that the formation of plastein products was at a maximum when the concentration of protein hydrolysates ranged from 20 to 40%, and then fell sharply both above and below that range. In our previous work, the suitable substrate concentration for casein hydrolysates subjected to alcalase-catalyzed plastein reaction was 35 to 40% by weight.Citation[25] Our presented results shed similarity to these reported results. Meanwhile, reaction temperature should be considered as an important factor. Based on the reported resultsCitation[9,Citation25,Citation26] and previous results from single factor experiments, soybean protein hydrolysates were modified at a fixed temperature 30°C in the presented work.
ACE-Inhibitory Activity of the Modified Product In Vitro
Based on the selected suitable conditions, soybean protein hydrolysates were modified with different reaction times (1 to 10 h) to study the impacts of plastein reaction or possible further hydrolysis on the ACE-inhibitory activity of the modified product. The ACE-inhibitory activities of eight prepared modified products were evaluated in vitro. The IC50 values of soybean protein hydrolysates and the modified products, together with the decreased amount of free amino groups, are given in .
Table 3 The ACE-inhibitory activities of soybean protein hydrolysates (SPH) and eight modified products (MP) of plastein reaction in vitro
During plastein reaction of soybean protein hydrolysates, the decrease amount of free amino groups of the modified product varied irregularly with reaction time. At the initial stage of plastein reaction (from beginning to 3 h of reaction time), the decrease amount of free amino groups of the modified product increased as plastein reaction progressed, indicating that condensation between the peptides in soybean protein hydrolysates occurred. When reaction time prolonged from 4 to 10 h, the decreased amount of free amino groups of the modified product showed a trend to decrease, implying the occurrence of hydrolysis in the modified product. Comparison of soybean protein hydrolysates with eight modified products showed an increase in ACE inhibition for the modified products, for the IC50 values of eight modified products ranging from 0.64 to 1.11 mg/mL, and were lower than that of soybean protein hydrolysates (IC50 = 1.45 mg/mL). This result reveals that no matter how much condensation or hydrolysis occurred in the modified product, plastein reaction improved the ACE-inhibitory in vitro of soybean protein hydrolysates subjected to plastein reaction. The data in also indicate that the modified product with higher ACE-inhibitory activity had a higher reaction extent (e.g., higher decreased amount of free amino groups) in principle, although the modified product MP5 had the highest ACE-inhibitory activity. It stated that the decreased amount of the free amino groups of the modified product had influence on the ACE-inhibitory activity in vitro. Also, comparison of the IC50 of MP6, MP7, and MP8 with that of MP4 showed that further hydrolysis of the modified product could impair its ACE inhibition. More studies are needed to investigate the relationship between the extent of plastein reaction and biological activity of the modified product.
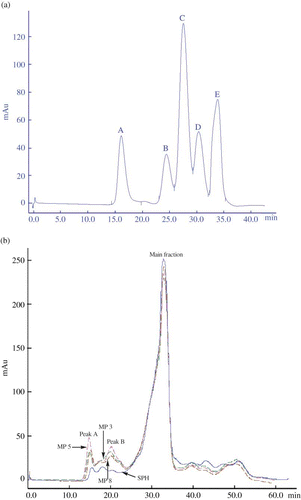
The analysis result from size exclusion chromatography is given in , which shows the peptide profiles of soybean protein hydrolysates and three modified products, MP3, MP5, and MP8. Based on the distribution profiles and molecular weights of the standards analyzed (), it was estimated that the molecular weights of the main peptide fraction of soybean protein hydrolysates were in the range of 0.2 to 5.7 kDa. Meanwhile, some peptide fractions with molecular weights about 41 to 69 kDa also existed in soybean protein hydrolysates because precipitation at the isoelectric point was not applied during the preparation of soybean protein hydrolysates. It also could be observed in that some protein-like plasteins existed in the modified products analyzed. The plasteins appeared mainly as two peaks (labeled as peaks A and B) with elution time about 15 and 20 min and greater peak area in the peptide profiles, and should be considered as the result of condensation of the peptides. The molecular weights of the main peptide fraction in the modified product, MP5, were in the range of 0.2 to 5.7 kDa, and that of new formed plasteins were about 41 to 72 kDa. A similar analysis result was also observed in MP3 or MP8 but with different peak areas (see other lines in ). The analysis results from software of AKTA Explorer 100 showed that the total peak area of the peptide fractions with molecular weight about 41 to 72 kDa in three modified products was in an order of MP5 > MP3 > MP8, implying that the formation of the plasteins in MP5, MP3, and MP8 was in the same order. As the ACE-inhibitory activity of three modified products in vitro also ranged in an order of MP5 > MP3 > MP8 (), a primary conclusion could be drawn that it might be due to the formation of high molecular weight protein-like plasteins that improve the ACE-inhibitory activity of the modified product. However, more work is recommended to reveal the nature of the plasteins.
Figure 3 Analysis of the standards (a), soybean protein hydrolysates (SPH) and three modified products (MP3, MP5, and MP8) (b) by size exclusion chromatography in an AKTA Explorer 100 equipped with a Superdex-75 column. The analysis was performed at a flow rate of 0.5 mL/min with 0.1 mol/L Na2HPO4–0.1 mol/L NaOH buffer (pH 12) and monitored at 280 nm. The DH of SPH was 16.6% and the decrease amount of free amino groups of MP3, MP5, and MP8 were 84.70, 75.79, and 44.58 μmol/g peptides, respectively. The standards were bovine serum albumin (66.2 kDa), cytochrome c (12.4 kDa), insulin (5.7 kDa), oxidized L-glutathione (0.6 kDa), and L-tyrosine (0.2 kDa), and appeared as peaks A to E in Fig. 3a, respectively. (Color figure available online.)
Ono et al.Citation[24] applied plastein reaction to improve the antioxidant activity of the hydrolysates from squid hepatopancreas with alcalase. The plasteins they prepared were soluble in water, stable against heat, and suppressed the proxidative effect of metals on lipid oxidation. Casein hydrolysates subjected to papain-catalyzed plastein reaction also displayed an improved radical scavenging activity in our study.Citation[26] Our previous work showed that when casein hydrolysates were subjected to alcalase-catalyzed plastein reaction for 4 or 5 h, the ACE-inhibitory activity of modified product in vitro was improved significantly.Citation[25] The IC50 value of casein hydrolysates was 47 μg/mL, while that of the modified casein hydrolysates was lowered to 0.6 or 0.5 μg/mL. The past and present work confirmed that plastein reaction might be applicable as effective approach to enhance the ACE-inhibitory activity of protein hydrolysates in vitro.
CONCLUSIONS
Soybean protein hydrolysates were prepared from soybean protein isolates by alcalase-catalyzed hydrolysis to a degree of hydrolysis of 16.6%, and showed the ACE-inhibitory activity in vitro with IC50 value of 1.45 mg/mL. Soybean protein hydrolysates were modified by alcalase-catalyzed plastein reaction and some reaction conditions were studied by response surface methodology with the decrease amount of free amino groups of the modified product as response. The reaction conditions selected were as follows: reaction temperature was fixed at 30°C, the concentration of soybean protein hydrolysates was 45% (w/w), the addition level of alcalase was 275 U/g peptides, and reaction time was 3 to 4 h. Size exclusion chromatography analysis showed that some plasteins with molecular weights ranging from 41 to 72 kDa were formed in the modified product. Comparison of the ACE-inhibitory activity of soybean protein hydrolysates and the modified products showed that plastein reaction could improve the ACE-inhibitory activity in vitro of the modified products, because the IC50 values of the modified products ranged from 0.64 to 1.11 mg/mL and were lower than that of soybean protein hydrolysates. Also, the decreased amount of the free amino groups of the modified product had influence on the ACE-inhibitory activity in vitro. The result implied that plastein reaction might be served as an effective approach to enhance ACE-inhibitory activity of soybean protein hydrolysates in vitro.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the financial support from the National Natural Science Foundation of China (No. 30972132), the Program for Innovative Research Team of NEAU (No. CXT007-1-1), and Innovative Research Team of Higher Education of Heilongjiang Province (No. 2010td11). The authors also thank the anonymous reviewers and editors for their constructive works and valuable suggestions to this article.
REFERENCES
- Fahmi , A. , Morimura , S. , Guo , H.C. , Shigematsu , T. , Kida , K. and Uemura , Y. 2004 . Production of angiotensin I converting enzyme inhibitory peptides from sea bream scales . Process Biochemistry , 39 ( 10 ) : 1195 – 1200 .
- Zhang , J.H. , Tatsumi , E. , Ding , C.H. and Li , L.T. 2006 . Angiotensin I-converting enzyme inhibitory peptides in douchi, a Chinese traditional fermented soybean product . Food Chemistry , 98 ( 3 ) : 551 – 557 .
- Barbosa-Filho , J.M. , Martins , V.K.M. , Rabelo , L.A. , Moura , M.D. , Silva , M.S. , Cunha , E.V.L. , Souza , M.F.V. , Almeida , R.N. and Medeiros , I.A. 2006 . Natural products inhibitors of the angiotensin converting enzyme (ACE). A review between 1980–2000 . Revista Brasileira de Farmacognosia , 16 ( 3 ) : 421 – 446 .
- Atkinson , A.B. and Robertson , J.I.S. 1979 . Captopril in the treatment of clinical hypertension and cardiac failure . Lancet , 2 ( 8147 ) : 836 – 839 .
- Wu , J.P. and Ding , X.L. 2002 . Characterization of inhibition and stability of soy-protein-derived angiotensin I-converting enzyme inhibitory peptides . Food Research International , 35 ( 4 ) : 367 – 375 .
- Suh , H.J. , Whang , J.H. and Lee , H. 1999 . A peptide from corn gluten hydrolysate that is inhibitory toward angiotensin I converting enzyme . Biotechnology Letters , 21 ( 12 ) : 1055 – 1058 .
- Abubakar , A. , Saito , T. , Kitazawa , H. , Kawai , Y. and Itoch , T. 1998 . Structural analysis of new antihypertensive peptides derived from cheese whey protein by proteinase K digestion . Journal of Diary Science , 81 ( 12 ) : 3131 – 3138 .
- Miguel , M. , Contreras , M.M. , Recio , I. and Aleixandre , A. 2009 . ACE-inhibitory and antihypertensive properties of a bovine casein hydrolysate . Food Chemistry , 112 ( 1 ) : 211 – 214 .
- Lee , J.S. , Yoo , M.A. , Koo , S.H. , Baek , H.H. and Lee , H.G. 2008 . Antioxidant and ACE inhibitory activities of soybean hydrolysates: effect of enzyme and degree of hydrolysis . Food Science and Biotechnology , 17 ( 4 ) : 873 – 877 .
- Rho , S.J. , Lee , J.S. , Chung , Y.I. , Kim , Y.W. and Lee , H.G. 2009 . Purification and identification of an angiotensin I-converting enzyme inhibitory peptide from fermented soybean extract . Process Biochemistry , 44 ( 4 ) : 490 – 493 .
- Farzamirad , V. and Aluko , R.E. 2008 . Angiotensin-converting enzyme inhibition and free-radical scavenging properties of cationic peptides derived from soybean protein . International Journal of Food Sciences and Nutrition , 59 ( 5 ) : 428 – 437 .
- Chiang , W.D. , Tsou , M.J. , Tsai , Z.Y. and Tsai , T.C. 2006 . Angiotensin I-converting enzyme inhibitor derived from soy protein hydrolysate and produced by using membrane reactor . Food Chemistry , 98 ( 4 ) : 725 – 732 .
- Belitz , H.D. , Grosch , W. and Schieberle , P. 2009 . Food Chemistry , 4th revised and extended , Berlin Heidelberg , , Germany : Springer-Verlag .
- Yamashita , M. , Arai , S. , Tsai , S.J. and Fujimaki , M. 1971 . Plastein reaction as a method for enhancing the sulfur-containing amino acid level of soybean protein . Journal of Agricultural and Food Chemistry , 19 ( 6 ) : 1151 – 1154 .
- Williams , R.J.H. , Brownsell , V.L. and Andrews , A.T. 2001 . Application of the plastein reaction to mycoprotein: I. Plastein synthesis . Food Chemistry , 72 ( 3 ) : 329 – 335 .
- Lozano , P. and Combes , D. 1991 . α-Chymotrypsin in plastein synthesis: Influence of substrate concentration on enzyme activity . Biotechnology and Applied Biochemistry , 14 ( 2 ) : 212 – 221 .
- Combes , D. and Lozano , P. 1992 . “ α-Chymotrypsin in plastein synthesis. Influence of water activity ” . In Annals of New York Academy of Sciences Vol. 672 , 409 – 414 . (Enzyme Engineering XI)
- Andrews , A.T. and Alichanidis , E. 1990 . The plastein reaction revisited: Evidence for a purely aggregation reaction mechanism . Food Chemistry , 35 ( 4 ) : 243 – 261 .
- Stevenson , D.E. , Morgan , K.R. , Fenton , G.A. and Moraes , G. 1999 . Use of NMR and mass spectrometry to detect and quantify protease-catalyzed peptide bond formation in complex mixtures . Enzyme and Microbial Technology , 25 ( 3–5 ) : 357 – 363 .
- Ashley , D.V.M. , Temler , R. , Barclay , D. , Dormond , C.A. and Jost , R. 1983 . Amino acid-enriched plasteins: A source of limiting amino acids for the weanling rat . Journal of Nutrition , 113 ( 1 ) : 21 – 27 .
- Yamashita , M. , Arai , S. , Imaizumi , Y. , Amano , Y. and Fujimaki , M. 1979 . A one-step process for incorporation of L-methionine into soy protein by treatment with papain . Journal of Agricultural and Food Chemistry , 27 ( 1 ) : 52 – 56 .
- Yamashita , M. , Arai , S. and Fujimaki , M. 1976 . A low-phenylalenine, high-tyrosine plastein as an acceptable dietetic food . Journal of Food Science , 41 ( 5 ) : 1029 – 1032 .
- Fujimaki , M. , Arai , S. and Yamashita , M. 1977 . Enzymic protein degradation and resynthesis for protein improvement in food proteins . Advances in Chemistry , 160 : 156 – 184 .
- Ono , S. , Kasai , D. , Sugano , T. , Ohba , K. and Takahashi , K. 2004 . Production of water soluble antioxidative plastein from squid hepatopancreas . Journal of Oleo Science , 53 ( 5 ) : 267 – 273 .
- Zhao , X.H. and Li , Y.Y. 2009 . An approach to improve ACE inhibitory activity of casein hydrolysates with plastein reaction catalyzed by alcalase . European Food Research and Technology , 229 ( 5 ) : 795 – 805 .
- Zhao , X.H. , Wu , D. and Li , T.J. 2010 . Preparation and the radical scavenging activity of papain catalyzed casein plasteins . Dairy Science and Technology , doi: 10.1051/dst/2009054
- Sarath , G. , De La Motte , R.S. and Wagner , F.W. 1989 . “ Protease assay methods ” . In Proteolytic Enzymes, A Practical Approach , Edited by: Beynon , R.J. and Bond , J.S. 25 – 55 . Oxford , , UK : IRL Press .
- International Dairy Federation (IDF) . 1993 . Determination of the nitrogen (Kjeldahl method) and calculation of the crude protein content , Brussels , , Belgium : IDF Standard 20B. International Dairy Federation .
- Church , F.C. , Swaisgood , H.E. , Porter , D.H. and Catignani , G.L. 1983 . Spectrophotometric assay using o-phthaldialdehyde for determination of proteolysis in milk and isolated milk proteins . Journal of Dairy Science , 66 ( 6 ) : 1219 – 1227 .
- Spellman , D. , McEvoy , E. , O'Cuinn , G. and FitzGerald , R.J. 2003 . Proteinase and exopeptidase hydrolysis of whey protein: Comparison of the TNBS, OPA and pH stat methods for quantification of degree of hydrolysis . International Dairy Journal , 13 ( 6 ) : 447 – 453 .
- Adler-Nissen , J. 1979 . Determination of the degree of hydrolysis of food protein hydrolysates by trinitrobenzenesulfonic acid . Journal of Agricultural and Food Chemistry , 27 ( 6 ) : 1256 – 1261 .
- Chu , K.T. and Ng , T.B. 2004 . First report of a glutamine-rich antifungal peptide with immunomodulatory and antiproliferative activities from family Amaryllidaceae . Biochemical and Biophysical Research Communications , 325 ( 1 ) : 167 – 173 .
- Murray , B.A. , Walsh , D.J. and FitzGerald , R.J. 2004 . Modification of the furanacryloyl-L-phenylalanyl-glycylglycine assay for determination of angiotensin-I-converting enzyme inhibitory activity . Journal of Biochemical and Biophysical Methods , 59 ( 2 ) : 127 – 137 .
- Shalaby , S.M. , Zakora , M. and Otte , J. 2006 . Performance of two commonly used angiotensin-converting enzyme inhibition assays using FA-PGG and HHL as substrates . Journal of Dairy Research , 73 ( 2 ) : 178 – 186 .
- Marambe , P.W.M.L.H.K. , Shand , P.J. and Wanasundara , J.P.D. 2008 . An in-vitro investigation of selected biological activities of hydrolysed flaxseed (Linum usitatissimum L.) proteins . Journal of the American Oil Chemists' Society , 85 ( 12 ) : 1155 – 1164 .
- Kuba , M. , Tana , C. , Tawata , S. and Yasuda , M. 2005 . Production of angiotensin I-converting enzyme inhibitory peptides from soybean protein with Monascus purpureus acid proteinase . Process Biochemistry , 40 ( 6 ) : 2191 – 2196 .
- Tovar-Pérez , E.G. , Guerrero-Legarreta , I. , Farrés-González , A. and Soriano-Santos , J. 2009 . Angiotensin-I-converting enzyme-inhibitory peptide fractions from albumin 1 and globulin as obtained of amaranth grain . Food Chemistry , 116 ( 2 ) : 437 – 444 .
- Megías , C. , Yust , M.M. , Pedroche , J. , Lquari , H. , Girón-Calle , J. , Alaiz , M. , Millán , F. and Vioque , J. 2004 . Purification of an ACE inhibitory peptide after hydrolysis of sunflower (Helianthus annuus L.) protein isolates . Journal of Agricultural and Food Chemistry , 52 ( 7 ) : 1928 – 1932 .
- Ahn , S.W. , Kim , K.M. , Noh , D.O. , Yu , K.W. and Suh , H.J. 2000 . Isolation of angiotensin I converting enzyme inhibitory peptide from soybean hydrolysate . Food Science and Biotechnology , 9 ( 6 ) : 378 – 381 .
- Sukan , G. and Andrews , A.T. 1982 . Application of the plastein reaction to caseins and to skim milk powder. I. Protein hydrolysis and plastein formation . Journal of Dairy Research , 49 ( 2 ) : 265 – 278 .
- Pallavicini , C. , Finley , J.W. , Stanley , W.L. and Watters , G.G. 1980 . Plastein synthesis with α-chymotrypsin immobilised on chitin . Journal of Science of Food and Agriculture , 31 ( 3 ) : 273 – 278 .