Abstract
Gas chromatography coupled to mass spectrometry using a static head space technique was applied to analyze the volatile aromatic compounds of various melons collected from different locations in Turkey. Some physicochemical and sensory analyses were also made. A total of 33 volatile compounds were identified. Differences in the physicochemical and sensory properties of various Turkish melon samples were observed. The multivariate analyses by the volatile compounds separated the three varieties into groups successfully. The most abundant volatile compounds as mean value in all melon varieties were ethyl acetate (22.10%), acetaldehyde (13.65%), and ethanol (23.25%). The results showed that Cantaloupe melons were more preferred by panelists, which had relatively high levels of esters being responsible for strong fruit flavor, like melon, as well as high total soluble solid content and low titratable acidity compared with Inodorus and Simama.
INTRODUCTION
Turkey is the second biggest melon (Cucumis melo L.) producing country after China, with 1,765,605 tons in a 103,000 ha area.Citation[1] Turkey also has valuable genetic resources for melon. HarlanCitation[2] described Turkey as a microcenter of melon, and all subspecies of melon are present in Turkey.Citation[3] However, to the authors' knowledge, the aromatic volatile compounds of Turkish melons have not been studied. This may have a critical importance as lack of the characteristic flavor in marketable melons is a common consumer complaint nowadays. Sweetness (sugar content) and volatile compounds are important parameters in the overall quality and consumer acceptability of melon fruits.Citation4–8 The unique flavor (taste and odor) of a melon variety is the result of a complex balance between volatile and non-volatile chemical compounds, originating from carbohydrates, amino acids, and fatty acids;Citation[9] as a consequence, each product has a characteristic and unique composition of volatile compounds.Citation[10] Therefore, high soluble solids concentration at harvest does not always correspond with high overall fruit quality and should be used in conjunction with the evaluation of aroma.Citation[11] In the melon fruits, volatile compounds responsible for taste and odor are mainly esters, aldehydes, and alcohols.Citation12–16 Aldehydes are formed as a result of the enzymatic degradation of lipids and/or unsaturated fatty acids, such as linoleic and linolenic via lipoxygenase activity, or from amino acids (such as acetaldehydes that come from alanine).Citation[17] Aldehydes are considered to be transitory compounds in fruits because they are further transformed to their corresponding alcohols by alcohol dehydrogenase or to acids.Citation[18] These alcohols are then utilized for the production of esters in reactions catalyzed by alcohol acetyl-transferases (AATs), which is a key enzyme in aroma biosynthesis.Citation[16]
Alcohols of lower molecular weight have a sweet, spirituous odor that intensifies up the series to C9, which has an unpleasant oil–note.Citation[9] The esters make a major contribution to characteristic aroma of the fruit.Citation[4, Citation11, Citation19] The aliphatic esters can also be produced from free fatty acids, such as linoleic and linolenic acids, via generation of short-chain compounds through oxidation.Citation[16, Citation20] Some amino acids, including alanine, c-aminobutyric acid, and valine, are likely precursors of acetates, butyl esters, and 2-methylpropyl esters, respectively.Citation[14]
Chromatographic methods enable the identification and quantification of an individual aroma component. The development of gas chromatography-mass spectrometry (GC-MS) has allowed a detailed analysis of the volatile compounds in melon varieties believed to be responsible for its flavor. Although there were many studies on volatile compounds in various melons, no study has been carried out on volatile compounds of melons grown in Turkey. This may be due to the absence of an accurate analytical method for identification of volatile compounds in melons, which has possibly restricted development of definitive information on aroma compounds. Studies on chemical composition of melons are also scarce and data in the literature about melon varieties in Turkey referred only to its gross composition.Citation[21] Thus, the specific objectives of the present study were: (1) to identify major aroma compounds in melon varieties obtained from the different locations in Turkey; (2) to determine whether melon sensory attributes can be related with aroma composition since sensory and instrumental quality characteristics may predict consumer acceptability of melon eating quality; and (3) if the melon variety groups of C. melo var. cantaloupensis, C. melo var. inodorus, and C. melo var. dudaim can be grouped based on their volatile compounds.
MATERIALS AND METHODS
Materials
Eleven commercial samples of melon accessions belonging to three different subspecies (Cucumis melo var. cantaloupensis, C. melo var. inodorus, and C. melo var. dudaim) were sampled randomly from various locations in Turkey (). All melon samples were cleaned with tap water, were cut in longitudinal sections, and manually peeled with a stainless steel knife. The slices were used for sensory and chemical analyses. Sensory analysis was performed after arrival at the laboratory. At the same time, the samples were prepared for the physicochemical analyses (flesh color, soluble solids content, TA, pH, and volatile compounds). Chemicals were supplied from Merck (Darmstadt, Germany). Two fruits of each melon accessions were sampled for aroma analyses. The four slices from each fruit were cut into small pieces and placed in a chilled mortar and ground with a pestle. The homogenized flesh was used for the determination of total soluble solids (TSS) content, titratable acidity (TA), pH, and volatile compounds.
Table 1 Subspecies, names, properties, and sources of melon accessions (Cucumis melo L.) studied
Method
The TSS content and pH from juice extracts were measured in duplicate using a Link hand refrectometer (RHB-32 model, Fuzhou, Fujian, China) and an Orion pH meter (Thermo, Beverly, MA, USA), respectively. TA was determined in duplicate according to the Official Methods of Analysis.Citation[22] Results were expressed as g citric acid/100 g melon. Flesh color was measured on a latitudinal cut using a Minolta Chromameter (model CR-400; Tokyo, Japan) calibrated with a manufacturer-supplied white calibration plate. The L (dark = 0 and light = 100), a (red = +a and green = −a), and b (yellow = +b and blue = −b) values were measured. The L*, a*, and b* sreading was taken from each of nine melon pieces of each replicate sample. Results were expressed as Chroma (C* = [(a*)² + (b*)²]0.5), hue angle (hab = tan−1[(b*)(a*)−1]), and white index (WI = 100 − [(100 − L)2 + a2 + b2]0.5).
Volatile Compound Analysis
The extraction and characterization of the volatile compounds were carried out by means of static head space (HS)–(GC)-(MS) analysis, which is able to detect most of the volatile aroma compounds. Volatile samples were prepared in duplicate from each replicate fruit. The four slices from each fruit were cut into small pieces and placed in a chilled mortar and ground with a pestle. Ten grams of juice of the homogenized flesh using a Pasteur pipette was immediately transferred in a 20-mL HS vial (Agilent, Palo Alto, CA, USA), containing 3 g NaCl, to inhibit enzyme reactions. The vials were sealed using crimp-top caps with TFE/silicone headspace septa (Agilent) and immediately frozen at −20°C until use. Prior to analysis, frozen samples were thawed at 4°C overnight. At the time of HS-GC analysis, the vials were kept at 45°C for 30 min, then at 75°C for 10 min and stirred five times, and then kept at 75°C for 5 min. These sampling temperatures were chosen after preliminary trials at different temperatures. HS (250 μL) was injected with a gas-tight syringe onto the GC column. The volatile compounds were analyzed on Agilent model 6890 gas chromatography (GC) and 5973 N mass spectrometry (MS) (Agilent) equipped with a HP-INNOWAX capillary column (30 m × 0.32 mm i.d. × 0.25 μm film thickness). Helium was used as the carrier gas at a flow rate of 1.4 mL min−1. The injector temperature was 200°C, set for splitless injection. The oven temperature program was initially held at 45°C for 1 min and then programmed from 45°C by a ramp of 1.5°C min−1 up to 80°C and then at 10°C min−1 to reach a final temperature of 200°C, which held for 15 min. The mass selective (MS) detector was operating in the scan mode within a mass range of 33 to 330 m z−1 at 1 scan s−1, with electron energy of 70 eV. The interface line to MS was set at 250°C. The total analysis time was 42.33 min. The volatile compounds were preliminarily identified by a computer-matching of their mass spectral data supplemented with a Wiley7n.1 and Nist 02.L. GC-MS libraries, and then the identities of most were confirmed by GC retention time (RT) and MS ion spectra of authentic standards, such as ethanol, acetaldehyde, and acetoin. Results from the volatile analyses were expressed as the percentage of each compound integrated area relative to the total integration of compounds identified.
Sensory Analysis
Sensory analysis was determined by 12 experienced panelists (10 males and 2 females) who were familiar with the melon, and the average panelists' age was 35. The panel was performed by academic staff from Mustafa Kemal University. One peeled slice from each variety was placed on a single plate and presented to the tasters at room temperature. Each slice was coded by a digit-numerical code. Melons were evaluated for color (white to typical melon orange), odor (none to excellent odor), texture (mushy to firm), freshness (old to fresh), sweetness (none to very sweet), juiciness (none to very juicy), and overall acceptability (unacceptable to excellent) using a scale rating of 0–7.
Statistical Analysis
Data were analyzed using SAS procedures. The means and standard deviations were calculated using PROC TABULATE. PROC PRINCOMP was used to conduct principle component analysis (PCA) for eleven melon accessions analyzed for sensory attributes, physicochemical properties, and volatile components. The outputs of this analysis are eigenvalues, eigenvectors, and standardized principal component scores. The melon accessions were plotted using PROC P3G with principle component scores. Mean separations were examined using Tukey's studentized range test (t-test).
RESULTS
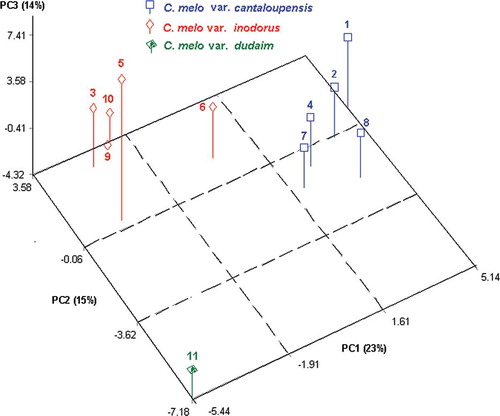
PCA was applied to whole data obtained from sensory, physicochemical, and volatile compound analyses. This multivariate statistical technique allows clustering and grouping of observations with a similar properties group. According to statistical results, the melons were divided into three groups (). As shown in , melons 1, 2, 4, 7, and 8 (C. melo var. cantaloupensis), melons 3, 5, 6, 9, and 10 (C. melo var. inodorus), and melon 11 (C. melo var. dudaim-Simama) were made up of groups I, II, and III, respectively. There were statistically significant differences in aroma compounds and sensory attributes of various melon varieties. Therefore, the evaluations and comparisons of all physicochemical and sensory parameters of 11 melon samples analyzed were performed over three groups.
Figure 1 Results of principal component analysis (PCA) for 11 melon varieties. (Color figure available online.)
Physicochemical parameters of melons are presented in . Cantaloupes had high values for both TSS and pH, while Simama had the lowest values for these parameters. TA was significantly higher in Simama than that in Cantaloupe and Inodorus melons (P < 0.05). Another important marketable (consumer acceptance) quality attribute is melon flesh color. Melon flesh color is a measure of whiteness index (WI, black = −100 and white = +100), chroma (C*; color intensity) and hue angle (hab; color purity). Fruit flesh of Simama had a lower hab, more red than yellow hue, i.e., more orange-colored, than fruit flesh of Cantaloupe samples (). Inodorus melons had a negative hab, more green colored. The orange color was also noticeably more intense (higher Chroma) in flesh of Cantaloupe fruit than in flesh of Inodorus and Simama. Pieces from Inodorus had a significantly higher whiteness index than pieces from Cantaloupe and Simama (P < 0.05).
Table 2 Quality parameters of the three melon variety groups (n = 10)
There were many volatile compounds (>30) identified in the melon varieties; they were grouped according to chemical classes in . Most of these compounds are commonly presented in fruits. Whereas some volatile compounds were newly identified in melons, which are cyclic thioether, 1,3-trans-5-cis-octatrien, N-ethyl-1,3-dithioisoindoline, naphthalene, and acetic acid, it would be very difficult to compare them now.
Table 3 The relative percentages of volatile compounds in three melon variety groups
As shown in , there were significant differences in the volatile composition of the three melon groups as both qualitative and quantitative. Esters were the main components, accounting for 79.9 and 50% of the total volatile identified in Cantaloupe and Inodorus melons, respectively. However, striking differences were observed in volatiles recovered from Simama, which contained only nine volatile compounds compared with Cantaloupe and Inodorus. Ethanol made up 58.77% of the total of volatile compounds in Simama. The esters and ketone (acetoin) were the second largest class in this melon (about 15% of total) (). Of the esters, acetate esters were predominant over non-acetates. Cantaloupe melons had the highest percentage of total acetate esters (59.97%), followed by Inodorus (39.60%) and Simama (14.36%). Interestingly, propanol-metoxy-acetate (2-acetoxy-1-methoxy propane) was found in Simama only. The predominant aldehyde identified in all melons was acetaldehyde. Inodorus melons had a very high content of acetaldehyde and also pentanal and hexanal at low levels. Cantaloupe did not contain pentanal (). From the alcohols, ethanol was the main compound found in the head-space, which was the most abundant compound of volatiles identified in Simama. From carboxylic acids, only acetic acid was presented in Simama. As for hydrocarbons, they were found at lower percentages than the other volatile compounds. Inodorus melons had the highest percentage of hydrocarbons with 3.68%. Hydrocarbon naphthalene was found in Simama variety only. Ethers of lower molecular weight are extremely volatile and have a sweet, spirituous odor. Diethyl ether was determined in Inodorus at trace levels and a relative amount of chloroform was higher than diethyl ether.
Panelists' scores for sensory evaluation of melon varieties are presented in . None of the melons received the maximum score of 7 (excellent) described by the sensory evaluation form. Panelists distinctly preferred the overall quality of melon pieces from Cantaloupe compared to those from Inodorus and Simama (). Cantaloupe was scored the highest for texture, color, juiciness, sweetness, and overall acceptability. Odor score of Simama was similar to that of Cantaloupe. Simama was scored the lowest for texture, freshness, and sweetness; that is, Simama had the softest flesh. The firmness scores of all melon varieties showed a similar trend to TSS and an inverse relation to that of juiciness.
Table 4 Sensory analysis of the three melon variety groups (n = 10)
Panelists' scores showed that, although Simama was acceptable in terms of odor, it was unacceptable for sweetness with a score of 0.17. Sweetness scores of melons were consistent with TSS, TA, pH values, and some aromatic volatiles. However, the odor score for Simama was inconsistent with the results of HS-GC analysis. The orange color (score of 5.49) was noticeably more intense (higher chroma) in Cantaloupe melons compared with the other varieties. The color measurements by Minolta were parallel with the color scores. Simama recovered the lowest score for overall acceptability since this melon had a whitish, flaccid, and insipid pulp and was barely edible.
DISCUSSION
The results showed that there were significant differences in physicochemical properties, volatile aromatic compounds, and sensory quality among melon cultivars belonging to different subspecies of melon (P < 0.05). The similar results were previously obtained by Senesi et al.,Citation[11] Aubert and Pitrat,Citation[13] and Beaulieu.Citation[23] The high levels of pH and TSS in Cantoloupe melons are generally considered as indicators of high sugar content.Citation[7, Citation24] The mean values of TSS and TA in Cantaloupe samples was in agreement with results previously obtained by others.Citation[7, Citation11, Citation25] pH values of Inodorus and Simama were similar to that obtained by Simandjuntak et al.Citation[25] for over-ripped Honeydew melon and Hernandez-Gomez et al.Citation[6] for melon (C. melo L.). Therefore, the reason for much higher TA in Simama may be due to the high activity of enzyme or it's over-ripeness.
In melons, color values are related to β-carotene (yellow to range pigment) and chlorophyll (green pigment) depending on the variety since the flesh color of Cantaloupe, Inodorus, and Dudaim group melons are mainly “orange,” “green-white,” and “white,” respectively.Citation[26] Chroma and hue angle values were the highest in Cantaloupe melons with the low acidity, and the lowest in Simama with high acidity, since acid hydrolysis and/or increase in activity of enzymes may have resulted in the degradation of both β-carotene and chlorophyll pigments.Citation[25]
With regard to aroma compounds, esters were the most abundant compound in Cantaloupe samples having a high chroma value, pH, and TSS. That result was consistent with the finding of Beaulieu and Lea.Citation[27] Esters are likely to be key contributors to unique aroma of melons.Citation[16] They have a low perception threshold concentration, which is 10-fold lower than their alcohols precursor.Citation[28] The principal ester found in all melons was ethyl acetate. This finding was in agreement with the results of others.Citation[5, Citation11, Citation13, Citation17] Ethyl acetate contributed to aroma complexity and had a positive impact at a very low level,Citation[6] whereas its high level was responsible for an off-flavor note as “solvent” and “ethereal,” particularly in Cantaloupes.Citation[5, Citation17] Apart from ethyl acetate, propyl acetate (odor like fruity, pear, and raspberry) 2-methyl propyl acetate (sweet, fruity), butyl acetate (flavor like fruity, sweet, and grape), 2-methyl-1-butyl acetate, hexyl acetate (fruity), ethyl-2-methyl butanoate (powerful green, fruity apple-like odor), ethyl butanoate (fruity odor with pineapple undertone and sweet), and ethyl pentanoate (fruity and apple) responsible for various taste and odorCitation[7, Citation15, Citation29] were the most abundant aliphatic esters in the Cantoloupe variety over Inodorus. The esters mentioned above were important contributors to melon flavor; that is, they were responsible for the strong fruity notes in melons.Citation[4, Citation13, Citation14, Citation17, Citation19] Butyl acetate, methyl 2-methyl butanoate, and ethyl butanoate were especially characterized by sweetness, and fruity flavor notesCitation[7, Citation29, Citation30] were significantly high in Cantaloupe melons (P < 0.05). This result could explain why Cantaloupe melons were scored by high sweetness. In general, ethyl-, methyl-, and propyl-acetates and propionates are “odor” characteristics and ethyl-, methyl-butanoate and hexanoates, butyl and methyl acetates are “taste” characteristics.Citation[15] Esters mentioned might serve as flavor tools for breeders in improving sensory quality of melons in addition to soluble solids (sugar). Interestingly, propanol-metoxy-acetate (2-acetoxy-1-methoxy propane) detected in Simama was only found in Queen Anne's Pocket melon, which was called the Fragrant melon in France. Simama like Queen Anne's Pocket melon may also contain less number and amount of volatiles in pulp than those in the skin.Citation[13] It was reported by Umano et al.Citation[31] that acetoxy esters increased in the pineapple during ripening.
As for aldehydes, the main aldehyde identified in all melons was acetaldehyde with sharp flavor note (). This finding was confirmed by the results reported by others.Citation[5, Citation11, Citation12, Citation17, Citation19] Of the straight-chain aldehydes, pentanal and hexanal detected in Inodorus melons are responsible for green like-grass and herbaceous aromas.Citation[7] Actually, n-hexanal confers a beamy flavor and is used as a general index of degree of fatty acid oxidation.Citation[32] Pentanal is responsible for distinguishing some non-climacteric melons and climacteric melons.Citation[17] In our analyses, aldehydes were the most abundant compound in Inodorus. Beaulieu and GrimmCitation[19] reported that most aldehydes (especially less than C8) were dominant in immature melon samples and cucumbers, and were not detected or recovered in only trace levels from mature fruit. This finding also confirmed why Inodorus melons recovered the lowest odor and the highest freshness scores.
In melons, ethanol was called a “negative compound” being responsible for the fermented flavor noteCitation[5] and some branched alcohols (2-methyl, propanol, 2-methyl butanol) are aromatic at low levels.Citation[13] Cantaloupe melons, being much more preferred by panelists, contained 2-methyl propanol, 2-methyl butanol at very low levels as well as ethanol, whereas Inodorus and Simama had only ethanol. Senesi et al.Citation[11] found that a high ethanol concentration has been associated with advanced melon ripeness with off-flavor, acetaldehyde can easily be degraded to ethanol by alcohol dehydrogenase synthesized by some microorganism. The results of this study showed that the percentage of acetaldehyde in all melons decreased when the ethanol level increased. Acetoin (3-hydroxy-2-butanone) and acetic acid were detected only in Simama variety with a high level. Acetoin is essentially odorless,Citation[32] and has been previously detected in Protea and Vector melon cultivars in ItalyCitation[11] and Queen Anne's pocket melon in France.Citation[13] It was also reported to be an essential oil constituent of several other foods, including vegetables, especially in guar bean and French beans.Citation[33] Acetic acid (CH3COOH) is by far the most important of the carboxylic acids, which produces unpleasant odors with strongly pungent, penetrating, and high concentration that is an indicator of poor quality.Citation[6, Citation9] Simama samples were evaluated with “sour” and “unacceptable” flavor by panelists. It, therefore, is a possible contributor to the particular taste (sour) of Simama melon.
Aromatic (naphthalene) and unsaturated (1,3–trans–5–cis octatriene) hydrocarbons were detected in very low levels in melons. Hydrocarbons, based on double (naphthalene) ring structure, are more aromatic. Naphthalene was detected only in Simama, which had a high whiteness index and low hab value. Some authors have claimed that hydrocarbons with ring structure could originate from the degradation of the carotene.Citation[34, Citation35] The unsaturated hydrocarbons, such as 1,3–trans–5–cis octatriene, 2–methyl,1–4–pentadien may also be significant contributors to desirable aroma.Citation[34] It has been reported that some hydrocarbons, such as cis- and trans-isomers of octane could originate from oxidation of unsaturated fatty acids.Citation[36] From hydrocarbons, 1,3-trans-5-cis octatriene identified only in Inodorous was previously found in essential oil of Glechoma hederaracea L. by Mockutë et al.Citation[37]
On the other hand, it has been previously reported that C9 compounds, such as (Z) 6-nonen-1-ol and (Z),6-nonenal, are the principal odorants in muskmelon flavor,Citation[29, Citation38, Citation39] whereas these were not identified in this study and some earlier studies.Citation[4, Citation5, Citation11, Citation20] It is believed that some of the C9 compounds formerly designated as flavor active in the Cucurbitacea family (e.g., cucumber and honeydew) may not be present in all melon varieties. The differences found in the aroma profile may be due to the varietal/genetic variations, postharvest fruit handling, maturity at harvest, breeding programs, and partial differences in aroma extraction technique and chromatographic conditions since there were differences in aroma compounds of melon varieties analyzed under the same conditions in this study.
Sensory attributes play a key role in determining consumer preference. Hence, elucidation of sensory causing components of melon fruit is of paramount importance to melon producers. In general, scores of sensory parameters of melons were parallel to physicochemical properties and flavor character of aromatic volatiles, whereas the mean odor score of Simama with a very unique odor, although, was similar to that of Cantaloupe, and it contained only nine volatile compounds. This may be due to suppression in recovery of minor volatile compounds of high ethanol level and/or the low detection limit of some odor active volatile compounds. For example, in wine, ethanol can suppress volatile of esters. Similarly, polyphenols, important nonvolatile constituents of red wine in particular, may interact with some flavor compounds to alter their volatility and flavor release.Citation[40] However, the mechanisms of these interactions and their effects on sensory perception have not yet been fully characterized.
From the volatile compounds analyzed, acetic acid, acetoin, naphthalene, and propanol-methoxy acetate were found only in Simama. These compounds, together with the lowest TSS, the highest TA, and ethanol, may explained why Simama melon was the lowest scored for “sweetness,” “texture,” and “freshness” by panelists. On the other hand, Cantaloupes were scored the highest sweetness (sugar content) and more preferred by the preference panel. Consumers' acceptance of melons is not only driven by sweetness,Citation[41] but also an acceptable taste, i.e., by the presence of aromatic volatiles. However, TSS above 8% is not always directly associated with melon sweetness, flavor, or overall acceptability.Citation[8] The present study showed that increase in the relative amounts of volatile compounds (butyl acetate, methyl-2-methyl butanoate, ethyl butanoate, and ethyl hexanoate) being responsible for sweet flavor caused an increase in sweetness score of melons. It is also worth noting that the high content of TSS and the low level of TA are equally essential to produce a good flavor. Additionally, the overall acceptability of melons with relatively much higher or lower aldehyde (especially acetaldehyde) contents was low. The same results were reported by Beaulieu and GrimmCitation[19] since presence of aldehydes could be either endogenous or a result of maceration, and they may cause off-flavor defects. These authors stated that particular esters and aldehydes could be used as flavor quality markers. Overall, melon flavor intensity seems to be closely related to esters and aldehydes and, to a lesser extent, to some alcohols. In this study, the authors suggest that the ratio of particular esters (except for ethyl acetate) to aldehydes can be used as an indicator for “balanced” and “pleasant” flavor in melons since that ratio was 4.4 for Cantaloupe, 1.0 for Inodorus, and 0.39 for Simama. This order was parallel to panelists' scores for overall acceptability.
CONCLUSIONS
Optimum fruit sensory quality was related to the ratio of particular esters to aldehydes, sugar content, and acid accumulation. An important result of this work is that a static head space method can be used as both a quality assessment system and a mean to distinguish in melon varieties. Therefore, volatile compounds determined using this technique might serve as flavor tools for breeders in improving sensory quality and determining of harvest maturity of melons in addition to soluble solids content. Furthermore, a long-term goal of this research is to determine the correlations between individual aroma compound and flavor attributes in melons grown under the same growing and climatic conditions.
ACKNOWLEDGMENTS
The authors wish to thank Associate Professor Dr. Suat Şensoy for providing Simama melons and Associate Professor Dr. Sedat Serçe for statistical analysis. GC-MS in this work was supported by the T.R. Prime Ministry State Planning Organization (DPT) project (Project no: 02 K 120860).
REFERENCES
- FAO. Food and agricultural commodities production. Countries by commodity. Other melons (inc. cantaloupes). 2007 http://www.faostat.fao.org/site/339/default.aspx (http://www.faostat.fao.org/site/339/default.aspx)
- Harlan , J.R. 1951 . Anatomy of gene centres . The American Naturalist , 85 : 97 – 103 .
- Sarı , N. , Tan , A. , Yanmaz , R. , Yetisir , H. , Balkaya , A. , Solmaz , I. and Aykas , L. May 21–24 2008 . “ General status of cucurbit genetic resources in Turkey ” . In Proceedings of IXth EUCARPIA Meeting on Genetics and Breeding of Cucurbitaceae May 21–24 , 21 – 32 . Avignon , , France
- Fallik , E. , Ikali-Tuvia , S. , Horev , B. , Copel , A. , Rodov , V. , Aharoni , Y. , Ulrich , D. and Schulz , H. 2001 . Characterisation of ‘Galia’ melon aroma by GC and mass spectrometric sensor measurements after prolonged storage . Postharvest Biology and Technology , 22 : 85 – 91 .
- Lo Scalzo , R. , Papadimutri , C. , Bertola , G. , Maestrelli , A. and Torreggiani , D. 2001 . Influence of cultivar and osmotic dehydration time on aroma profiles of muskmelon (Cucumis melo, cv reticulatus Naud) spheres . Journal of Food Engineering , 49 : 261 – 264 .
- Hernandez-Gomez , L.F. , Ubeda-Iranzo , J. , Garcia-Romero , E. and Brionez-Perez , A. 2005 . Comparative production of different melon distillates: Chemical and sensory analysis. Food Chemistry , 90 : 115 – 125 .
- Saftner , R.A. , Abbort , J.A. , Lester , G. and Vinyard , B. 2006 . Sensory and analytical comparison of orange-fleshed honeydew to cantaloupe and green-fleshed honeydew for fresh-cut chunk . Postharvest Biology and Technology , 42 : 150 – 160 .
- Saftner , R.A. and Lester , G.E. 2009 . Sensory and analytical characteristics of a novel hybrid muskmelon fruit intended for the fresh-cut industry . Postharvest Biology and Technology , 51 : 327 – 333 .
- Reineccius , G. 2006 . Flavor Chemistry and Technology , 489 New York , NY : Taylor & Francis .
- Eskin , N.A.M. 1990 . Biochemistry of Foods , 2nd , 539 New York , NY : Academic Press .
- Senesi , E. , Lo Scalzo , R. , Prinzivalli , C. and Testoni , A. 2002 . Relationships between volatile composition and sensory evaluation in eight varieties of netted muskmelon Cucumis melo L. var. reticulatus Naud) . Journal of Agricultural and Food Chemistry , 82 : 655 – 662 .
- Horvat , R.J. and Senter , S.D. 1987 . Identification of additional volatile compounds from cantaloupe . Journal of Food Science , 52 : 1097 – 1098 .
- Aubert , C. and Pitrat , M. 2006 . Volatile compounds in the skin and pulp of Queen Anne's Pocket Melon . Journal of Agricultural and Food Chemistry , 54 : 8177 – 8182 .
- Kourkoutas , D. , Elmore , J.S. and Mottram , D.S. 2006 . Comparison of volatile compositions and flavour properties of cantaloupe, Galia and honeydew muskmelons. Food Chemistry , 97 : 95 – 102 .
- Beaulieu , J.C. and Lancaster , V.A. 2007 . Correlating volatile compounds, sensory attributes, and quality parameters in stored fresh-cut cantaloupe . Journal of Agricultural and Food Chemistry , 55 : 9503 – 9513 .
- Khanom , M.M. and Ueda , Y. 2008 . Bioconversion of aliphatic and aromatic alcohols to their corresponding esters in melons (Cucumis melo L. cv. Prince melon and cv. Earl's favorite melon) . Postharvest Biology and Technology , 50 : 18 – 24 .
- Obando-Ulloa , J.M. , Morenob , E. , Garcia-Mas , J. , Nicolai , B. , Lammertyn , J. , Monforte , A.J. and Fernández-Trujillo , J.P. 2008 . Climacteric or non-climacteric behavior in melon fruit Aroma volatiles . Postharvest Biology and Technology , 49 : 27 – 37 .
- Manriquez , D. , El Sharkawy , I. , Flores , F.B. , El Yahyaoui , F. , Regad , F. , Bouzayen , M. , Latché , A. and Pech , J.C. 2006 . Two highly divergent alcohol dehydrogenases of melon exhibit fruit ripening-specific expression and distinct biochemical characteristics . Plant Molecular Biology , 61 : 675 – 685 .
- Beaulieu , J.C. and Grimm , C.C. 2001 . Identification of volatile compounds in cantaloupe at various developmental stages using solid phase microextraction . Journal of Agricultural and Food Chemistry , 49 : 1345 – 1352 .
- Bauchot , A.D. , Mottram , D.S. , Dodson , A.T. and Jonh , P.J. 1998 . Effect of aminocyclopropane-1-carboxylic acid oxidase antisense gene on the formation of volatile esters in cantaloupe Charentais melon (Cv. Vedrandais) . Journal of Agricultural and Food Chemistry , 46 : 4787 – 4792 .
- Şalk , L. , Deveci , M. and Polat , S. 2008 . Özel Sebzecilik.Namık Kemal Üniversitesi, Ziraat Fakültesi, Bahçe Bitkileri Bölümü, Tekirdağ , : 488
- AOAC . 1990 . Official Methods of Analysis , Washington , DC : AOAC International .
- Beaulieu , J. 2006 . Volatile changes in cantaloupe during growth, maturation, and in stored fresh-cuts prepared from fruit harvested at various maturities . Journal of the American Society for Horticultural Science , 131 : 127 – 139 .
- Corey , K.A. and Schlimme , D.V. 1988 . Relationship of rind gloss and groundspot color to flesh quality of watermelon fruits during maturation . Science Horticultural , 34 : 211 – 218 .
- Simandjuntak , V. , Barrett , D.M. and Wrolstad , R.E. 1996 . Cultivar and frozen storage effects on muskmelon (Cucumis melo) colour, texture and cell wall polysaccharide composition . Journal of the Science of Food and Agriculture , 71 : 291 – 296 .
- Portnoy , V. , Benyamini , Y. , Bar , E. , Harel-Beja , R. , Gepstein , S. , Giovannoni , J.J. , Joseph Burger , A.A. , Tadmor , Y. , Lewinsohn , E. and Katzir , N. 2008 . The molecular and biochemical basis for varietal variation in sesquiterpene content in melon (Cucumis melo L.) rinds . Plant Molecular Biology , 66 : 647 – 661 .
- Beaulieu , J.C. , Lea , J.M. , Eggleston , G. and Peralte-Inga , Z. 2007 . Sugar and organic acid variations in commercial cantaloupes and their inbred parents . Journal of the American Society for Horticultural Science , 126 : 611 – 617 .
- Preininger , M. and Grosh , W. 1994 . Evaluation of key odorants of the neutral volatiles of Emmantaler cheese by the calculation of odor activity values . Leben Wissen Technology , 27 : 237 – 244 .
- Hayata , Y. , Takayuki , S. , Maneerat , C. , Li , X. , Kozuka , H. and Sakamato , K. 2003 . Evaluation of aroma compounds contributing to muskmelon flavor in porapak Q extracts by aroma extract dilution analysis . Journal of Agricultural and Food Chemistry , 51 : 3415 – 3418 .
- Chin , S.T. , Nazimah , S.A.H. , Que , S.Y. , Che Man , Y.B. , Abdul Rahman , R. and Mat Hashim , D. 2007 . Analysis of volatile compounds from Malaysian durians (Durio zibethinus) using headspace SPME coupled to fast GC-MS . Journal of Food Composition and Analysis , 20 : 31 – 44 .
- Umano , K. , Hagi , Y. , Nakaraha , K. , Shoji , A. and Shibamoto , T. 1992 . Volatile constituents of green and ripened pineapple (Ananas comosus) . Journal of Agricultural and Food Chemistry , 40 : 599 – 603 .
- Lindsay , R.C. 2008 . “ Flavors ” . In Fennema's Food Chemistry , Edited by: Damodaran , S. , Parkin , K.L. and Fennema , O.R. 640 – 683 . New York : CRC Press .
- Chatterjee , S. , Sharma , J. , Variyar , P.S. and Sharma , A. 2009 . Free and glycosidically bound volatiles of some common Indian vegetable . EJEAF Chemistry , 8 : 613 – 620 .
- Irigoyen , A. , Ortigosa , M. , Juansaras , I. , Oneca , M. and Torre , P. 2007 . Influence of an adjunct culture of Lactobacillus on the free amino acids and volatile compounds in a Roncal-type ewe's-milk cheese . Food Chemistry , 100 : 71 – 80 .
- Baron , L.J.R. , Yolando , R. , Aramburu , M. , Gil , P. , Perz-Elortonda , P.J. , Albisu , M. , Najera , A.I. , Renobales , M. and Fernandez-Garcia , F. 2007 . Volatile composition and sensory properties of industrially produced Idiazabal cheese . International Dairy Journal , 17 : 1401 – 1414 .
- Shipe , W.F. , Bassette , R. , Deane , D.D. , Dunkley , W.L. , Hammond , E.G. and Harper , W.J. 1978 . Off–flavor of milk: Nomenclature, standards and bibliography . Journal of Dairy Science , 61 : 855 – 869 .
- Mockutë , D. , Bernotienë , G. and Judpentiënë , A. 2005 . Chemical composition of essential oils of Glechoma hederacea L. growing wild in Vilnius district . Chemistry IJA , 16 : 47 – 50 .
- Homatidou , V.I. , Karvouni , S.S. , Dourtoglou , V.G. and Poulos , C.N. 1992 . Determination of total volatile components of Cucumis melo L. variety Cantaloupensis . Journal of Agricultural and Food Chemistry , 40 : 1385 – 1388 .
- Schieberle , P. , Ofner , S. and Grosch , W. 2006 . Evaluation of potent odorants in cucumbers (Cucumis sativus) and muskmelons (Cucumis melo) by aroma extract dilution analysis . Journal of Food Science , 55 : 193 – 195 .
- Deibler , K.D. and Delwiche , J. 2003 . Handbook of Flavor Characterization: Sensory Analysis, Chemistry, and Physiology , 384 New York : CRC Press. Taylor & Francis .
- Beaulieu , J.C. and Lea , J.M. 2003 . Aroma volatile differences in commercial orange fleshed cantaloupes, the inbred parental lines and stored fresh-cuts . Acta Horticulturea , 628 : 809 – 815 .