Abstract
Seven kinds of bush plants, namely, bush tomato (BT), lemon myrtle (LM), wild lime (WL), finger lime (FL), wattle seed (WS), Davidson's plum (DP), and Kakadu plum (KP) were investigated for antioxidant capacity by 2,2-diphenyl-1-picrylhydrazyl radical, Trolox equivalent antioxidant capacity assay, or 2,2'-azinobis-93-ethyl-benzothiazoline-6-sulfonic acid radical, total polyphenols, and flavonoids. It was found that there was a positive correlation between antioxidant activities examined by the two methods. However, there was a negative correlation between total phenol and each of the antioxidant activity tests; for example, Davidson's plum contained the phenolic content as high as 890 mg GAE/100 g while low antioxidant activities were detected (23 TE/100 g and 45% for TEAC and % DPPH, respectively). For the qualitative flavonoids test, bush tomato contained feulic acid, caffeic acid, naringenin, and hesperetin. Lemon myrtle contained catechin, epicatechin, vanilic acid, myricetin, kampferol, and naringenin. Finger lime contained caffeic acid and vanilic acid. Wild lime contained epicatechin, vanilic acid, luteolin, and naringenin. Kakadupum contain catechin and naringenin. Davidson's plum contained naringenin and hesperetin. Wattle seed contained naringenin. However, some other compounds could not be identified because there was no standard to confirm the retention time available. Absorbance was changed for the detection of anthocyanins in Davidson's plums from 220–400 to 525 nm. It was shown by liquid chromatography mass spectrometry that six major anthocyanidins (delphinidin, cyanidin, petunidin, pelargonidin, peonidin, and malvidin) attached with the sugar molecules (hexose and pentose) were found and the major anthocyanin was cyaniding-hexose-pentose. This study suggests that regarding the antioxidant capacity, these Australian Native plants have potential as functional food ingredients.
INTRODUCTION
Recently, theret has been an overwhelming number of researches on food science and nutrition with regard to functional food. The term “functional food” refers to an antioxidant-rich diet, which has a potential in lowering the risk of cardiovascular disease, diabetes, arthritis, and cancer.Citation[1] There has also been a considerable interest in plants with a high antioxidant capacity diet. Australian native plants are considered to be potential sources of antioxidants.Citation2–4 Citation Citation4] The examples are ascorbic acid in Kakadu plumCitation[5] and flavonoids in Davidson's plum.Citation[6,Citation7] In addition, lemon myrtle was found to deliver antibacterial and antifungal activity.Citation[8] Thus, the native food ingredients may be utilized as captivating antioxidants for the preservation of foods and have application for human health.Citation[2]
Since polyunsaturated fatty acids are oxidized by either enzymatic or autoxidation in free radical chain reactions, antioxidants can react against these two reactions. Two types of antioxidants are categorized upon the ability to prohibit the oxidation process in different stages. Primary antioxidants react against the oxidation reaction by breaking chain reaction and/or scavenging free radicals. Secondary antioxidants work by deactivation of metal, inhibition of the breakdown of lipid hydroperoxides, regeneration of primary antioxidants, and singlet oxygen quenching.Citation[9]
Antioxidant activity can be determined by measuring relatively stable. For the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical method, the remaining DPPH·, after the reaction between DPPH· solution and donating antioxidant (AH), is measured at 517 nm at a certain time.Citation[10,Citation11] This method could be considered very rapid, and no expensive reagents or sophisticated instruments are required.Citation[10,Citation12] Trolox equivalent antioxidant capacity (TEAC) is one of the spectrophotometric methods that have been used to determine the total antioxidant activity of solutions of pure substances, aqueous mixtures, and beverages.Citation[13] TEAC assay measures the ability of an antioxidant to quench a radical cation, which is generated by 2,2'-azinobis-93-ethyl-benzothiazoline-6-sulfonic acid (ABTS). Various types of TEAC assay have also been described.Citation[14]
Recent work on antioxidant activity on Australian native plants focused on those methods used to evaluate lipid oxidation by the spectrophotometric method. Forbes-Smith and PatonCitation[2] examined the antioxidant activity of native plants by using the β-carotene bleaching agar diffusion test and linoleic acid reaction test. Netzel et al.Citation[7] tested some Australian native fruits for their antioxidant activity (DPPH), ascorbic acid, total phenolic content, and anthocyanins. However, more work on bioactive compounds including antioxidant level is required to promote the Australian native food industry on nutritional benefits of native fruits and seeds. This study was conducted to evaluate antioxidant capacity, total phenolic content, and flavonoids of seven Australian native food plants.
MATERIALS AND METHODS
Chemicals
General chemicals were supplied by the School of Agriculture and Food Sciences, the University of Queensland: ABTS, 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt; Trolox, 6-hydroxxy-2,5,7,8-tetramethylchromane-2-carboxylic acid; DPPH, 2-Di(4-tert-octylphenyl)-1-picrylhydrazyl; and Folin–Ciocalteu reagent were purchased from Sigma-Aldrich (NSW, Australia). The standard reagents: Kaemperol, Luteolin, Naringenin, Myricetin, and Hesperetin were also from Sigma-Aldrich (Sydney, Australia). The standard reagents: (−)-epicatachin, caffeic acid, ferulic acid, cinnamic acid, vanilic acid, (+) catechin, quercetin, and anthocyanidin standards (cyaniding, delphinidin, pelargonidin) were supplied by Analytical Services (University of Queensland).
Sample Preparation
Samples were collected from different producers who produce native food plants. One kilogram of whole as well as a kilo gram of ground bush tomato in an air tight plastic bag were collected at the production site of the producer (Rod Honna, Alice Springs, NT, Australia). Kakadu plums were brought frozen from the producer site (Ray Hall, Winnellie, NT, Australia). Frozen wild lime was collected from a distributor (Robins Foods, Braeside, VIC, Australia). The whole fruits were packed into a plastic bag, which was stored in a box topped with dried ice. Frozen Davidson's plum, finger lime, and fine-ground dried lemon myrtle (1 kg each) were sent from the producer (Sibylla Hess-Buschrann, Lismore, NSW, Australia).
All samples, except dried bush tomato and lemon myrtle, were cut into thin pieces. Stones were removed for Kakadu plum and Davidson's plum. Sample slides were placed onto the trays and kept frozen prior to freeze drying. The freeze dryer (Alpha 1-4 LSC, Martin Christ, Gefriertrocknungsanlagen GmbH, Germany) was warmed up for 15–20 min before use. Samples were put into the chamber and the machine was run overnight (at 20°C and 1 mbar). Moisture content was calculated after samples were taken out of the freeze dryer and vacuumed in an oven until the weight was constant. Samples were kept in the freezer for further analysis.
Solvent Extraction of Australian Native Plants
Dried sample (∼1.0 g) was added into a 50-mL polypropylene centrifuge tube and mixed with 15 mL of hexane. It was then mixed on a rotating device for 35 min and centrifuged at 25,000 rpm for 5 min. The extraction was done twice and the supernatant was combined. The combined solvent was evaporated off under a nitrogen stream on a hot plate. The residue sample was then dried under a vacuum oven to remove the tract solvent.
The extracting solvent was prepared by mixing methanol, acetone, and water (7/7/6) with the addition of 0.05% ascorbic acid. Solvent (20 mL) was then added into the dried residue and the mixture was mixed on a rotator for 35 min. The mixture was centrifuged at 25,000 rpm for a further 10 min. The extraction was done twice and the supernatant was combined. The combined supernatant was concentrated in the rotary evaporator to remove the organic component. The concentrated extracts were frozen before drying in the freeze dryer. The extracts were store below 5°C for further analysis. The addition of 0.05% of ascorbic acid to eliminate dissolved oxygen in the extracting solvent was only used for the flavonoid's extraction. For the test of phenolic content, antioxidant capacity by DPPH radical, and TEAC, the extracting solvent was used alone.
Antioxidant Activity Determination by DPPH Radical Method
Dry extract was dissolved in methanol. Samples with concentration series of 50, 100, 500, and 1000 μg/mL as well as BHT (positive control) with concentrations of 50, 100, 500, and 1000 μg/mL were prepared on the day of analysis. DPPH (1 mM) solution was also freshly prepared. A sample of (4.0 mL) each concentration was added to a 20-mL test tube and 1 ml of DPPH solution was added on top of the samples. The samples with DPPH were mixed on a vortex mixer for 1 min and incubated in the dark for 35 min at room temperature. After incubation, the absorbance at 517 nm (Ultrospec III UV/Vis, Pharmacia, The Netherlands) was measured.Citation[15] Methanol (4.0) was used as a negative control. % RSA was calculated from the following equation:
Antioxidant Activity Determination by TEAC Assay
Similarly to DPPH assay, the same sample concentration series was prepared. ABTS radical solution (ABTS°+) was prepared by mixing ABTS (7 mmol/L) with 2.45 mmol/L of potassium persulfate (K2S2O8),Citation[13] which obtained a molar ratio of 1:0.035. The solution was left stand at room temperature to allow complete reaction and a stable absorbance for 12–24 h. To prepare the working solution, the mixture was then diluted with ethanol to obtain the absorbance at 0.700 ± 0.020 at 734 nm and the dilution was recorded.
An adequate dilution (10 μL) of the sample was added to 1 mL of diluted ABTS°+ solution and the absorbance reading was taken immediately within 1 min after initial mixing for 1–15 min at 734 nm. A dose-response curve was derived for Trolox (0, 25,100,400 mg/L). The percentage of exhibition of both standards and the samples were calculated when the absorbance readings were constant (after 10 min). Values were expressed as Trolox equivalent (TE) per gram of the sample.
Phenolic Content by Folin-Ciocalteu Method
Appropriately diluted extracts (1.0 mL) that were diluted with methanol or standard solutions of gallic acid (20, 40, 60, 80, and 100 mg/L) were added to a 25-mL volumetric flask containing 9 mL of deionized H2O (DI).One milliliter of 10% Folin-Ciocalteu reagent was added to the mixture and shaken for 5 min. Then, 10 mL of 7% Na2CO3 was added to the mixture. The solution was finally brought to volume with DIH2O using a 25-mL volumetric flask. The incubation was taken for 90 min at room temperature. The absorbance was read at 750 nm using DIH2O as a blank.Citation[16] Total phenolic contents were expressed as mg gallic acid equivalents (GAE)/100 g sample.
Qualitative Screening of Flavonoids in Bush Plants
Mixed standard 1 was prepared by dissolving (−)-epicatachin, caffeic acid, ferulic acid, cinnamic acid, vanillic acid, and (+) catechin. Each of them was prepared at a concentration of 50 μg/mL in 20% acetronitrile (ACN). The concentration of 25 μg/mL of mixed myricetin, quercetin, luteolin, and kampferol in 20% ACN were prepared and named as mixed standard 2. Fifty μg/mL of hesperetin and naringeninin 20% ACN were also mixed (mixed standard 3). Dried extract was redissolved with 2 mL of 20% ACN. The samples were then dissolved in solution by a sonicator filled with warm water. The liquid samples were filtrated through a 0.45-μm nylon filter and then injected into liquid chromatography mass spectrometry (LCMS) (Alliance 2690 HPLC, Waters, USA) using the Electrospray Ionisation in positive mode (ESI+). The compounds were distinguished using a mass scan detector and were confirmed with the retention time of the standards. The separation of the compounds was readily achieved on an X-terra column (150 × 2.1 mm, φ 3.5 μm) with the gradient mobile phase (A: 2% ACN 0.1% formic acid; B: 80% ACN 0.1% formic acid). Total running time was 35 min with a flow rate of 0.25 mL/min. The flavonoid compounds were detected individually with wavelengths ranging from 220–400 nm. However, as Davidson's plum contains a high amount of anthocyanins and the anthocyanins are detected at 525 nm, the standards and the methods of detection were slightly changed.
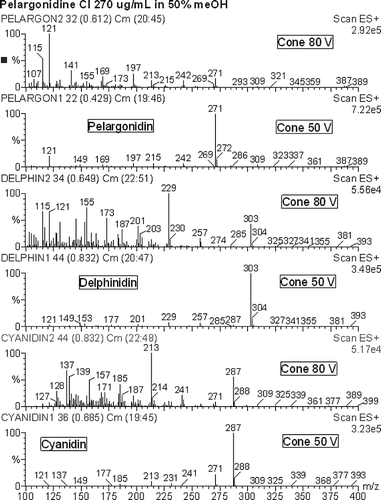
For the analysis of anthocyanin (anthocyanidins conjugated with sugars) in Davidson's plum, the conditions for ionization of the reference anthocyanidins (cyanidin, delphinidin, and pelargonidin) was optimized for ESI+ mode taking advantage of the + ionizable site present in the flavium ring structure. A cone voltage was adjusted from 25, 50, to 80 V and a better condition was chosen. In the absence of reference anthocyanidins for other compounds, the standards of cyaniding and delphinidin were used to qualify the peak area for all anthocyanin present.
Statistical Correlation Between the Tests for Antioxidant Activity and Total Phenols
Statistical correlation between each of the antioxidant activity tests (DPPH radical and TEAC) and total phenols was analyzed by Pearson analysis using Minitab® statistic software, version 14 (Minitab, USA).
RESULTS AND DISCUSSION
Radical scavenging activity (%RSA) by DPPH method of some of the bush plants is shown in . At the higher concentration (i.e., 1000 μg/mL), all bush plant samples gave relatively high activity (∼50–90%). The highest radical scavenging activity was Kakadu plum followed by lemon myrtle, finger lime, bush tomato, wild lime, wattle seed, and Davidson's plum, which possibly is due to its high ascorbic acid content (406–5320 mg/100 edible potion).Citation[17,Citation18] Moreover, DPPH assay reacts rapidly to ascorbic acid since the condition of the assay does not have an adverse effect to ascorbic acid and iso ascorbic acid.Citation[12] Unpublished data also agree with this (Dyah, personal communication). However, Davidson's plum showed the lowest activity. This might be due to the fact that Davidson's plum also contains a purple pigment that interferes with the initial color of DPPH solution (purple).Citation[12] This antioxidant study was then confirmed by TEAC assay. The effect of time on the suppression of the absorbance of the radical was also studied. It was observed that the absorbance of the samples appeared to be constant after 10 min (data not shown). Consequently, the percentage of the exhibition of the samples was calculated at 10 min and expressed as Trolox equivalent. It was discovered () that wattle seed gave the highest antioxidant capacity followed by Kakadu plum, lemon myrtle, wild lime, bush tomato, finger lime, and Davidson's plum, respectively. In relation to % RSA conveyed by DPPH radical method, Kakadu plum still showed the highest antioxidant activity. In contrast, the highest amount of phenols was Davidson's plum followed by bush tomato, finger lime, wild lime, Kakadu plum, lemon myrtle, and wattle seed, respectively. Positive correlation between antioxidant expressed by DPPH and ABTS radical method was found (P = 0.14). The total phenolic content is generally responsible for antioxidants and is well correlated with other antioxidant assays.Citation[19, Citation20] However, in our study, a converse correlation was found when comparing the amount of total phenol with the antioxidant capacity and these two methods (P < 0.05) ().
Table 1 Percent radical scavenger activity (DPPH), antioxidant capacity, and total phenol in bush plants
Table 2 Pearson's correlations between antioxidant potential by DPPH radical, TEAC, and total phenols
Further analysis on other bioactive compounds and flavonoids was evaluated using LCMS. The common standards were prepared, which were comprised of (-)-epicatachin, caffeic acid, ferulic acid, cinnamic acid, vanilic acid, (+) catechin, myricetin, quercetin, luteolin, kampferol, hespertin, and naringinin. illustrates the compounds found in some bush plants. Flavonone (hesperetin, naringenin) was found in bush tomato. Some of the compounds from flava-3-ols, flavonols, and flavononeds were seen in lemon myrtle. Some compounds in flavan-3-ols, flavones, flavonones group were found in wild lime, wattle seed, and Davidson's plum was found compounds from flavonones. Kakadu plum contained (+) catechin and naringinin, which were a member of flavan-3-ols and flavonones, respectively. Finger lime contained caffeic and vanillic acid. However, there were some other peaks in the samples that cannot be identified and some expected compounds, such as epicatechingallate and epigallocatechingallate in Davidson's plumCitation[6] have yet been evaluated due to the limitation of the standards.
Table 3 Molecular weight and retention time of some compounds found in some bush plants
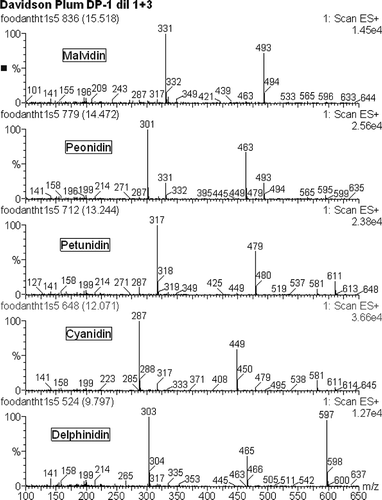
The conditions for ionization of the reference anthocyanidins (cyanidin, delphinidin, pelargonidin) was optimized for ESI+ mode taking advantage of the + ionizable site present in the flavium ring structure. For the anthocyanidins tested, a Cone voltage of 50 V was found to be essential for efficient ionization. Increasing the Cone voltage to 80 V resulted in considerable fragmentation with useful fragments for constructing a MS Library (). However, an initial test with a native fruit sample where the compounds are present as anthocyanins showed that a Cone voltage of 50 V was too high and a Cone voltage of 25 V gave the anthocyanin M+ molecular ion plus fragment ions for the sequential loss of sugar molecules and finally the anthocyanidin M+ ion ().
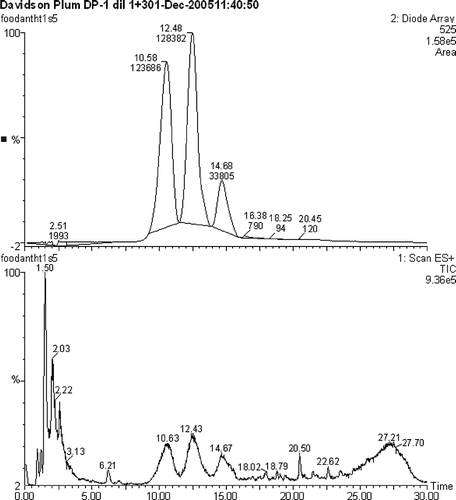
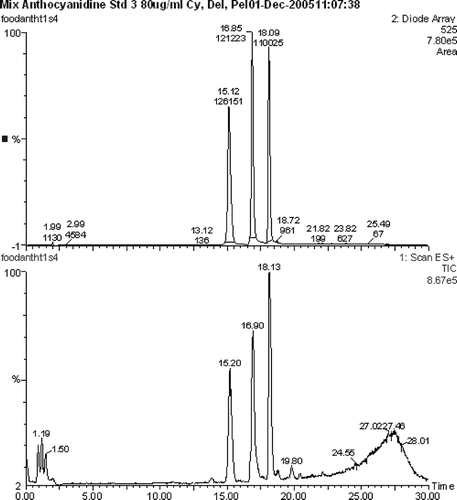
The separation of the test anthocyanidin reference compounds was readily achieved on an X-Terra column (150 × 2.1 mm, φ 3.5 μm) with a gradient starting at 95% A and 0.1% formic acid (). However, when the native fruit sample was run, the corresponding anthocyanins eluted earlier and were not fully resolved (). Further development of the gradient program or choice of a different column may achieve separation.
Figure 4 Chromatogram of anthocyanidin standards. Delphinidin RT 15.20 min, Cyanidin RT 16.90 min, and Pelargonidin RT 18.13 min.
For quantification purposes the absorbance at 525 nm, which is associated with the aglycone component (anthocyanidin) of the molecule, was initially investigated using the three reference anthocyanidins. The use of the absorbance of the aglycone component to quantify the corresponding anthocyanins is based on an equi-molar basis and is similar to that used for the quantification of the naturally occurring isoflavones in soy flour where they are normally present as glycosidated compounds (isoflavones conjugated with sugars) but only standards for the free isoflavones are readily available. It has been demonstrated that the UV absorbance of the free isoflavones are equivalent on a molar basis to the corresponding glycosidated compounds.Citation[21] The detector response factors were found to be similar for cyanidin and delphinidin but different for pelargonidin. In the absence of reference anthocyanidins for the other compounds identified an average response factor for cyanidin and delphinidin was used to quantify the peak areas for all anthocyanins present.
showed the relative proportion of anthocyanins in Davidson's plum. The highest proportion shared between Delphinidin and Cyanidin attached with the same sugar molecule (Hexose–pentose). Petunidin-hexose-pentose, Peonidin-hexose, and malvidin-hexose were found to be relatively the same amount. Only a little palargonidin-hexose was found in Davidson's plum. To compare with the other fruits, blueberries and cherries also contain anthocyanins. Malvidin, delphinidin, and cyanidin were the three most identified compounds. Further development work may be undertaken to use the integrated peak areas for each of the anthocyanidin M+ in SIM Mode run under Cone voltage conditions to fully fragment the anthocyanins to the anthocyanidins.
Table 4 Anthocyanins identified in Davidson's plum
CONCLUSION
The antioxidant activity conveying by two different methods (DPPH radical and TEAC) were compared, and it was found that there was an incremental correlation between the methods. Kakadu plum gave a high level of antioxidant activity by both methods because of the high content of ascorbic acid, which reacts rapidly against DPPH radical. Davidson's plum showed the lowest antioxidant activity for both techniques as the purple pigment interferes with activity absorption reading. The study of the effect of time on the suppression of ABTS radical for TEAC assay showed that the absorbance at 734 nm of all samples was stable after 10 min, and thus, it was used for calculation of the percentage of exhibition.
Selected bush plants were also tested for total poly phenol content. There was a negative correlation between antioxidant capacity by both techniques and total phenols content. In contrast, Davidson's plum gave the highest amount of total phenolic content. This can be elucidated that further investigation on flavonoid and anthocyanin was required.
Some flavonoids were found in the bush plants, which may also perform as natural antioxidants. The anthocyanins in Davidson's plum test gave the relative proportion of the anthocyanins. Delphinidin-hexose-pentose was predominantly found in Davidson's plum. Overall, Australian native plants tested contain promising antioxidant levels as well as the variety of flavonoids, which could be used as functional ingredients in processed food. Further direction was to study the stability of these bioactive compounds during food processing.
REFERENCES
- Saluk-Juszczak , J . 2010 . Anthocyanins as components of functional food for cardiovascular risk prevention . PostepyHigieny I MedycynyDoswiadczalnej , 64 : 451 – 458 .
- Forbe-Smith , M. and Paton , J.E. 2002 . Innovative products from Australian native food , Rural Industries Research and Development Corporation: ACT . 02/109
- McDonald , J. K , Caffin , N.A. , Sommano , S. and Cocksedge , R. 2006 . The effect of post harvest handling on selected native food plants , Rural Industries Research and Development Corporation: ACT . 06/021
- Tan , A.C. , Konczak , I. , Sze , D.M.Y. and Ramzan , I. 2010 . Towards the discovery of novel phytochemicals for disease prevention from native Australian plants: An ethnobotanical approach . Asia Pacific Journal of Clinical Nutrition , 19 : 330 – 334 .
- Ahmed , A. and Johnson , K.A. 2000 . Horticultural development of Australian native edible plants . Australian Journal of Botany , 48 : 417 – 426 .
- Wilkins , C.K. and Bohm , B.A. 1977 . Flavonoids of Davidsoniapruriens . Phytochemistry , 16 ( 1 ) : 144 – 145 .
- Netzel , M. , Netzel , G. , Tian , Q.G. , Schwartz , S. and Konczak , I. 2006 . Sources of antioxidant activity in Australian native fruits. Identification and quantification of anthocyanins . Journal of Agricultural and Food Chemistry , 54 : 9820 – 9826 .
- Wilkinson , J.M. , Hipwell , M. , Ryan , T. and Cavanagh , H.M.A. 2003 . Bioactivity of Backhousiacitriodora: Antibacterial and antifungal activity . Journal of Agricultural and Food Chemistry , 51 : 76 – 81 .
- Gordon , M.H. 1990 . “ The mechanism of antioxidant action in vitro ” . In Food Antioxidants , 1 – 18 . London : Elsevier Applied Science .
- Koleva , I.I. , Van Beek , T.A. , Linssen , J.P.H. , Kortenska , V. , Handjieva , N. and Evstatievga , L.N. 2003 . Antioxidant activity screening of extracts from Sideritis species (Labiatae) grown in Bulagaria . Journal of the Science Food and Agriculture , 83 : 809 – 819 .
- Koleva , I.I. , Van Beek , T.A. , Linssen , J.P.H. , de Groot , A. and Evstatieva , L.N. 2002 . Screening of plant extracts for antioxidant activity: a comparative study on three testing methods . Phytochemical Analysis , 13 : 8 – 17 .
- Brand-William , W. , Cuvelier , M.E. and Berset , C. 1995 . Use of a free radical method to evaluate antioxidant activity . Lebensmittel-Wissenschaft and Technology , 28 : 25 – 30 .
- Re , R. , Pellegrini , N. , Proteggente , A. , Pannala , A. , Yang , M. and Rice-Evans , C. 1999 . Antioxidant activity applying an improved ABTS radical cation decolorization assay . Free Radical Biology and Medicine , 26 ( 9–10 ) : 1231 – 1237 .
- Schlesier , K. , Harwat , M. , Bohm , V. and Bitsch , R. 2002 . Assessment of antioxidant activity by using different in vitro methods . Free Radical Research , 36 ( 2 ) : 177 – 187 .
- Duan , X. , Su , X. , Shi , J. , You , Y. , Zhao , M. , Li , Y. , Wang , Y. and Jiang , Y. 2008 . Inhibitory effect of anthocyanin extract from seed coat of black bean on pericarp browning and lipid peroxidation of litchi fruit during storage . Journal of Food Science and Biochemistry , 32 : 415 – 430 .
- Singleton , V.L. , Orthofer , R. , Lamuela-Raventós , R.M. and Lester , P. 1999 . “ Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent ” . In Methods in Enzymology , 152 – 178 . Academic Press, USA .
- Brand Miller , J. , James , K.W. and Maggiore , P.M.A. 1993 . Tables of Composition of Australian Aboriginal Foods , Canberra , , Australia : Aboriginal Studies Press .
- Sommano , S. , Caffin , N. , McDonald , J. and Kerven , G. 2011 . Measurement of ascorbic acid in Australian native plants . International Food Research Journal , 18 ( 3 ) : 1017 – 1020 .
- Okmen , B. , Sigva , H.O. , Mutlu , S. , Doganlar , S. , Yemenicioglu , A. and Frary , A. 2009 . Total antioxidant activity and total phenolic contents in different Turkish eggplant (Solanum Melongena L.) cultivars . International Journal of Food Properties , 12 ( 3 ) : 616 – 624 .
- Zujko , M.E. and Witkowska , A.M. 2011 . Antioxidant potential and polyphenol content of selected food . International Journal of Food Properties , 14 ( 2 ) : 300 – 308 .
- Barnes , S. , Kirk , M. and Coward , L. 1994 . Isoflavones and their conjugates in soy foods: Extraction conditions and analysis by HPLC-Mass Spectrometry . Journal of Agriculture and Food Chemistry , 42 : 2466 – 2474 .