Abstract
Effect of different indigenous processing methods on the levels of certain bioactive compounds in the seed materials of velvet bean (Mucuna pruriens L. DC), a promising wild type legume collected from different agro-ecological locations of the world, was investigated. Ten different accessions of velvet bean seeds were found to contain appreciable levels of certain bioactive compounds, such as total free phenolics (5.24–8.65 g/100 g DM), tannins (1.77–3.49 g/100 g DM), L-Dopa (4.30–6.23 g/100 g DM), and phytic acid (1.17–2.37 g/100 g DM). In general, velvet bean accession with black coloured seed coat (collected from Zimbabwe, Mexico, and Guinea) registered high levels of total free phenolics and tannins, whereas the maximum level of L-Dopa was detected in Guatemala accession. Furthermore, among the different indigenous processing methods employed in the present study, sprouting and oil-frying can be considered as mild and suitable treatment for the consumption of velvet bean grains as a natural source of health promoting bioactive compounds.
INTRODUCTION
Legume grains have been playing a key role in the traditional diets of human beings throughout the world. They are an excellent source of protein, dietary fibre, starch, micronutrients, and bioactive compounds with a low level of fat. The total per capita consumption of legume grains has been increased markedly over the past two decades in the US, due to increased attention to beans being classified as functional foods.Citation[1] Accumulation of chemical, biochemical, clinical, and epidemiological evidences indicates a positive correlation between the consumption of legume seeds and decreasing incidence of several chronic diseases, such as cancer, cardiovascular diseases, obesity, and diabetes.Citation[2] Such obvious health benefits of legume seeds are attributed to the presence of certain bioactive compounds, such as phenolic acids, flavonoids, and tannins.Citation[3] Therefore, at present the studies on bioactive compounds, which are responsible for health promoting/disease preventing effect, are being increased in addition to the evaluation of nutritive profiles of legume grains.
Apart from common legume seeds, the earlier research efforts revealed the nutritive potential of certain promising under-utilized/wild legume seeds, including the pulses of tribal utility.Citation[4] Among the various under-utilized legumes, the Mucuna pruriens (velvet bean, VB) seeds merit a wider use as a food legume in many tropical and sub-tropical countries.Citation[5] It is widespread in the Southern and South-eastern Asian regions and is also cultivated as a green manure/cover crop in some parts of Africa and South America. In India, the mature VB seeds are being traditionally consumed by certain ethnic groups, particularly the Kanikkar, Lambadi, Uraali, and Dravidian tribes living in Tamilnadu, Kerala, Karnataka, and Andhrapradesh States. Similarly, the seed materials of VB are being eaten by the native people in different parts of the world like Benin, Brazil, Ghana, Guatemala, Guinea, Kenya, Malawi, Mexico, Nigeria, Philippines, Sri Lanka, and Zimbabwe.Citation[6] Few reports indicate that the VB seeds possess appreciable levels of certain bioactive compounds, such as total free phenolics, tannins, phytic acid, and L-Dopa (3, 4-Dihydroxyphenylalanine, a non-protein phenolic amino acid), in addition to high protein content (26–29%) and other nutrients.Citation[7] Hence, in recent years, research efforts are under-way for the utilization of VB seeds in the dietary management of Parkinsonism, diabetes, obesity, etc.
Actually, in older days, the total free phenolics, tannins, L-Dopa, and phytic acid were considered as antinutritional substances and their presence in food/feed-stuffs was reported to be undesirable from the nutritional point of view. But, nowadays, the health beneficial role of such bioactive compounds has been explored by a large number of research studies. Particularly, these bioactive compounds were demonstrated to possess many favourable medicinal properties, including potential antioxidant activity.Citation[3,Citation8,Citation9] As a consequence of health beneficial effects, the presence of such bioactive compounds in the diet has been viewed in a positive light in recent years by both scientists and consumers and resulted in a push to procure foods with specific health benefits, such as functional foods.
Generally, before consumption, all the legume grains are subjected to appropriate processing methods in order to improve their nutritive quality. But, most of the common processing methods have been shown to reduce the levels of bioactive compounds in commercial legume grains, while increase on the levels of tannins, catechins, and polyphenols was reported during cooking by Vidal-Valverde et al.Citation[10] Also, in VB seeds, a significant level of loss of bioactive compounds was reported during soaking, cooking, and autoclaving treatments.Citation[11] So, it is very important to identify a suitable processing method(s), which will cause minimum loss of bioactive compounds in VB seeds. In this connection, in the present study, an attempt has been made to analyse the effect of certain indigenous processing techniques, particularly those that are used by Indian tribal groups, on the levels of bioactive compounds of VB seeds collected from different parts of the world.
MATERIALS AND METHODS
Chemicals
Poly-vinyl-polypyrrolidone, (+)-catechin hydrate, vanillin, tannic acid, L-Dopa, and phytic acid were procured from Sigma-Aldrich Chemicals (St. Louis, MO, USA); Sephadex LH-20 was obtained from Pharmacia Fine Chemicals (Uppsala, Sweden); anion exchange resin was purchased from Bio-Rad (Hercules, CA, USA), and all other chemicals were received from Merck (Darmstadt, Germany).
Collection of Seed Samples
The details on collection of seed materials of VB from different agro-climatic locations of the world were given in . After removing the immature and damaged seeds, the mature seeds were dried under a shaded condition for 2 days. Then the seed materials were randomly divided into five batches and the first batch was stored without any treatment, which is considered as raw seeds and the remaining four batches were processed as described below.
Table 1 Data on collection of seed materials of velvet bean from different agro-ecological locations of the world
Processing Methods
Soaking in tamarind solution and cooking
The tamarind solution was prepared by dissolving 50 g of tamarind pulp in 500 ml of distilled water (pH 2.75). The whole seeds of VB (50 g) were soaked in tamarind solution in the ratio of 1:10 (w/v) and kept in dark for 8 h at 25°C. The soaked samples were rinsed and then cooked with distilled water (in the ratio of seed to water, 1:10 w/v) at 85–90°C on a hot plate until they became soft when felt between the fingers (about 45 min). This treatment was adapted based on the fact that the Kanikkar tribe living in Kerala state, India has been following this method for the consumption of VB seeds.
Soaking in alkaline solution and cooking
The whole seeds of VB (50 g) were soaked in 500 ml of 0.2% NaHCO3 solution (pH 8.6) for 8 h in the dark at room temperature (25°C) in the bean to alkaline solution ratio of 1:10 (w/v). After soaking, the alkaline solution was drained off and the seeds were cooked with distilled water at 85–90°C for about 45 min. This method of VB processing is common among the Dravidian tribal sect living in Tirunelveli District, Tamilnadu state, India.
Sprouting and oil-frying
Clean red soil (100 g) was taken in a tray and made into a paste with distilled water in the ratio of 1:5 (w/v). Then, 50 g of VB seeds was added into the red-soil suspension and mixed well. The tray was covered with a moist cloth and kept for 7 days in the dark at 25°C. Then the sprouts were separated and thoroughly washed with tap water. The sprouts, thus obtained, were fried with Biskin oil (100% sunflower oil) at 85–90°C on a hot plate for about 15 min. This treatment was based on the practice of the Lambadi ethnic group of Karnataka and Andhrapradesh states, India.
Open-pan roasting
The VB seeds (50 g) were taken in an iron pot along with acid-treated clean and fine sand to prevent the burning of seed coat and also to ensure the uniform distribution of heat. The seed materials were roasted for 30 min at 120–130°C. Then the seeds were separated by using a sieve, and allowed to cool to room temperature. In general, the Uraali tribal group that lives near the Kadambur hills of Sathyamangalam, Erode District, Tamilnadu state, India has been using open-pan roasting for the consumption of VB seeds in their regular diet.
Preparation of Seed Flour
All the processed, as well as raw, samples were frozen at −80°C and freeze-dried for 48 h. Then the samples were first cracked with the help of a wooden hammer into small pieces and, subsequently, powdered in a seed mill (Siemens, Munich, Germany) to 1 mm particle size, freeze-dried for 24 h, and stored at 9°C until further use.
Analysis of Bioactive Compounds
Total free phenolics
The total free phenolics were extracted from VB seed flour by taking 1 g of defatted seed flour with 10 ml of 100, 80, and 50% methanol and 70% acetone acidified with 1% conc. HCl in an ultra-sonic bath (Bandelin Sonorex, RK–514 H, Berlin, Germany) for 30 min. After centrifugation, all the supernatants were pooled and made up to a known volume. The extract was treated with 1 g of poly-vinyl-polypyrrolidone at 0°C for 30 min. Then the contents were purified by using a solid phase cartridge (SPC) (Strata-x-33 um polymeric sorbent, L100-1105, 200 mg/6 ml sample, 8B-S100-FCH-S from Phenomenex, Torrance, CA, USA). The total free phenolics were eluted with 10 ml of 50 and 100% methanol and used for estimation according to the method of Singleton et al.Citation[12] Based on the standard curve prepared with (+)-catechin hydrate (20–100 μg), the amount of total free phenolics in the extract was calculated and expressed in g/100 g seed flour on a dry matter basis.
Tannins
The tannins were extracted from VB seeds by taking 1 g of defatted seed flour sequentially with 100, 90, 80, and 70% acetone solutions acidified with 1% conc. HCl. After centrifugation, all the supernatants were pooled together and made up to a known volume with acetone. Then the extract was purified by using Sephadex LH-20 column chromatography (96 × 1.6 cm) with acetone:water (50:50, v/v) as a solvent.Citation[2] After collecting 20 fractions (5 ml each), the active fractions were identified and pooled together and used for quantification by using the vanillin reagent method.Citation[13] The aliquot (500 μl) was taken in a test tube and treated with 5 ml of 0.5% vanillin and 5 ml of 4% HCl. After standing for 30 min at room temperature, the contents were mixed well and the absorbance was measured at 500 nm in a UV-visible spectrophotometer (Lambda 35, Perkin-Elmer, Champaign, IL, USA). The standard curve was prepared by taking different concentrations of tannic acid and the level of tannins was calculated.
L-Dopa
Finely ground VB seed flour (1 g) was treated with 10 ml of petroleum ether and kept in an ultra-sonic bath for 30 min. Then the defatted pellet was extracted with 10 ml of 0.1 N HCl. The contents were vortexed for 10 min at room temperature (25°C) and kept in an ultra-sonic bath for 30 min under ice bath conditions and, subsequently, it was kept on a magnetic stirrer for 1 h at room temperature. The supernatant was collected by centrifugation (13,000× g, 15 min) and the extraction procedure was repeated twice and all the supernatants were pooled and diluted to a known volume and used for further analysis. The L-Dopa content was quantified by measuring the ultra-violet light absorption at 282 nm in a UV-visible spectrophotometer after correction for background absorption according to Brain.Citation[14] The L-Dopa content of VB samples was calculated by using the standard curve prepared with synthetic L-Dopa and expressed in g/100 g seed flour on dry matter basis.
Phytic acid
The phytic acid was extracted from raw and differentially processed VB seeds by taking 1 g of defatted seed flour with 10 ml of 2.4% HCl and incubated for 10 min in an ultra-sonic bath. Then the contents were centrifuged at 13,000× g for 5 min and the supernatant was collected. Similarly, the residue was re-extracted twice and all the supernatants were pooled together and made up to a known volume with distilled water. The extract was purified by using an anionic-exchange column chromatography (0.7 cm × 15 cm) containing 0.5 g of anion-exchange resin (100–200 mesh, chloride form; AG1-X4, Bio-Rad Co., Hercules, CA, USA). The phytic acid was eluted with 2 M HCl and used for quantification according to the Latta and Eskin method.Citation[15] The purified extract (100 μl) was diluted to 3 ml with distilled water and 1 ml of Wade reagent (0.03% FeCl3.6H2O and 0.3% sulfosalicylic acid) was added. The contents were vortexed and centrifuged at 3500× g for 5 min. Then the absorbance of the supernatant was measured at 500 nm. The phytic acid content was calculated by using the standard curve prepared with synthetic phytic acid.
Statistical Analysis
All the data were analyzed and expressed as means ± standard deviation of five separate determinations (n = 5). The statistical analysis was carried out by using SPSS for Windows (SPSS Inc., Chicago, IL, USA, version 11.0). Values of analyzed compounds were found to be normally distributed by using the Kolmogorov-Smirnov-test. Means of the groups regarding different processing methods were compared by one-way ANOVA and Dunnett post-hoc test using the raw VB seeds as a control. Two-tailed P values < 0.05 were considered statistically significant.
RESULTS AND DISCUSSION
Total Free Phenolics
The phenolic compounds constitute one of the most numerous and ubiquitously distributed groups of plant secondary metabolites, which are ranged from simple molecules (e.g., phenolic acids, phenyl-propanoids, and flavonoids) to highly polymerized compounds (e.g., lignins and melanins). Nowadays, the phenolic compounds are demonstrated to prevent the development of many chronic diseases, such as atherosclerosis, diabetes, cancer, etc. Such protective effect of phenolics might be associated with their powerful antioxidant and free radical scavenging properties.Citation[3] The seed coat of legume grains are reported to contain numerous types of phenolics, which play an important protective role against oxidative damage in a consumer's body.Citation[2]
The total free phenolics content of raw VB accessions collected from various parts of the world were found to range between 5.24 and 8.65 g/100 g seed flour DM (). These values are lower when compared to previous reports on different cultivars of eggplant (0.06–0.13 g/100 g)Citation[16] and certain legume grains,Citation[17] such as broad bean (2.39 g/100 g DM); pea (2.26–3.48 g/100 g DM); white bean (1.08 g/100 g DM); black bean (4.40 g/100 g DM); and common bean (1.88–2.53 g/100 g DM), but comparable with that of faba bean (5.59 g/100 g DM); Adzuki bean (8.97 g/100 g DM); red bean (5.54–9.36 g/100 g DM); red lentil (5.80 g/100 g DM); green lentil (6.76 g/100 g DM), and brown bean (9.14 g/100 g DM).
Figure 1 Level of bioactive compounds in ten different accessions of velvet bean seeds: (a) total free phenolics, (b) tannins, (c) L-Dopa, and (d) phytic acid.
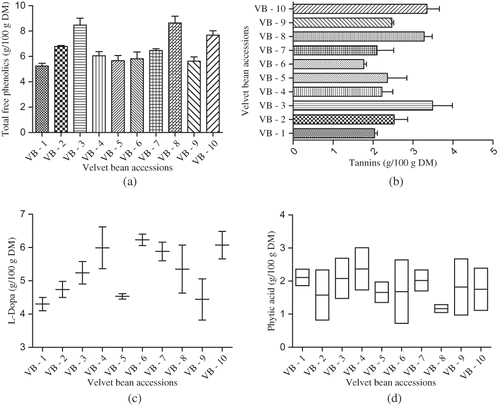
In general, the total free phenolics content of presently investigated VB accessions appear to be higher when compared to the previous reports on the same speciesCitation[7] (3.1–4.9 g/100 g DM). This might be due to the repeated extraction of phenolic compounds by using both methanol and acetone as solvents, because recovery of phenolic compounds from legume grains mainly depends upon the type of solvent used and the duration of extraction. Acetone and methanol extracts of seed samples exhibited higher phenolic yield when compared to either methanol or acetone used alone.Citation[18]
It is interesting to notice that the VB accessions with black-coloured seed coat (VB accessions 3, 8, and 10) registered a significantly (p < 0.05) higher level of total free phenolics when compared to white-coloured and mottled seed coat VB accessions (). Relationships between seed coat colour and phenolics level are still controversial. While Barampama and SimardCitation[19] found a positive relationship between the seed coat colour and phenolic content, Guzman-Maldonado et al.Citation[20] did not find any relationship. However, there are some reports available with high correlation between cultivar lines and phenolic content.Citation[21] In addition to seed coat colour, it is well documented that the quantity of phenolic compounds in seed samples is influenced by soil; environmental conditions; genotype (cultivar/variety); agronomic practices (irrigation, fertilization, and pest management); maturity level at harvest; and post-harvest storage. For instance, low temperatures during the onset and duration of seed fill were shown to increase the isoflavone content by several fold in soybean.Citation[22] Since VB grows wildly in adverse environmental conditions, such as drought, poor soil, etc., a high phenolic content contributes to the resistant function.
Although the dietary intake of phenolics varies considerably among the geographical regions, it is estimated that the daily intake of total free phenolics ranged from 20 mg to 1 g, which is higher than the intake of vitamin E. Hence, in recent years, food technologists are keen to harness the nutritional benefits of phenolics, namely, its antioxidant or free radical scavenging, food preservative, antimicrobial, anti-mutagenic, therapeutic and pharmaceutical properties.
Tannins
Besides simple phenolics mainly found in cellular vacuoles, some polymerized form of phenolics with varying degrees of solubility, such as tannins, are also noticed in legume seeds. Tannins are defined as a unique group of phenolic metabolites of relatively high molecular weight. Concerning chemical structure, they can be divided into four groups: condensed tannins, hydrolyzable tannins, phlorotannins, and complex tannins.Citation[23] Tannins possess ideal structural chemistry for better free radical scavenging activity and, hence, they exhibit more effective antioxidant activity under in vitro conditions than tocopherols and ascorbic acid.Citation[24] The free radical scavenging power of tannins is closely connected with their spatial confirmation and degree of polymerization. Further, both the hydrolysable and condensed tannins are demonstrated to possess more effective and greater antioxidant activity than simple phenolics.
The tannins content of raw VB seeds were found to fall between 1.77 and 3.49 g/100 g DM (). These values are found to be higher when compared to previous reportsCitation[17] on green pea (0.003–0.17 g/100 g DM); yellow pea (0.15 g/100 g DM); chickpea (0.18 g/100 g DM); lentil (0.012–0.88 g/100 g DM); red kidney bean (0.012–0.55 g/100 g DM); black bean (0.04–0.67 g/100 g DM); and common bean (0.02–0.13 g/100 g DM). Such a high level of tannins in VB seed accessions when compared to the literatureCitation[7] might be due to the type of solvent used for extraction in the present analysis (acidified acetone). Similarly, Troszynska et al.Citation[2] reported the maximization of extraction of tannins from yellow pea seed coats when acetone was used as a solvent compared to methanol.
It is noticeable that the black colour seed coated VB accessions collected from Zimbabwe, Mexico, and Guinea registered a significantly (p < 0.05) higher level of tannins. It is postulated that higher levels of condensed tannins or proanthocyanidin are seen in coloured beans than in yellow- or white-coloured beans. Since, the level of phenolics was relatively low in pale-coloured seeds; it is possible to assume that the major phenolics in dark-coloured coated seeds could be proanthocyanidins. Recent studies have demonstrated a quantitative pattern of heredity for tannins content and that tannins level is also associated with seed coat colour inheritance. Several factors, such as plant type, cultivar, age of the plant or plant parts, stage of development, and environmental conditions were reported to govern the tannins content in legume grains. Presence of high content of tannins in VB seeds might be due to the metabolism of polyphenolic compounds or polymerization of existing phenolic compounds during development or maturation.
According to Serrano et al.,Citation[23] the mean daily intake of condensed tannins among US population (>2 years old) was estimated to be 53.6 mg/person/day, whereas 450 mg/person/day in the Spanish diet. There are a lot of epidemiological data, which suggested that tannins intake may prevent the onset of many chronic diseases. The positive biological effects, including antioxidant, anticarcinogenic, anti-mutagenic, antimicrobial, antiviral, and anti-diabetic properties, of tannins have been extensively studied.
L-Dopa
L-Dopa (L-3,4-Dihxdroxyphenylalanine) is a non-protein phenolic amino acid, mainly used in the treatment of Parkinson's disease, since it is the precursor of dopamine.Citation[6] L-Dopa has also been investigated as a dietary supplement to manage hypertension, renal failure, and liver cirrhosis. Furthermore, the protective effects of L-Dopa on small bowel injury, ulcer, gastro-intestinal diseases, diabetes, as well as antioxidant stress, were scientifically proved by earlier studies.Citation[7] The seed materials of VB are naturally considered as a rich source of L-Dopa, and earlier research studies indicated that the L-Dopa extracted from VB seeds exhibited twice the anti-Parkinsonian activity than the synthetic L-Dopa.Citation[6]
The raw seed materials of different VB accessions of the present study showed the L-Dopa content of 4.30–6.23 g/100 g DM, while the Guatemala accession recorded the maximal level (). In India, the L-Dopa content of VB seeds was reported between 1.5 and 9% from the previous studies.Citation[6] The L-Dopa content of VB seeds is found to be comparable with that of certain under-utilized legumes, such as Mucuna cochichinensis Citation[25] (6.11 g/100 g DM); M. rajada Citation[25] (5.35 g/100 g DM), and M. veracruz Citation[25] (7.12 g/100 g DM). However, these values are higher than that of an earlier reportCitation[26] on Cassia floribunda (1.57 g/100 g DM); C. obtusifolia (1.34 g/100 g DM); Canavalia ensiformis (2.64 g/100 g DM); and C. gladiata (2.83 g/100 g DM).
The L-Dopa content varies considerably at a significant level (p < 0.05) among the VB accessions of the present investigation. The VB seed samples collected from Guatemala recorded a maximum level of L-Dopa content (6.23 g/100 g DM), while the lower level was observed in a USA VB accession. Such a wide variability in L-Dopa content among VB accessions was reported to be caused by both environmental effect and genetic nature. Presence of more L-Dopa was noticed in the VB plants growing near the equator (within 10°) than the plants cultivated far away from equatorial regions in earlier investigations.Citation[6] Further, the L-Dopa synthesis is reported to be high in plants growing at low latitudes near the equator.Citation[7] It was also hypothesized that variation in the intensity of light and backscattered ultraviolet radiation, both generally more near the equator, may be among the factors explaining why the L-Dopa content was found to be high in VB plants growing at low latitudes.
Phytic Acid
Phytic acid (myo-inositol hexaphosphate) is widely found in cereals, nuts, legumes, oil seeds, pollen, and spores, constituting about 1–5% and, generally, the legume seeds are regarded as the major source of dietary phytate.Citation[8] In recent years, the phytic acid is considered as an antioxidant, anti-carcinogenic, hypoglycaemic, and hypolipidemic agent, in addition to the fact that a high phytate diet can be effectively used in the treatment of hyper-calciuria and kidney stones in human beings.Citation[27] The raw VB seeds of different accessions were found to contain 1.17–2.37 g/100 g DM of phytic acid (). These values are comparable with that of an earlier reportCitation[27] on Phaseolus vulgaris (0.61–2.38 g/100 g DM); Vicia faba (0.51–1.77 g/100 g DM); Pisum sativum (0.22–1.22 g/100 g DM); Vigna unguiculata (0.37–2.90 g/100 g DM); Cicer arietinum (0.28–1.60 g/100 g DM); and Lens culinaris (0.27–1.51 g/100 g DM).
Even though a considerable level of variation on the phytic acid content was noticed among the VB accessions, it was not at a significant level. Furthermore, such variability might be attributed to both genetic and environmental conditions. In general, the cultivar, which contains an appreciably high amount of proteins, is observed to be associated with high phytic acid content. Hence, as the protein content increases, the phytate level is also found to increase in the seed samples. Al-Numair et al.Citation[28] reported that the amount of phytic acid always exceeds that of phosphorus for all the legume cultivars, which indicates that the ratio would be more than 100%. Generally, in legume seeds, the phytic acid level is positively correlated with total phosphorous, correlation coefficients being greater than 0.90. Factors that affect the total phosphorous content, such as soil, available phosphorous, and fertilizer, can also influence the phytic acid concentration.
The estimated daily phytic acid intake of human population was about 750 mg in the USA; 600–800 mg/day in the UK; 393 mg/day among Canadian children; 2000–2200 mg/day in Nigeria; 1890 and 569 mg/day in Malawi and New Guinea, respectively; and 1487 mg/day in East India.Citation[27] But, historically it has been considered as an antinutrient and postulated to impede the bioavailability of minerals. Nevertheless, the research studies conducted by Grases et al.Citation[29] showed that there is no negative effect on the mineral balance and element bioavailability due to the oral administration of phytic acid, even in the second generation rats.
Effect of Treatments on Total Free Phenolics
Reduction of significant level (p < 0.05) of total free phenolics was noticed in most of the VB accessions during soaking in tamarind solution + cooking treatment (24–46%) (). Soaking in tamarind solution may lead to the softening of cell wall tissues under an acidic environment, which is usually accompanied by release of bounded phenolic compounds, and, hence, may be leached into the soaking medium. Ross et al.Citation[30] have also reported that water uptake during processing can also cause a decrease in total phenolics content of pinto and navy beans.
Table 2 Effect of certain indigenous processing methods on the total free phenolics content of velvet bean seeds.1–3
Soaking in alkaline solution + cooking treatment resulted in a significant loss of total free phenolics (36–65%) (). This is in agreement with that of previous reportsCitation[31,Citation32] on Vigna radiata (32%), Cajanus cajan (50%), and Vigna unguiculata (37%). Such significant levels of reduction of total free phenolics during this treatment might be because of the leaching out of this compound into the soaking medium due to increased permeability of the seed coat under alkaline environment or due to solubilisation of this compound in alkaline solution under the influence of concentration gradient and also due to degradation of phenolics with the high temperature during subsequent cooking.
It is important to recognize that a slight increase of total free phenolics (4–11%) was observed during sprouting + oil-frying. Similarly, sprouting for 2 days + autoclaving was reported to increase the total free phenolics by 9, 20, 27, and 50% in wheat, buckwheat, corn, and oats, respectively.Citation[9] A very high level of elevation on the level of total free phenolics was noticed in Vigna radiata (217%) after 7 days of germination.Citation[33] Further, ZielinskiCitation[34] reported that germination of Glycine max caused an increase of total free phenolics from 2.6 to 3.1 mg/g DM. Earlier research studies indicated that a major portion of total free phenolics was stored in seeds as soluble conjugate or insoluble forms. Hence, the small level of increase noticed in VB seeds under sprouting + oil-frying treatment might be due to mobilization of stored phenolics by the activation of polyphenol oxidase enzyme during sprouting and also due to release of free phenolics from a bounded form by the breakdown of cellular constituents and cell walls during subsequent thermal process (oil-frying). Furthermore, dissociation of conjugated phenolic forms due to oil-frying followed by some polymerization/oxidation of phenolic constituents may also be responsible for the increase. A significant level of reduction of total free phenolics was noticed during open-pan roasting (24–44%) in all the VB accessions, except accession 5 (). Similarly, dry-heat treatment was reported to decrease the total free phenolics content in cowpea seedsCitation[35] (48–60%). Degradation of total free phenolics as a result of direct heat exposure could be a reason for this reduction observed in VB seeds under open-pan roasting.
Effect of Treatments on Tannins
Soaking in tamarind solution + cooking caused a significant level (16–57%) of reduction of tannins in most of the VB accessions (). Such loss of tannins during this treatment could be attributed to leaching out of this compound into the acidic soaking medium and chemical transformation or decomposition of tannins and also the formation of tannins-protein complex under acidic soaking medium as well as by thermal conditions during subsequent cooking.
Table 3 Effect of certain indigenous processing methods on the tannins content of velvet bean seed samples.1–3
Soaking in alkaline solution + cooking resulted in 24–52% decrease of tannins and it was significant in most of the VB accessions. Such a level of loss of tannins under this treatment was found to be lower when compared to earlier reportsCitation[36,Citation37] on Bauhinia purpurea (69–78%) and Phaseolus vulgaris (68%). Such losses may be a function of increased permeability of the seed coat, which leads to leaching out of this compound due to the alkaline environment and subsequent degradation during cooking.
Alternatively, a slight increase on the levels of tannins was recorded (1–8%) during sprouting + oil-frying treatment in VB seeds (). These results are in agreement with those reported by Fernandez-Orozco et al.,Citation[33] who found a high level of increase of tannins (53%) in lupin sprouts. Khattak et al.Citation[38] reported a small rise on the tannins content of chickpea, while several authors observed a large level of increase of tannins in soybean and other beans during germination. Similarly, Duenas et al.Citation[39] have also noticed a significant level of increase on both flavonoid and non-flavonoid polyphenolic compounds during germination of lupin seeds. It may be probably due to the polymerization of existing phenolic compounds into insoluble and high molecular weight polymers like tannins.
Open-pan roasting caused a significant level of loss of tannins (31–45%) in VB accessions (). This is in agreement with that of an earlier report on loss of tannins (54–72%) in light brown colour seed-coated Vigna unguiculata Citation[35] as well as Mucuna urens Citation[40]. Such a substantial reduction of tannins in VB seeds might be due to the fact that some of the polyphenolic compounds, such as tannins, are known to accumulate in the cellular vacuoles and the direct heat may denature them. Other reasons could be Maillard reaction, caramelization, chemical oxidation of tannins, and modernisation.
Effect of Treatments on L-Dopa
Soaking in tamarind solution + cooking resulted in a significant level of removal of a non-protein amino acid, the L-Dopa (24–44%) in all the investigated VB accessions (). Similarly, Srivastava and KhokharCitation[41] also observed the significant effect of tamarind solution on another non-protein amino acid, ß-ODAP in Lathyrus sativus seeds. However, the level of L-Dopa loss appears to be lower in relation to reduction of phenolics and tannins in the respective samples under this treatment, which could be explained by two factors: (1) Permeability of the seed coat along with the diffusion rate of L-Dopa and (2) presence of L-Dopa in the intact cell compartments of cotyledons rather than the seed coat.
Table 4 Effect of certain indigenous processing methods on the L-Dopa level of velvet bean seed samples.1–3
A drastic level of reduction of L-Dopa was observed in the seed materials of VB (48–67%) during soaking in alkaline solution + cooking treatment of the present study, which is in consonance with that of an earlier report on the same legumeCitation[5] (81%). Furthermore, D'Mello and WalkerCitation[42] also reported that the treatment of Canavalia ensiformis seeds with alkaline solution (potassium-bi-carbonate) at 80°C resulted in decline of another non-protein amino acid, L-Canavanine to a negligible level. Such obvious reduction of L-Dopa in VB seeds under this treatment is most likely due to enhanced leaching out of this compound by the increased seed coat permeability caused by the alkaline soaking medium and also due to the chemical conversion of L-Dopa into melanin pigment under alkaline conditions in addition to partial denaturation by heat under cooking.
Sprouting + oil-frying treatment was found to cause a significant level of loss of L-Dopa (9–23%) in most of the VB seeds (). These results suggested that the L-Dopa degrading enzymes, such as polyphenol oxidase, could be synthesized upon germination of VB seeds to metabolize the L-Dopa. From the earlier research works, it was reported that only a short-term germination of legume grains is sufficient to capture a good amount of L-Dopa, after which it may be mobilized to some other products that are not yet identified.Citation[9] Hence, the raise noticed in total free phenolics and tannins contents after sprouting + oil-frying treatment of the present study ( and ) could be mobilized from L-Dopa. It is postulated that during initial stages of germination most of the phenolics may have been diverted towards antioxidant function and L-Dopa production, when the need for lignifications was minimal. But, as germination proceeds, the L-Dopa content has reduced markedly, indicating that the precursor metabolites are potentially diverted from L-Dopa production towards the synthesis of phenolics and tannins, which are required for the lignifications process associated with growth. This may be one of the reasons explaining why the L-Dopa content was reduced after sprouting + oil-frying treatment in VB seeds.
Open-pan roasting also resulted in moderate reduction of L-Dopa (6–17%) in VB accessions. This is in consonance with that of a previous report on the reduction of L-Dopa content in Entada scandens Citation[43] (27%). Such loss of L-Dopa under this treatment might be due to either partial oxidation of this compound or racemisation under high temperature. Another reason could be that there may be a chance of modification of chemical properties of L-Dopa, since the VB seeds were subjected to a very high temperature (120–130°C).
Effect of Treatments on Phytic Acid
Significant reduction of phytic acid was noticed during soaking in tamarind solution + cooking treatment (25–47%) in VB seeds (). These results are in agreement with the previous findingsCitation[41] in different lines of Lathyrus sativus seeds. These losses are mainly due to leaching out of this compound into the acidic soaking medium and the leaching is particularly favoured when the compound possesses low molecular weight and ionic character. Deshpande and CheryanCitation[44] also reported that the loss of phytic acid can be enhanced by increasing the ionic concentration of the soaking medium.
Table 5 Effect of certain indigenous processing methods on the phytic acid content of velvet bean seed samples.1–3
Soaking in alkaline solution and cooking treatment resulted in 18–37% of loss of phytic acid in VB seeds. Similarly, 27% of reduction of phytic acid was noticed in Bauhinia purpurea seeds.Citation[36] Nonetheless, a high level of removal of phytic acid was also reported in Phaseolus vulgaris during this treatment.Citation[37] Such level of loss of phytic acid in VB seeds is likely due to either leaching out of this compound into the alkaline soaking medium and/or formation of insoluble complexes between phytic acid and other nutrients, such as phytate-protein and phytate-protein-mineral complexes.
Sprouting and oil-frying exhibited a non-significant level of removal of phytic acid in VB accessions (11–27%) (). These results are comparable with that of earlier reportsCitation[31,Citation45] on Cajanus cajan (17%) and Vigna radiata (13%). Similarly, Al-Numair et al.Citation[28]reported that germination reduces the phytic acid content by 18–34% in Vicia faba and 23–36% in Phaseolus vulgaris. Recently, germination and cooking treatments are reported to reduce 13 and 15% of phytic acid, respectively, in cowpea seed flour.Citation[8] But, on the other hand, a high level of reduction of phytic acid is also observed in certain common legume grains during germination.Citation[38] The removal of phytic acid during germination in legume grains is attributed to the enzymatic (phytase) hydrolysis of phytic acid followed by diffusion. Phytase activity is detected in all the sprouts of cereal and legume grains. Since the seeds need a lot of energy for their sprouting process, the naturally occurring phytase enzyme becomes active upon sprouting. Consequently, the phytase hydrolyzes the phytic acid into phosphate and myo-inositol phosphate, which represent an important primary source of energy for seed sprouting.
A moderate level of reduction of phytic acid (5–14%) was noticed in VB seeds upon open-pan roasting (). These results are lower when compared to an earlier report on Entada scandens Citation[43] (49%) and horse-eye bean (Mucuna urens).Citation[40] Such minimal loss of phytic acid during this treatment might be partially due to degradation of myo-inositol hexa-phosphate (phytic acid) into penta- and tetra-phosphates under high temperature (120–130°C). Prolonged input of energy is necessary to denature this heat-stable compound.
CONCLUSION
Ten different accessions of VB seeds collected from various agro-ecological regions of the world were found to contain appreciable levels of bioactive compounds, such as total free phenolics, tannins, L-Dopa, and phytic acid. Among the VB accessions, significantly high levels of total free phenolics and tannins were noticed in VB seeds collected from Zimbabwe, Mexico, and Guinea, while Guatemala VB accession registered a maximum level of L-Dopa. Considering the effect of different indigenous processing methods, soaking in tamarind solution + cooking as well as soaking in alkaline solution + cooking treatments represent more aggressive practices and exhibited a drastic level of loss of all the investigated compounds. Furthermore, open-pan roasting also demonstrated a significant level of reduction of total free phenolics, tannins with moderate loss of L-Dopa and phytic acid. Nonetheless, sprouting + oil-frying was found to slightly increase the content of total free phenolics and tannins and also caused only minimal loss of L-Dopa and phytic acid. Hence, such viable and mild processing methods could be recommended for the consumption of VB seeds in order to increase the dietary intake of health beneficial bioactive compounds. Implementation of such suitable processing techniques will increase opportunities for the versatile utilization of VB seeds with high levels of bioactive compounds in the dietary management of certain chronic diseases, such as Parkinsonism, diabetes, etc.
ACKNOWLEDGMENTS
One of the authors (VV) is thankful to the Alexander von Humboldt (AvH) Foundation, Bonn, Germany for the award of Post Doctoral Research Fellowship. The authors are also grateful to Professor A.A. Teixeira, University of Florida, USA and Professor J.B. Castillo Caamal, University of Yucatán, Mexico for their immense help in the collection of VB seed samples.
REFERENCES
- Luthria , D.L. and Pastor-Corrales , M.A. 2006 . Phenolic acids content of fifteen dry edible bean (Phaseolus vulgaris L.) varieties . Journal of Food Composition and Analysis , 19 : 205 – 211 .
- Troszynska , A. , Estrella , I. , Lopez-Amores , M.L. and Hernandez , T. 2002 . Antioxidant activity of pea (Pisum sativum L.) seed coat acetone extract . LWT—Food Science and Technology , 35 : 158 – 164 .
- Siddhuraju , P. and Manian , S. 2007 . The antioxidant activity and free radical-scavenging capacity of dietary phenolic extracts from horse gram (Macrotyloma uniflorum (Lam.) Verdc.) seeds . Food Chemistry , 105 : 950 – 958 .
- Janardhanan , K. , Vadivel , V. and Pugalenthi , M. 2003 . “ Biodiversity in Indian under-exploited/tribal pulses ” . In Improvement Strategies for Leguminosae Biotechnology , Edited by: Jaiwal , P.K. and Singh , R.P. 353 – 405 . The Netherlands : Kluwer Academic Publishers .
- Vadivel , V. and Pugalenthi , M. 2010 . Evaluation of growth performance of broiler birds fed with diet containing different levels of effectively processed velvet bean seeds collected from South India . Livestock Science , 127 : 76 – 83 .
- Pugalenthi , M. and Vadivel , V. 2007 . L-Dopa (L-3,4-Dihydroxyphenylalanine): A non-protein toxic amino acid in Mucuna pruriens seeds . Food , 1 : 322 – 343 .
- Pugalenthi , M. , Vadivel , V. and Siddhuraju , P. 2005 . Alternative food/feed perspectives of an under-utilized legume Mucuna pruriens var. utilis—A review . Plant Foods for Human Nutrition , 60 : 201 – 218 .
- Herken , E.N. , Ibanoglu , S. , Oner , M.D. , Bilgicli , N. and Guzel , S. 2007 . Effect of storage on the phytic acid content, total antioxidant capacity and organoleptic properties of macaroni enriched with cowpea flour . Journal of Food Engineering , 78 : 366 – 372 .
- Randhir , R. , Kwon , Y.I. and Shetty , K. 2008 . Effect of thermal processing on phenolics, antioxidant activity and health-relevant functionality of select grain sprouts and seedlings . Innovative Food Science and Emerging Technology , 9 : 355 – 364 .
- Vidal-Valverde , C. , Frias , J. , Prodanov , M. , Tabera , J. , Ruiz , R. and Bacon , J. 1993 . Effect of natural fermentation on carbohydrates, riboflavin and trypsin inhibitor activity of lentils . Food Research and Technology , 197 : 449 – 452 .
- Vadivel , V. and Pugalenthi , M. 2008 . Removal of antinutritional/toxic substances and improvement in the protein digestibility of velvet bean seeds during various processing methods . Journal of Food Science and Technology , 45 : 242 – 246 .
- Singleton , V.L. , Orthofer , R. and Lamuela-Raventos , R.M. 1999 . Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent . Methods in Enzymology , 299 : 152 – 178 .
- Price , M.L. , Scoyoc , S. and Butler , L.G. 1978 . A critical evaluation of the vanillin reaction as an assay for tannin in sorghum grain . Journal of Agricultural and Food Chemistry , 26 : 1214 – 1217 .
- Brain , K.R. 1976 . Accumulation of L-Dopa in cultures from Mucuna pruriens . Plant Science Letters , 7 : 157 – 161 .
- Latta , M. and Eskin , M. 1980 . A simple and rapid colorimetric method for phytate determination . Journal of Agricultural and Food Chemistry , 28 : 1313 – 1315 .
- Okmen , B. , Sigva , H.O. , Mutlu , S. , Doganlar , S. , Yemenicioglu , A. and Frary , A. 2009 . Total antioxidant activity and total phenolic contents in different Turkish eggplant (Solanum Melongena L.) cultivars . International Journal of Food Properties , 12 : 616 – 624 .
- Amarowicz , R. and Pegg , R.B. 2008 . Legumes as a source of natural antioxidants . European Journal of Lipid Science and Technology , 110 : 865 – 878 .
- Agboola , S.O. , Mofolasayo , A.A. , Watts , B.M. and Aluko , R.E. 2009 . Some functional properties of yellow field pea (Pisum sativum L.) seed flours and the in vitro bioactive properties of their polyphenols . Food Research International , 43 : 582 – 588 .
- Barampama , Z. and Simard , R.E. 1993 . Nutrient composition, protein quality and antinutritional factors of some varieties of dry beans (Phaseolus vulgaris L) grown in Burundi . Food Chemistry , 47 : 159 – 167 .
- Guzman-Maldonado , G.H. , Castellanos , J. and De Mejia , E.M. 1996 . Relationship between theoretical and experimentally detected tannin content of common bean (Phaseolus vulgaris L.) . Food Chemistry , 55 : 333 – 335 .
- De Mejia , E.G. , Guzman-Maldonado , G.H. , Acosta-Gallegos , J.A. , Reynoso-Camacho , R. and Ramirez-Rodriguez , A. 2003 . Effect of cultivar and growing location on the trypsin inhibitors, tannins, and lectins of common beans (Phaseolus vulgaris L.) grown in the semi-arid high lands of Mexico . Journal of Agricultural and Food Chemistry , 51 : 5962 – 5966 .
- Kim , J.A. , Jung , W.S. , Chun , S.C. , Yu , C.Y. , Ma , K.H. and Gwag , J.G. 2006 . A correlation between the level of phenolic compounds and the antioxidant capacity in cooked-with-rice and vegetable soybean (Glycine max L.) varieties . European Journal of Food Research and Technology , 224 : 259 – 270 .
- Serrano , J. , Puupponen-Pimi , R. , Dauer , A. , Aura , A.M. and Saura-Calixto , F. 2009 . Tannins: Current knowledge of food sources, intake, bioavailability and biological effects . Molecular Nutrition and Food Research , 53 : S310 – S329 .
- Shukla , S. , Metha , A. , John , J. , Singh , S. , Mehta , P. and Vyas , S.P. 2009 . Antioxidant activity and total phenolic content of ethanolic extract of Caesalpinia bonducella seeds . Food and Chemical Toxicology , 47 : 1848 – 1851 .
- Adebowale , Y.A. , Adeyemi , A. and Oshodi , A.A. 2005 . Variability in the physicochemical and antinutritional attributes of six Mucuna species . Food Chemistry , 89 : 37 – 48 .
- Vadivel , V. and Janardhanan , K. 2005 . Nutritional and antinutritional characteristics of seven South Indian wild legumes . Plant Foods for Human Nutrition , 60 : 69 – 75 .
- Schlemmer , U. , Frolich , W. , Prieto , R.M. and Grases , F. 2009 . Phytate in foods and significance for humans: Food sources, intake, processing, bioavailability, protective role and analysis . Molecular Nutrition and Food Research , 53 : S330 – S375 .
- Al-Numair , K.S. , Ahmed , S.E.B. , Al-Assaf , A.H. and Alamri , M.S. 2009 . Hydrochloric acid extractable minerals and phytate and polyphenols contents of sprouted faba and white bean cultivars . Food Chemistry , 113 : 997 – 1002 .
- Grases , E. , Simonet , B.M. , Pere , J. , Costa-Bauz , A. and Prieto , R.M. 2004 . Effect of phytate on element bioavailability in the second generation of rats . Journal of Trace Elements in Medicine and Biology , 17 : 229 – 234 .
- Ross , K.A. , Zhang , L. and Arntfield , S.D. 2010 . Understanding water uptake from the induced changes occurred during processing: Chemistry of pinto and navy bean seed coats . International Journal of Food Properties , 13 : 631 – 647 .
- Grewal , A. and Jood , S. 2006 . Effect of processing treatments on nutritional and antinutritional contents of green gram . Journal of Food Biochemistry , 30 : 535 – 546 .
- Onwuka , G.I. 2006 . Soaking, boiling and antinutritional factors in pigeon peas (Cajanus cajan) and cowpea (Vigna unguiculata) . Journal of Food Processing and Preservation , 30 : 616 – 630 .
- Fernandez-Orozco , R. , Frias , J. , Zielinski , H. , Piskula , M.K. , Kozlowska , H. and Vidal-Valverde , C. 2008 . Kinetic study of the antioxidant compounds and antioxidant capacity during germination of Vigna radiata cv. emerald, Glycine max cv. jutro and Glycine max cv. merit . Food Chemistry , 111 : 622 – 630 .
- Zielinski , H. 2003 . Contribution of low molecular weight antioxidants to the antioxidant screen of germinated soybean seeds . Plant Foods for Human Nutrition , 58 : 1 – 20 .
- Siddhuraju , P. and Becker , K. 2007 . The antioxidant and free radical scavenging activities of processed cowpea (Vigna unguiculata (L.) Walp.) seed extracts . Food Chemistry , 101 : 10 – 19 .
- Vijayakumari , K. , Pugalenthi , M. and Vadivel , V. 2007 . Effect of soaking and hydrothermal processing methods on the levels of antinutrients and in vitro protein digestibility of Bauhinia purpurea L. seeds . Food Chemistry , 103 : 968 – 975 .
- Shimelis , E.A. and Rakshit , S.K. 2007 . Effect of processing on antinutrients and in vitro protein digestibility of kidney bean (Phaseolus vulgaris L.) varieties grown in East Africa . Food Chemistry , 103 : 161 – 172 .
- Khattak , A.B. , Zeb , A. , Bibi , N. , Khalil , S.A. and Khattak , M.S. 2007 . Influence of germination on phytic acid and polyphenols content of chickpea (Cicer arietinum L.) sprouts . Food Chemistry , 104 : 1074 – 1079 .
- Duenas , M. , Hernandez , T. , Estrella , I. and Fernandez , D. 2009 . Germination as a process to increase the polyphenol content and antioxidant activity of lupin seeds (Lupinus angustifolius L.) . Food Chemistry , 117 : 599 – 607 .
- Umoren , U.E. , Effiong , O.O. , Onyilagha , J.C. , Ekpe , E.D. and Okiror , S.O. 2008 . Changes in nutritional characteristics of the horse-eye bean [Mucuna urens (L.) Medik] subjected to different processing methods . International Journal of Food Properties , 11 : 901 – 909 .
- Srivastava , S. and Khokhar , S. 1996 . Effects of processing on the reduction of β-ODAP (β-N-oxylyl-L-2,3-diaminopropionic acid) and antinutrients of khesari dhal, Lathyrus sativus . Journal of Agricultural and Food Chemistry , 71 : 50 – 58 .
- D'Mello , J.P.F. and Walker , A.G. 1991 . Detoxification of jack beans (Canavalia ensiformis): Studies with young chicks . Animal Feed Science and Technology , 33 : 117 – 127 .
- Vadivel , V. , Pugalenthi , M. and Megha , M. 2008 . Biological evaluation of protein quality of raw and processed seeds of gila bean (Entada scandens Benth.) . Tropical and Subtropical Agroecosystem , 8 : 125 – 133 .
- Deshpande , S.S. and Cheryan , M. 1983 . Changes in the phytic acid, tannin and trypsin inhibitor activity on soaking of dry beans . Nutrition Reports International , 27 : 371 – 378 .
- Oloyo , R.A. 2004 . Chemical and nutritional quality changes in germinating seeds of Cajanus cajan L . Food Chemistry , 85 : 497 – 502 .