Abstract
Extracts from dried stem of Opuntia dillenii and rhizome of Zingiber officinale were evaluated for antimicrobial activities by extraction in non-polar (petroleum ether and chloroform) and polar solvents (methanol and water). Bacillus subtilis and Staphylococcus aureus showed considerable susceptibility to all extracts of Opuntia dillenii and Zingiber officinale. Ether and chloroform extracts of Opuntia dillenii showed improved antimicrobial activity against Escherichia coli (gram negative) as compared to that of its methanolic and water extracts. On the other hand, methanolic and water extracts of Zingiber officinale showed better susceptibility for Escherichia coli. Salmonella typhi (gram negative) was found to be resistant to all extracts of both Opuntia dillenii and Zingiber officinale. Our results indicated that both Opuntia dillenii and Zingiber officinale have potent antimicrobial activity that is also based on solvent polarity during extraction.
INTRODUCTION
The recent emergence of resistance in bacterial pathogens, both nosocomially and in the community against the most commonly used antibiotics, is one of the very serious problems that medical science is facing today.[Citation1] Rapid emergence of resistance against clinically important antimicrobials, including macrolides,[Citation2] fluoroquinolones,[Citation3] and β-lactam antibiotics,[Citation4–6 have been repeatedly reported over the past decades. This, on one side, mandates a more responsible approach to antibiotic use, but on the other side, it boosts up the urge to discover new antibiotics to solve the problems of antibiotic resistance.
Food has been a tremendous source of drug discovery for centuries. Up until now, a lot of work has been published regarding the chemical characterization and properties of different food items.[Citation7–10 The most important of all foods that have been used as natural remedies to cure ailments since pre-historic times are the spices and herbs. It is estimated that about 80% of the world population relies on botanical preparations as medicines to treat their health problems. Scientific experiments since the late 19th century have documented the antimicrobial properties of some spices, herbs, and their components.[Citation11,Citation12] Numerous studies have been published on the antimicrobial activities of plant extracts.[Citation2,Citation13,Citation14] However, the results for these different studies are difficult to compare directly, usually because of the low number of plant samples tested, different test methods and diverse bacterial strains and sources of antimicrobial samples used.[Citation15] In the present study, we focused the antimicrobial effect of polar and non-polar extracts of two medicinal plants, i.e., Opuntia dillenii and Zingiber officinale.
Anti-diabetic, anti-inflammatory, and analgesic activity of Opuntia dillenii (chhitarthohar) has already been explained elsewhere.[Citation16–18 The oral administration of Opuntia dillenii phylloclade extract to male rats caused a significant decrease in the weights of testes, epididymides, seminal vesicle, and ventral prostate, and the production of spermatid was also reduced by 88.06% in Opuntia-treated rats.[Citation19] The crude extract prepared from fruits of Opuntia dillenii is effective against gastrointestinal and liver disturbances (diabetes, hepatitis, intestinal spasm, etc.). It is also an effective remedy for cough, bronchial troubles, and asthma.[Citation16] It is worthwhile to confirm whether polar and non-polar extracts of Opuntia dillenii has the antimicrobial activities.
Zingiber officinale (ginger) is one of the oldest herbs and it is one of the earliest spices to be known in the east. Ginger consists of thick scaly rhizomes of the plant Zingiber officinale, belonging to the family Zingiberaceae. The plant is cultivated in warm tropical climates, particularly southeastern Asia. It is now extensively cultivated in Pakistan, India, China, Africa, Mexico, and Hawaii. There are numerous studies on the composition and activities of Zingiber officinale. The extracts of ginger rhizome are reported to have cytotoxic effect,[Citation20] anti-emetic,[Citation21] anti-inflammatory activity,[Citation22–25 and antimicrobial properties of ginger essential oil.[Citation26–28 However, polar and non-polar solvent extractions of Zingiber officinale are not studied so vastly with reference to its antimicrobial activity. Therefore, the present study was designed to evaluate the antibacterial effect of polar and non-polar extracts of Opuntia dillenii and Zingiber officinale. Four different solvents (having different polarity) were used for the extraction purpose in order to separate constituents according to their polarity. These solvents include petroleum ether, chloroform, methanol, and water. The aim of this study was to determine the antimicrobial activity of the Opuntia dillenii and Zingiber officinale extracts against Staphylococcus aureus, Bacillus subtilis, Escherichia coli, and Salmonella typhi and to compare the relative antimicrobial activity of polar and non-polar extracts of these two plants.
MATERIALS
Collection of Plant Material
Opuntia dillenii and Zingiber officinale were obtained from the Botanical Gardens of Government College University, Lahore, Pakistan.
Microorganisms
Bacillus subtilis, Staphylococcus aureus, and Escherichia coli were isolated from food and Salmonella typhi was isolated from the fecal samples of a typhoid patient obtained from the laboratory of Mayo Hospital, Lahore, Pakistan by using a method directed by Surinder et al.[Citation29] All four bacteria were later identified up to species level by using biochemical tests[Citation29] and the Api 20E system (bio-Merieux).[Citation30]
METHODS
Preparation of Plant Extracts
Fresh plant samples were cleaned, dried in vacuum desiccators, ground into a fine powder, and passed through a sieve (24-mesh). Dried plant samples were further dried in vacuum desiccators (Millipore, Sydney, Australia) and passed through the above mentioned sieve. Powdered samples (20 g) were extracted with 500 ml of methanol (99.9%), distilled water, chloroform (37%), and petroleum ether (40–60%) separately at room temperature (25°C) for 24 h in a shaking water bath. The extracts were filtered by Minisart (Sartorius, Hannover, Germany) 0.2 μm syringe filters in safety cabinets. The filtrates were concentrated by vacuum desiccators and stored at 4°C temperature until use.
Inoculation of Media
Bacterial “Master Suspensions” were prepared in phosphate buffered saline (PBS) and colony forming units (CFU) per milliliter of these suspensions was calculated by using a viable count method. Nutrient agar was weighed and mixed with distilled water in a concentration of 2.8 g per 100 ml (as advised by the manufacturer) in an autoclavable conical flask. The media was autoclaved at 15 pound pressure and 121°C temperature for 15 to 20 min. After the media was cooled (but still molten), bacterial suspension from “Master Suspension” was taken (and mixed with the media) in such a quantity that each milliliter of the media contained 106 CFU.
Antimicrobial Activity Assay
An agar–well diffusion method was employed for determination of antibacterial activities.[Citation31–33 The dried extracts of Opuntia dillenii and Zingiber officinale were weighed and dissolved in dimethyl sulfoxide (1 ml solvent for each 10 mg of dried sample) and sterilized by Minisart (Sartorius) 0.2 μm syringe filters in a laminar flow hood. Media was prepared and 20 ml of media was poured into each Petri dish. Wells of 1 cm diameter were cut in Petri dishes and in one well, plant extract 200 μl (containing 2000 μg) and in second well, plant extract 400 μl (containing 4000 μg) were taken. Dimethyl sulfoxide (DMSO) was used as negative control and Penicillin G (640 μg/well) and Gentamicin (400 μg/well) were used as positive reference standards to determine the sensitivity of each microbial species tested.[Citation31] The inoculated plates were incubated at 37°C for 24 h. Antimicrobial activity was evaluated by measuring the diameter of inhibition zone (DIZ) of the tested bacteria. DIZ was expressed in millimeters. All tests were performed in five replicates.[Citation31]
Statistical Analysis
Statistical Package for Social Sciences (SPSS Inc., Chicago, IL, USA) version 11.5 was used for statistical analysis. Differences between means were tested by Fisher's Protected LSD and a value of P < 0.05 was considered to be statistically significant. Data are presented as mean ± SEM.
RESULTS AND DISCUSSION
Zingiberacea (ginger family) is a family of perennial herbs that is famous for many of its species, for instance, Zingiber cassumunar, Zingiber nimmonii, Zingiber mioga, Aframomum danielli, Curcuma longa, and Zingiber officinale that have known medicinal importance.[Citation34–36 Zingiber officinale is well reported for its anti-inflammatory,[Citation37,Citation38] anti-allergic,[Citation39] hypoglycemic, analgesic, antipyretic,[Citation38] and antirhinoviral properties.[Citation40] Antimicrobial effect of both the essential oils isolated from Zingiber officinale[Citation26] and the ginger paste exhibits considerable antimicrobial activity against a range of gram positive and gram negative bacteria.[Citation41] There is, however, no appreciable data available regarding the inter-comparison of antimicrobial activity of non-polar and polar extracts of Zingiber officinale. Opuntia dillenii is one of the famous species of the Cactaceae family that has been used for centuries in traditional medicine. This plant is well reported for its analgesic, anti-inflammatory, and antidiabetic properties.[Citation16–18 However, there is no significant data available regarding the anti-microbial activity of Opuntia dillenii. Therefore, in vitro antimicrobial activity of Opuntia dillenii and Zingiber officinale was evaluated against Bacillus subtilis, Staphylococcus aureus, Escherichia coli, and Salmonella typhi. Different solvents based on polarity (petroleum ether, chloroform, methanol, and water) were found to be valuable for antimicrobial activity extraction.
Effect of Solvents Used on Antimicrobial Activity
A plant extract in a single solvent contains a large number of constituents with different properties. Extraction of an herb by using different solvents with different polarity is an effective tool used by many workers to separate hundreds of herbal constituents on their polarity basis.[Citation42] In the present study, the two plants were extracted in petroleum ether, chloroform, methanol, and water. The antimicrobial activity against Bacillus subtilis, Staphylococcus aureus, Escherichia coli, and Salmonella typhi as shown by inhibition zones in a well diffusion study is presented in and . Petroleum ether and chloroform, the non-polar solvent extracts, prove better (P < 0.05) than methanol and water, the polar solvent extracts against Bacillus subtilis and Staphylococcus aureus (). For Escherichia coli, non-polar solvents extraction for Opuntia dillenii gave better inhibition zones similar to that against Bacillus subtilis and Staphylococcus aureus. But Zingiber officinale extraction by methanol or water (polar solvents) gave optimal inhibition zones (). In the case of Salmonella typhi, neither extraction of Opuntia dillenii nor Zingiber officinale gave a proper in vitro inhibition zone ( and ).
Figure 1 Mean diameter of inhibition zone (n = 5) for gram positive bacteria by different extraction sources at concentration of 4 mg/well.
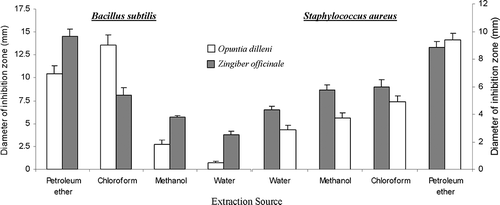
Figure 2 Mean diameter of inhibition zone (n = 5) for gram negative bacteria (Escheria coli) by different extraction sources at concentration of 4 mg/well.
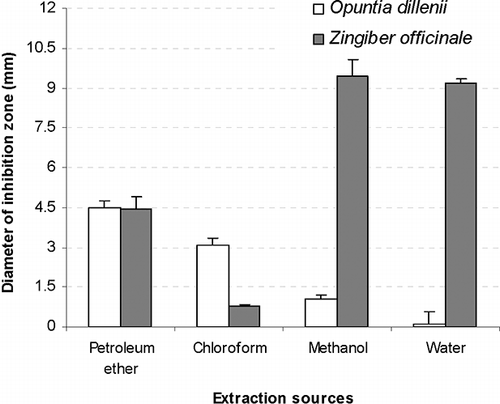
The activities of Opuntia and Zingiber are mostly dose dependent.[Citation16,Citation34] The in vitro antimicrobial effect of solvent extraction versus dose dependency was evaluated by altering per well extract concentrations. Zones of inhibition against Bacillus subtilis and Staphylococcus aureus by petroleum ether or chloroform at 2 mg per well concentration prove better than even double concentration (4 mg per well) extracted by methanol or water. Zingiber officinale gave reasonable antimicrobial activity against Escherichia coli and an opposite trend was observed. For Escherichia coli, the extraction by methanol or water at a concentration of 2 mg/well gave a similar inhibition zone to the extractions by petroleum ether at a concentration of 4 mg per well ().
Antimicrobial Effect of Opuntia dillenii
Against Bacillus subtilis, the chloroform extraction of Opuntia dillenii gave a maximum inhibitory zone (13.56 ± 1.14). Petroleum ether extraction gave the best antimicrobial performance of Opuntia dillenii for Staphylococcus and its 4 mg per well concentration was found to be close to Penicilline (P > 0.05). For Escherichia coli and Salmonella typhi, none of the Opuntia dillenii extraction gave a considerably good antimicrobial effect (). The present study demonstrated that the antimicrobial activity of non-polar extracts of Opuntia dillenii was considerable against Bacillus subtilis and Staphylococcus aureus. The diameters of inhibitory zones (DIZ) against Bacillus subtilis were 10.47 and 13.57 mm, respectively, with petroleum ether and chloroform extracts. DIZ against Staphylococcus aureus were 9.40 and 4.90 mm, respectively, for petroleum ether and chloroform extracts. Methanolic and water extracts, however, showed weak activity against Bacillus subtilis and Staphylococcus aureus. Activity against Escherichia coli and Salmonella typhi was poor with slight activity of non-polar extracts against Escherichia coli (4.48 and 3.18 mm for petroleum ether and chloroform extracts, respectively) and almost zero activity against Salmonella typhi. Non-polar solvents (petroleum ether and chloroform) showed comparatively more antibacterial activity than the polar solvents (methanol and water).
Table 1 In vitro antimicrobial activity of Opuntia dillenii extracted in different solvents
As demonstrated by Jiang et al.[Citation43] that the stem of Opuntia dillenii contains C29-5β-sterols, opuntisterol [(24R)-24-ethyl-5β-cholest-9-ene-6β,12α-diol] and opuntisteroside [(24R)-24-ethyl-6β-[(β-d-glucopyranosyl)oxy]-5β-cholest-9-ene-12α-ol], as well as nine known compounds, β-sitosterol, taraxerol, friedelin, methyl linoleate, 7-oxositosterol, 6β-hydroxystigmast-4-ene-3-one, daucosterol, methyl eucomate, and eucomic acid. All of the above constituents are non-polar and are more soluble in petroleum ether and chloroform than in methanol and water.[Citation43] Therefore, the antibacterial activity of Opuntia dillenii may be due to one or more of these constituents.
Antimicrobial Effect of Zingiber officinale
shows the antimicrobial pattern by Zingiber officinale. Zingiber gave maximum inhibitory zones against Bacillus subtilis and its Petroleum ether extraction prove best, but even at a concentration of 4 mg per well the mean zone of inhibition was lower than our positive controls (Penicillin P < 0.05; Gentamicine P > 0.05). For Escherichia coli, Zingiber officinale extractions by high polar solvents (methanol, water) gave 9 to 11 mm inhibition zones at 4 mg concentration. Zingiber officinale extractions by low polar solvents (petroleum ether, chloroform) gave small zones of inhibitions. Against Salmonella typhi mostly zones of inhibitions by Zingiber officinale were similar to our negative control (DMSO, P > 0.05).
Table 2 In vitro antimicrobial activity of Zingiber officinale extracted in different solvents
It is evident from the results that antimicrobial activity of Zingiber officinale is better in non-polar solvents, i.e., petroleum ether (14.56 mm DIZ against Bacillus subtilis and 8.84 mm DIZ against Staphylococcua aureus) and chloroform (8.14 mm DIZ against Bacillus subtilis and 5.98 mm DIZ against Staphylococcus aureus) against the two gram positive bacteria; however, its activity in polar solvents (methanol and water) varies, i.e., it has good antibacterial activity against Escherichia coli (9.42 mm DIZ with methanol; 9.20 mm DIZ with water extract) but no antibacterial activity against Salmonella typhi (). It indicated that the activity of the Zingiber officinale against Bacillus subtilis and Staphylococcus aureus was mainly due to its non-polar constituents and its activity against Escherichia coli was mainly due to its polar constituents. Activity of ginger extracts was better against Bacillus subtilis as compared to that against Staphylococcus aureus. The extracts of Zingiber officinale contain hundreds of both polar and non-polar constituents, including Coumaric acid, Farnesene, Gingediol, Shikimic acid, Shogaol, Terpine, Zingiberene, Zingibenene, Mentha-1-5-dien-7-ol, Mentha-2-8-dien-1-ol, Mentha-1-5-dien-8-ol, Myrcene, Muurolene, Octa-2-6-diene-1-8-diol, 2-6-dimethyl, Octa-3-7-diene-1-6-diol, 2-6-dimethyl, Octa-3-7-diene-1-6-diol, 2-6-dimethyl, 8-hydroxy, and β-D-glucopyranoside.[Citation44] Isolation and investigation of these polar and non-polar constituents, which are responsible for antimicrobial activity of ginger, is to be demonstrated further.
CONCLUSION
The extracts of both Zingiber officinale (ginger) and Opuntia dillenii (chhitarthohar) in petroleum ether, chloroform, methanol, and water have antimicrobial properties. Bacillus subtilis and Staphylococcus aureus showed considerable susceptibility to all extracts of Opuntia dillenii and Zingiber officinale. Ether and chloroform extracts of Opuntia dillenii showed improved antimicrobial activity against Escherichia coli as compared to that of its methanolic and water extracts, while methanolic and water extracts of Zingiber officinale showed better susceptibility for Escherichia coli. Salmonella typhi was found to be resistant to all extracts of both herbs. This study not only provides a new insight on how the polarity of the solvent affects the antimicrobial activity of the herbal extracts, but it also demands further work on the isolation and purification of the constituents of the above mentioned herbs with special focus on their antimicrobial activity.
ACKNOWLEDGMENTS
The authors wish to thank Zahir Ud Din Khan (Government College University Lahore) for his help regarding the collection and identification of plants and also Asif Saeed (University College of pharmacy, Punjab University Lahore) for his help in developing the method of extraction of plants. A. Javeed, A. Riaz, and M. M. Mukhtar are supported by HEC Pakistan.
Notes
Muhammad Ihtisham Umar and Aqeel Javeed contributed equally to this work.
REFERENCES
- Martinez , J.L. and Baquero , F. 2002 . Interactions among strategies associated with bacterial infection: Pathogenicity, epidemicity, and antibiotic resistance . Clinical Microbiology Reviews , 15 : 647 – 679 .
- Roberts , M.C. 2004 . Resistance to macrolide, lincosamide, streptogramin, ketolide, and oxazolidinone antibiotics . Molecular Biotechnology , 28 ( 1 ) : 47 – 62 .
- Ferrara , A.M. 2005 . New fluoroquinolones in lower respiratory tract infections and emerging patterns of Pneumococcal resistance . Infection , 33 : 106 – 114 .
- Soichiro , A. , Tetsuro , M. , Yoji , Y. , Hisato , I. , Koichi , T. and Tetsuro , M. 2001 . Emergence of cephem- and aztreonam-high-resistant Neisseria gonorrhoeae that does not produce β-lactamase . Journal of Infection and Chemotherapy , 7 : 49 – 50 .
- Shahid , M. , Ensor , V.M. and Hawkey , P.M. 2009 . Emergence and dissemination of Enterobacteriaceae with plasmid-mediated CMY-6 and CTX-M-15 beta-lactamases in community in North-India . World Journal of Microbiology and Biotechnology , 25 : 1439 – 1446 .
- Kristof , K. , Szabo , D. , Marsh , J.W. , Cser , V. , Janik , L. , Rozgonyi , F. , Nobilis , A. , Nagy , K. and Paterson , D.L. 2007 . Extended-spectrum beta-lactamase-producing Klebsiella species in a neonatal intensive care unit: Risk factors for the infection and the dynamics of the molecular epidemiology . European Journal of Clinical Microbiology and Infectious Diseases , 26 : 563 – 570 .
- Pescador , P.J.C. , Garrido , C.A. , Chanona , P.J. , Farrera , R.R. , Gutierrez , L.G. and Calderon , D.G. 2009 . Effect of the addition of mixtures of glucose oxidase, peroxidase and xylanase on rheological and breadmaking properties of wheat flour . International Journal of Food Properties , 12 : 748 – 765 .
- Imran , P. , Faqir , M.A. and Masood , S.B. 2009 . Biochemical characterization of spring wheats in relation to grain hardness . International Journal of Food Properties , 12 : 910 – 928 .
- Kosanovic , M. , Hasan , M.Y. , Petroianu , G. , Marzouqi , A. , Abdularhman , O. and Adem , A. 2009 . Assessment of essential and toxic mineral elements in bitter gourd (Momordica charantia) fruit . International Journal of Food Properties , 12 : 766 – 773 .
- Rui , Y. , Wang , W. , Fazana , R. and Liu , Q. 2009 . Fatty acids composition of apple and pear seed oils . International Journal of Food Properties , 12 : 774 – 779 .
- Shelef , L.A. 1983 . Antimicrobial effects of spices . Journal of Food Safety , 6 : 29 – 44 .
- Shelef , L.A. , Jyothi , E.K. and Bulgarelli , M. 1984 . Effect of sage on growth of enteropathogenic and spoilage bacteria in sage containing broths and foods . Journal of Food Science , 809 : 737 – 740 .
- Arora , D.S. , Kaur , G.J. and Kaur , H. 2009 . Antibacterial activity of tea and coffee: Their extracts and preparations . International Journal of Food Properties , 12 : 286 – 294 .
- Mothershaw , A.S. and Jaffer , T. 2004 . Antimicrobial activity of foods with different physio-chemical characteristics . International Journal of Food Properties , 7 ( 3 ) : 629 – 638 .
- Shan , B. , Cai , Y.Z. , Brooks , J.D. and Corke , H. 2007 . The in vitro antibacterial activity of dietary spice and medicinal herb extracts . International Journal of Food Microbiology , 117 : 112 – 119 .
- Loro , J.F. , del Rio , I. and Perez-Santana , L. 1999 . Preliminary studies of analgesic and anti-inflammatory properties of Opuntia dillenii aqueous extract . Journal of Ethnopharmacology , 67 : 213 – 218 .
- Ahmed , M.S. , El Tanbouly , N.D. , Islam , W.T. , Sleem , A.A. and El Senousy , A.S. 2005 . Antiinflammatory flavonoids from Opuntia dillenii (Ker-Gawl) haw. flowers growing in Egypt . Phytotherapy Research , 19 : 807 – 809 .
- Zaika , L.L. 1988 . Spices and herbs: Their antimicrobial activity and its determination . Journal of Food Safety , 9 : 98 – 117 .
- Gupta , R.S. , Sharma , R. , Sharma , A. , Chaudhudery , R. , Bhatnager , A.K. , Dobhal , M.P. , Joshi , Y.C. and Sha , M.C. 2002 . Antispermatogenic effect and chemical investigation of Opuntia dillenii . Pharmaceutical Biology , 40 : 411 – 415 .
- Kim , J.S. , Lee , S.I. , Park , H.W. , Yang , J.H. , Shin , T.Y. , Kim , Y.C. , Choi , S.U. , Kwon , B.M. , Leem , K.H. , Jung , M.Y. and Kim , D.K. 2008 . Cytotoxic components from the dried rhizomes of Zingiber officinale Roscoe . Archives Pharmacal Research , 31 ( 4 ) : 415 – 418 .
- Ali , A. and Gilani , A.H. 2007 . Medicinal value of ginger with focus on its use in nausea and vomiting of pregnancy . International Journal of Food Properties , 10 : 269 – 278 .
- Penna , S.C. , Medeiros , M.V. , Aimbire , F.S.C. , Faria , N.H.C.C. , Sertie , J.A.A. and Lopes , M.R.A.B. 2003 . Anti-inflammatory effect of the hydralcoholic extract of Zingiber officinale rhizomes on rat paw and skin edema . Phytomedicine , 10 : 381 – 385 .
- Srivastava , K.C. and Mustafa , T. 1992 . Ginger (Zingiber officinale) in rheumatism and musculoskeletal disorders . Medical Hypotheses , 39 ( 4 ) : 342 – 348 .
- Black , C.D. , Herring , M.P. , Hurley , D.J. and O'Connor , P.J. 2010 . Ginger (Zingiber officinale) reduces muscle pain caused by eccentric exercise . The Journal of Pain , In Press. doi: 10.1016/j.jpain.2009.12.013
- Chrubasik , S. , Pittler , M.H. and Roufogalis , B.D. 2005 . Zingiberis rhizoma: A comprehensive review on the ginger effect and efficacy profiles . Phytomedicine , 12 : 684 – 701 .
- Martins , A.P. , Salgueiro , L. , Gonçalves , M.J. , da Cunha , A.P. , Vila , R. , Cañigueral , S. , Mazzoni , V. , Tomi , F. and Casanova , J. 2001 . Essential oil composition and antimicrobial activity of three Zingiberaceae from S . Tome e Principe. Planta Medica , 67 : 580 – 584 .
- Singh , G. , Kapoor , I.P. , Singh , P. , de Heluani , C.S. , de Lampasona , M.P. and Catalan , C.A. 2008 . Chemistry, antioxidant and antimicrobial investigations on essential oil and oleoresins of Zingiber officinale . Food and Chemical Toxicology , 46 : 3295 – 3302 .
- Konning , G.H. , Agyare , C. and Ennison , B. 2004 . Antimicrobial activity of some medicinal plants from Ghana . Fitoterapia , 75 : 65 – 67 .
- Surindra , S. , Vikram , C. and Sushma , S. 2009 . Bacterial contamination in drinking water: A case study in rural areas of northern Rajasthan, India . Environmental Monitoring and Assessment , 159 : 43 – 50 .
- Fethi , B.A. , Kamel , C. , Hela , K. and Amina , B. 2009 . RT-PCR assays for in vivo expression of Vibrio alginolyticus virulence genes in cultured gilthead Dicentrarchus labrax and Sparus aurata . Annals of Microbiology , 59 ( 1 ) : 63 – 67 .
- Bin , S. , Yi , Z.C. , John , D.B. and Harold , C. 2007 . The in vitro antibacterial activity of dietary spice and medicinal herb extracts . International Journal of Food Microbiology , 117 : 112 – 119 .
- Nair , R. , Vaghasiya , Y. and Chanda , S. 2008 . Antibacterial activity of Eucalpytus citriodora Hk. oil on few clinically important bacteria . African Journal of Biotechnology , 7 ( 1 ) : 025 – 026 .
- Swapna , L.P. and Kannabiran , K. 2006 . Antimicrobial activity and phytochemicals of Solanum trilobatum Linn . African Journal of Biotechnology , 5 ( 23 ) : 2402 – 2404 .
- Pithayanukul , P. , Tubprasert , J. and Wuthi , U.M. 2007 . In Vitro Antimicrobial Activity of Zingiber Cassumunar (Plai) Oil and a 5% Plai oil gel . Phytotherapy Research , 21 : 164 – 169 .
- Sabulal , B. , Dan , M. , J, A.J. , Kurup , R. , Pradeep , N.S. , Valsamma , R.K. and George , V. 2006 . Caryophyllene-rich rhizome oil of Zingiber nimmonii from South India: Chemical characterization and antimicrobial activity . Phytochemistry , 67 : 2469 – 2473 .
- Abe , M. , Ozawa , Y. , Uda , Y. , Yamada , F. , Morimitsu , Y. , Nakamura , Y. and Osawa , T. 2004 . Antimicrobial activities of diterpene dialdehydes, constituents from myoga (Zingiber Mioga Roscoe), and their quantitative analysis . Bioscience Biotechnology and Biochemistry , 68 : 1601 – 1604 .
- Sang , A. , Jun , K. and Hyung , S.Y. 2009 . Inhibition of homodimerization of Toll-like receptor 4 by 6-Shogaol . Molecules and Cells , 27 : 211 – 215 .
- Muhammad , N.G. and Anwarul , H.G. 2005 . Pharmacological basis for the medicinal use of ginger in gastrointestinal disorders . Digestive Diseases and Sciences , 50 ( 10 ) : 1889 – 1897 .
- Mari , M.Y. , Kaori , E. and Ikuo , S. 2007 . In vitro and in vivo anti-allergic effects of ‘benifuuki’ green tea containing O-methylated catechin and ginger extract enhancement . Cytotechnology , 55 : 135 – 142 .
- Denyer , C.V. , Jackson , P. , Loakes , D.M. , Ellis , M.R. and Young , D.A. 1994 . Isolation of antirhinoviral sesquiterpenes from ginger (Zingiber officinale) . Journal of Natural Products , 57 : 658 – 662 .
- Gupta , S. and Ravishankar , S. 2005 . A comparison of the antimicrobial activity of garlic, ginger, carrot, and turmeric pastes against Escherichia coli O157:H7 in laboratory buffer and ground beef . Foodborne Pathogens and Disease , 2 : 330 – 340 .
- Awatef , M.S. , Muhammad , Z.A. , Jagdish , N.S. and Ahmad , P.M.Y. 1999 . Anti-inflammatory activity of Crinum asiaticum plant and its effect on bradykinin-induced contractions on isolated uterus . Immunopharmacology , 43 : 311 – 316 .
- Jiang , J. , Li , Y. , Chen , Z. , Min , Z. and Lou , F. 2006 . Two novel C29-5beta-sterols from the stems of Opuntia dillenii . Steroids , 71 : 1073 – 1077 .
- Ross , I.A. 2005 . Zingiber officinale . Medicinal Plants of the World , 3 : 507 – 560 .