Abstract
Oxidative stress and upregulation of gastric H+, K+-ATPase enzyme activity have been known to cause ulcer pathogenicity for which safer drugs are yet to be identified. Aqueous extracts of seven commonly consumed vegetable sources were screened for inhibition of H+, K+-ATPase and antioxidant activities. Results indicated that Z. officinale (Ginger) followed by M. arvensis (Pudina) are potent gastroprotective sources with inhibition of H+, K+-ATPase of IC50 of 18.3 ± 0.7 and 25.2 ± 0.9 μg gallic acid equivalents/ml respectively, which is almost equivalent or better than the known inhibition of H+, K+-ATPase—Omeprazole (IC50 ˜27 μg/ml). Further, all these vegetable extracts showed multi-potent antioxidant activity, such as free radical scavenging, reducing power ability, and inhibition of lipid peroxidation, which are required to inhibit complex steps of ulcerations. On the basis of the absolute amounts and potency of inhibition of H+, K+-ATPase as well as antioxidant activity of individual phenolic acids, the relative percentage contribution of phenolic acids from different vegetable extracts to both inhibition of H+, K+-ATPase and antioxidant activity was calculated and data revealed that gentisic and protocatechuic acid contributes significantly to both inhibition of H+, K+-ATPase and antioxidant activity.
INTRODUCTION
Epidemiological studies have shown that consumption of fruits and vegetables are associated with the prevention of chronic diseases,Citation[1–Citation Citation3] such as cardiovascular, cancer, ulcer, diabetes, etc., which are induced by reactive oxygen species.Citation[4] Free radicals and other reactive oxygen species are continuously produced in vivo and are exacerbating factors in cellular injury and the aging process.Citation[5] The high energy of which acts on the cellular components at a molecular level, including proteins, lipid membranes, carbohydrate, and nucleic acids, ultimately results in oxidative damage of biomolecules.Citation[6] Although some preventive and defensive systems against the attack of reactive oxygen species exist in the body of living organisms, including human systems, they cannot eliminate harmful effects of such substances completely, particularly when their production is imbalanced over defense reactions. Exogenous supply of natural antioxidants via adequate intake from food sources has been found to be a safer option.
Antioxidants function against the free radical induced damage in the bodyCitation[7] and, hence, has been known to offer protection against the reactive oxygen species induced diseases. Innumerable data has been accumulating in the last two decades regarding the health beneficial effects of fruits and vegetables.Citation[8,Citation9] However, recent interest in food phenolics has increased greatly, because of their antioxidant and free radical scavenging abilities. Individual antioxidant compounds do not act alone.Citation[10] They act in combination with other antioxidants, as interactions among them can affect total antioxidant capacity, producing synergistic or antagonistic effects.Citation[11] Due to the difference in their chemical composition, vegetable extracts are found to exhibit different degrees of antioxidant activity with the defined capacity to prevent oxidative damage induced chronic diseases.Citation[12,Citation13]
Our studies,Citation[14] including studies from various other laboratories,Citation[15] revealed that effective proton–potassium ATPase inhibitors are potential anti-ulcerative agents, since they interfere with the cascade of events of gastric ulcerations. Since proton blockers act at the initial step of ulcer pathogenicity, subsequent steps of ulcerations can also be inhibited.Citation[16,Citation17] We have reported previously that phenolics from dietary sources were not only effective in inhibiting H+, K+-ATPase, but also H. pylori growth.Citation[18,Citation19] The current study, therefore, addresses the comparative study of some commonly consumed vegetables for their ability to inhibit H+, K+-ATPase and antioxidant activity. An attempt has also been made to identify the nature of phenolic acids present in them in order to precisely estimate their gastro-protective effect via inhibition of H+, K+-ATPase (PPAI) and antioxidant (AOX) activities that are essential for antiulcerative agents.
MATERIALS AND METHODS
Materials
Vegetables
Seven types of common and widely used vegetables, namely, Raphanus sativus (radish), Brassica oleracea (knol khol), Daucus carota (carrot), Solanum melongena (brinjal), Beta vulgaris (beetroot), Zingiber officinale (ginger), and Mentha arvensis (field mint) were purchased from a local vegetable market in Mysore, India. The vegetables were randomly sampled from the shelf.
Chemicals. α,α-Diphenyl-β-picrylhydrazyl (DPPH); 2-thiobarbituric acid (TBA); phenolic acid standards, such as gallic, tannic, caffeic, p-coumaric, ferulic, gentisic, protocatechuic, syringic, and vanillic acid; and synthetic antioxidants, such as butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT), were obtained from Sigma Chemical Co. (St. Louis, MO, USA). The HPLC column (Shimpak C18) was obtained from Shimadzu (Kyoto, Japan). Folin-Ciocalteu reagent, ferric chloride, trichloroacetic acid (TCA), sodium carbonate, ferrous sulphate, and ascorbic acid were of the highest quality and were purchased from Qualigens Fine Chemicals (Mumbai, India). High performance liquid chromatography (HPLC) grade solvents employed for HPLC analysis were purchased from Spectrochem Biochemicals (Mumbai, India).
Methods
Preparation of vegetable extracts
One kilogram each of fresh vegetables were cleaned, washed under running tap water, cut into small pieces, air dried, and powdered. Fifty grams of each defatted vegetable powder was taken in 200 ml (w/v) of deionized water, and stirred for 1 h at room temperature. The extracts were separated from the residue by centrifugation at 3000 g for 10 min at room temperature. The remaining residue was re-extracted twice and thus obtained supernatants were pooled, concentrated by using a rotary evaporator (Buchi rotavaporator, Flawil, Switzerland), and designated as aqueous extracts (AE). AE was stored at 4°C until used.
Total phenolic content
The total phenolic content of vegetable extracts was estimated by the Folin-Ciocalteu method.Citation[20] Vegetable extracts (0.1 ml) were mixed with 1 ml of 2-fold diluted Folin-Ciocalteu reagent and 2 ml of sodium carbonate solution (10 g/100 ml water). The absorbance was measured at 765 nm with a Shimadzu UV-visible spectrophotometer (Shimadzu, Kyoto, Japan) after incubating for 30 min at room temperature. Gallic acid was used as the reference standard and results were expressed as gallic acid equivalents (GAE) in milligram per gram dry weight (d.w.) of vegetable extracts.
H+, K+-ATPase assay
The fundic mucosa of sheep were scraped and homogenized in Tris buffer (40 mM, pH 7.4) containing sucrose (0.25 mM), HEPES (2 mM), MgCl2 (2 mM), and EDTA (2 mM). The homogenate was centrifuged at 10,000 g for 30 min, and the resulting supernatant was subsequently centrifuged at 10,000 g for 20 min. The supernatant was stored at −70°C until used. The protein concentration was determined using the Bradford methodCitation[21] with bovine serum albumin as the standard.
The H+, K+-ATPase activity was assayed in absence and presence of the vegetable extracts. The reaction mixture (1 ml) contained enzyme in Tris buffer (20 mM, pH 7.4), 2 mM MgCl2, and 2 mM KCl, and the reaction was initiated with the addition of 2 mM ATP and incubated for 30 min at 37°C. The inorganic phosphate released was measured spectrophotometrically, according to the method.Citation[18] Inhibition was calculated as the percent inhibition and inhibitory potency of vegetable extracts were expressed as IC50 (concentration required to inhibit 50% of activity). IC50 values were calculated from a typical dose-response curve of the activity of H+, K+-ATPase, which was quantitated as the amount of inorganic phosphate released per milligram protein per hour (μmol Pi/mg protein/h).
Measurement of Antioxidant Activity
Free radical scavenging activity
Free radical scavenging (FRS) activity of vegetable extracts was determined according to the method of Lai and colleagues.Citation[22] Briefly, 1 ml of DPPH (0.1 mM in methanol) was incubated with different concentrations of vegetable extracts (1 to 5 μg GAE). After 20 min of incubation in the dark, absorbance of the mixture was measured at 517 nm against methanol as the blank. The FRS activity was calculated using the formula:
Reducing power activity
The reducing power of vegetable extracts was determined according to Yen and Chen.Citation[23] Different amounts of vegetable extracts (2–10 μg GAE) in phosphate buffer (0.2 M, pH 6.6) were incubated with 0.5 ml of 1% potassium ferricyanide at 50°C for 20 min. The reaction was terminated by adding 10% TCA solution, centrifuged at 650 g for 10 min at room temperature. The supernatant was mixed with 0.1% ferric chloride, and the absorbance was measured at 700 nm. Increased absorbance of the reaction mixture indicated increased reducing power.
Inhibition of Lipid Peroxidation
A modified thiobarbituric acid reactive species (TBARS) assayCitation[24] was used to measure the lipid peroxide formed. Briefly, 0.5 mL of 10% rat liver homogenate in 0.1 M potassium chloride was incubated with 5 mM each of FeSO4 and ascorbic acid with or without vegetable extracts in a total volume of 1 ml in 0.1 M phosphate buffer (pH 7.4). After incubation at 37°C for 60 min, the reaction mixture was boiled with 2 ml of ice cold HCl (0.25 N) containing TCA (15%), TBA (0.38%), and BHT (0.5%) for 60 min. Samples were cooled, centrifuged at 3000 g for 10 min at room temperature, and the absorbance was measured at 535 nm. The percent antilipid peroxidative activity was calculated by the following formula:
Identification of Phenolic Acids by HPLC
Phenolic acids in vegetable extracts were analyzed using RP-HPLC (model LC-10A; Shimadzu Corp., Tokyo, Japan) employing a Phenomenex C18 column (250 × 4.6 mm; Sigma-Aldrich Corp., St. Lois, MO, USA) using a diode array UV-detector operating at λ max 280 nm. A solvent system consisting of water/acetic acid/methanol (80:5:15, v/v/v) was used as the mobile phase at a flow rate of 1 mL/min. The extracts were centrifuged at 4000 g and filtered in 0.2 micron filter prior to HPLC analysis. Phenolic acids in vegetable extracts were identified and quantified by matching the retention time and peak area against those of standards under similar experimental conditions.
Statistical Analysis
All of the experiments were carried out in triplicate (n = 3) and the results were expressed as mean ± standard deviation (SD). The correlation coefficients between total phenolics and the antioxidant activity were demonstrated by employing SPSS (version 10.0.2; SPSS Inc., Chicago, IL, USA) at a level of p ≤ 0.05.
RESULTS AND DISCUSSION
Total Phenolic Content
The total phenolic content was expressed as gallic acid equivalents (GAE) in milligram per gram dry weight of samples. Large variations in the total phenolic content were found among the vegetable sources investigated (). Phenolic content ranged from 1.0 ± 0.1 to 26.8 ± 1.0 mg GAE/g d.w. Z. officinale and M. arvensis showed relatively higher levels of phenolic content, while R. sativus, B. oleracea, D. carota, and S. melongena showed lower levels varying from 1.0 ± 0.1 to 3.8 ± 0.6 mg GAE/g d.w. and B. vulgaris contained moderate levels (11.3 ± 0.8 mg GAE/g d.w.).
Table 1 Total phenolic content, free radical scavenging activity, reducing power ability, inhibition of lipid peroxidation, and potency of inhibition of H+, K+-ATPase by phenolics of vegetable extracts
Inhibition of H+, K+-ATPase Activity
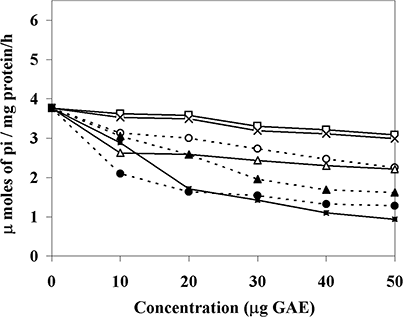
The fundamental factor in the pathogenesis of gastric and duodenal ulcers, besides reactive oxygen species, is the secretion of acid.Citation[25] Acid secretion by parietal cells generally is regulated through several stimulatory receptors, such as histamine H2, muscarinic M3, and gastrin; the final step is mediated by gastric hydrogen potassium ATPase or H+, K+-ATPase, the so-called proton pump.Citation[26] H+, K+-ATPase, which is found in parietal cells, is the enzyme primarily responsible for the acidification and subsequently ulcerations in the stomach. Therefore, the control of acid secretion may be essential for the treatment of the disease. Studies from our laboratory indicated that phenolic acids reduce gastric acid secretion in a structure dependent manner. Cinnamates and hydroxy cinnamates showed better activity than hydroxyl benzoates.Citation[18] Inhibition of the proton pump and antioxidant properties of phenolic acids, together with antimicrobial property were proposed as a mechanism for their gastroprotective activity.Citation[18,Citation19] In the current study, the tested extracts inhibited the H+, K+-ATPase with varied potency (). As indicated in , Z. officinale and M. arvensis exhibited good PPAI activity, B. vulgaris followed by S. melongena exhibited moderate; while D. carota, B. oleracea, and R. sativus showed lower activity. Approximately a 5- to 7-fold increase in activity was observed in Z. officinale and M. arvensis compared to R. sativus and B. oleracea. Omeprazole, a known PPA blocker, however, inhibited H+, K+-ATPase activity with IC50 of 27 μg/ml.
Figure 1 Inhibition of H+, K+-ATPase enzyme activity by vegetable extracts. Sheep parietal cell extract was employed as the enzyme source and was incubated with 10–50 μg of GAE of (−□−) R. sativus, (−×−) B. oleracea, (−○−) D. carota, (−Δ−) S. melongena, (−▲−) B. vulgaris, (−■−) Z. officinale, and (−•−) M. arvensis. The data are mean ± SD of three replicates (n = 3). Enzyme activity is represented as micromoles of inorganic phosphate released per milligram of enzyme protein per hour.
Evaluation of Antioxidant Activity
The antioxidant activities of vegetable extracts were measured in different assay systems, free radical scavenging, reducing power, and inhibition of lipid peroxidation assay systems.
Free radical Scavenging Activity
The free radical scavenging activity of vegetable extracts were tested using the DPPH method and the results were compared with the known antioxidant—BHA. Free radical scavenging activity was determined by calculating the concentration in μg GAE required to inhibit 50% activity—IC50. The highest activity was found in Z. officinale followed by M. arvensis, B. vulgaris, S. melongena, D. carota, B. oleracea, and R. sativus ().
Reducing Power Ability
The reducing power of all the vegetable extracts increased with increasing the amount of sample. Z. officinale and M. arvensis exhibited stronger reducing power when compared to B. oleracea and R. sativus (). The reducing capacity of a compound may serve as a significant indicator of its potential antioxidant activity.Citation[27] The reducing properties are generally associated with the presence of reductones.Citation[28] It is presumed that the phenolic compounds may act in a similar fashion as reductones by donating electrons and reacting with free radicals to convert them to more stable products and terminating the free radical chain reaction.Citation[23] The results indicated that the marked reducing power of vegetable extracts is the result of their antioxidant activity and, hence, general usage of these vegetables are valuable sources of natural antioxidants.
Inhibition of Lipid Peroxidation
The inhibition of lipid peroxidation in rat liver homogenate was assayed by measuring the lipid oxidation products, such as TBARS, since free radical scavenging is generally an accepted mechanism for antioxidants to inhibit lipid peroxidation. Results showed that the vegetable extracts inhibited TBARS formation in a concentration dependent manner. IC50 values for the inhibition of lipid peroxidation activity are presented in . The antioxidant activity of compounds is often described by their ability to delay the onset of auto oxidation by scavenging reactive oxygen species (ROS), or their ability to act as chain-breaking antioxidants by inhibiting the propagation phase of lipid auto oxidation.Citation[23] This effect is probably due to the presence of polyphenolic compounds in these extracts. Although D. carota extract contained a lower amount of total phenolics than that of S. melongena and B. vulgaris, its capacity to inhibit lipid peroxidation was equivalent to that of Z. officinale with IC50 of 8.8 ± 0.6 μg GAE/mL. This activity may have also been partly contributed by some constituents other than phenolics. β-Carotene, which reacts poorly for phenol reagent, may contribute to this activity in carrot.
Table 2 Vegetable extracts containing different phenolic acids given with their yield (μg/g)
Phenolic Acid Composition in Vegetable Extracts
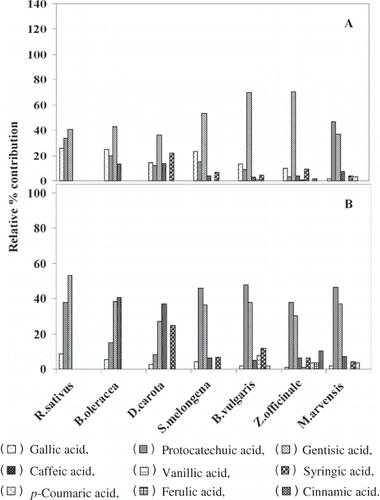
Phenolic acids constitute a major component of phenolics and contribute significantly to the H+, K+-ATPase inhibition and antioxidant activity. Hence, samples were analyzed on HPLC and types of phenolic acids (μg/g) present in them are given in . Cinnamic, caffeic, and ferulic acids, which have potent H+, K+-ATPase inhibitory activity, were found to be present in lower levels, while poorer PPAI, such as gentisic acid and protocatechuic acid, were present in significant levels. Based on the phenolic acid composition and absolute amounts, the relative percentage contribution to PPAI and AOX activity was determined. Results indicated that gentisic and protocatechuic acids are the major contributors to PPAI and AOX activity ( and ). The increase in the activity was due to the abundance of these components in aqueous extracts of vegetables.
Figure 2 (a) Relative percent contribution of individual phenolic acids found in vegetable extracts towards antioxidant activity; (b) relative percent contribution of individual phenolic acids found in vegetable extracts towards PPAI activity.
Interestingly, Z. officinale and M. arvensis, which showed good antioxidant PPA inhibitory activity, are being consumed traditionally among local populations in India and have been used since time immemorial as a dietary component in daily life, as a food spice, and an ayurvedic component. Chinese and traditional medicine systems have recorded Z. officinale as an important medicinal plant and it has been reported to exert antioxidant and antiulcer properties as reported by us in phenolic fractionCitation[18] and by others.Citation[29,Citation30] M. arvensis has also been used and valued for its aromatic and medicinal properties.Citation[31,Citation32]
It is indicated in the literature and from our study that PPA is up-regulated in ulcer conditions. Inhibition of the same, therefore, would result in gastroprotection or antiulcer property. The current study reveals that Z. officinale and M. arvensis have strong abilities to inhibit PPA and, hence, can be a potential antiulcerogenic source. AOX activity contribution from phenolic acid adds to the antiulcer potential, since oxidative stress is one of the potential risk factors for causation of ulcers.
CONCLUSION
The current study gathered experimental evidence on the most commonly used vegetables as natural antioxidants and gastro-protectants. Both H+, K+-ATPases inhibition and antioxidant property were found to be essential to prevent complex steps of ulcer pathogenicity. Z. officinale and M. arvensis extracts showed potent H+, K+-ATPase inhibitory and antioxidant properties; hence, they may be used as potential antiulcerogens. Studies are useful, particularly with respect to the ulcerated or ulcer prone population to consume reported vegetables for potential gastroprotection.
ACKNOWLEDGMENTS
The authors wish to thank Dr. V. Prakash, Director, Central Food Technological Research Institute, for his keen interest in the work and encouragement. The authors are also thankful to Dr. P.V. Salimath, Head, Department of Biochemistry and Nutrition, for his valuable suggestions. Smitha Jayaram thanks the Council for Scientific and Industrial Research, India for financial assistance.
REFERENCES
- Ames , B.N. , Shigenaga , M.K. and Hagen , T.M. 1993 . Oxidants, antioxidants and the degenerative diseases of aging . Proceedings of the National Academy of Sciences of the United States of America , 90 : 7915 – 7922 .
- Steinmetz , K.A. and Potter , J.D. 1996 . Vegetable, fruit and cancer prevention: A review . Journal of American Dietetic Association , 96 : 1027 – 1039 .
- Willet , W.C. 2002 . Balancing life-style and genomics research for disease prevention . Science , 296 : 695 – 698 .
- Southorn , P.A. and Powis , G. 1988 . Free radicals in medicine. II . Involvement in human-disease. Mayo Clinical Proceedings , 63 : 390 – 408 .
- Gulcin , I. , Tel , A.Z. and Kirecci , E. 2008 . Antioxidant, antimicrobial, antifungal, and antiradical activities of Cyclotrichium Niveum (BOISS.) Manden and Scheng . International Journal of Food Properties , 11 : 450 – 471 .
- Halliwell , B. , Gutteridge , J.M.C. and Cross , C.E. 1992 . Free radicals, antioxidants, and human disease: Where are we now? . Journal of Laboratory and Clinical Medicine , 119 : 598 – 619 .
- Namiki , M. 1990 . Antioxidant/antimutagens in food . CRC Critical Review Food Science and Nutrition , 29 : 273 – 300 .
- Cao , G. , Sofic , E. and Prior , R.L. 1996 . Antioxidant capacity of tea and common vegetables . Journal of Agricultural and Food Chemistry , 44 : 3426 – 3431 .
- Prior , R.L. and Cao , G. 2000 . Antioxidant phytochemicals in fruits and vegetables . Diet and health implications. Horticulture Science , 35 : 588 – 592 .
- Strazzulo , G. , De Giulio , A. , Tommonaro , G. , La Pastina , C. , Poli , A. , Nicolaus , B. , De Prisco , R. and Saturnino , C. 2007 . Antioxidative activity and lycopene and β-carotene contents in different cultivars of tomato (Lycopersicon Esculentum) . International Journal of Food properties , 10 : 321 – 329 .
- Niki , E. and Noguchi , N. 2000 . Evaluation of antioxidant capacity. What capacity is being measured by which method? . Life , 50 : 323 – 329 .
- Krinsky , N.I. 1989 . Antioxidant functions of carotenoids . Free Radical Biology and Medicine , 7 : 617 – 635 .
- Pool-Zobel , B.L. , Bub , A. , Liegibel , U.M. , Treptow-Van , L.S. and Rechkemmer , G. 1997 . Mechanisms by which vegetable consumption reduces genetic damage in humans . Cancer Epidemiology Biomarkers Prevention , 7 : 891 – 899 .
- Naik , Y. , Smitha , J. , Harish Nayaka , M.A. , Lakshman and Shylaja , M.D. 2007 . Gastroprotective effect of swallow root (Decalepis hamiltonii) extract: Possible involvement of H+, K+-ATPase inhibition and antioxidative mechanism . Journal of Ethnopharmacology , 112 : 173 – 179 .
- Bandyopadhyay , U. , Biswas , K. , Chatterjee , R. , Bandyopadhyay , D. , Chattopadhyay , I. , Ganguly , C.K. , Chakraborty , T. , Bhattacharya , K. and Banerjee , R.K. 2002 . Gastroprotective effect of Neem (Azardichta indica) bark extract: Possible involvement of H+, K+-ATPase inhibition and scavenging of hydroxyl radical . Life Sciences , 71 : 2845 – 2865 .
- Konturek , P.C. and Konturek , S.J. 1994 . Role of Helicobacter pylori infection in gastro-duodenal secretion and in pathogenesis of peptic ulcer and gastritis . Journal of Physiology and Pharmacology , 45 : 333 – 350 .
- Bandyopadhyay , D. and Chattopadhyay , A. 2006 . Reactive oxygen species-induced gastric ulceration: Protection by melatonin . Current Medicinal Chemistry , 13 : 1187 – 1202 .
- Siddaraju , M.N. and Shylaja , M.D. 2007 . Inhibition of gastric H+, K+-ATPase and Helicobacter pylori growth by phenolic antioxidants of Zingiber officinale . Molecular Nutrition and Food Research , 51 : 324 – 332 .
- Siddaraju , M.N. and Shylaja , M.D. 2007 . Inhibition of gastric H+, K+-ATPase and Helicobacter pylori growth by phenolic antioxidants of Curcuma amada . Journal of Agricultural and Food Chemistry , 55 : 7377 – 7386 .
- Singleton , V.L. and Rossi , J.A. 1965 . Colorimetry of total phenolics with phosphomolybdic–phosphotungstic acid reagents . American Journal of Enology Viticulture , 16 : 144 – 158 .
- Bradford , M.M. 1976 . A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding . Analytical Biochemistry , 72 : 248 – 254 .
- Lai , L.S. , Chou , S.T. and Chao , W.W. 2001 . Studies on the antioxidant activities of Hsian-tsao (Mesona procumbens Hemsl) leaf gum . Journal of Agricultural and Food Chemistry , 49 : 963 – 968 .
- Yen , G.C. and Chen , H.Y. 1995 . Antioxidant activity of various tea extracts in relation to their antimutagenicity . Journal of Agricultural and Food Chemistry , 43 : 27 – 32 .
- Ohkawa , M. , Ohishi , N. and Kunio , Y. 1979 . Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction . Analytical Biochemistry , 95 : 351 – 358 .
- Kishor , S.J. , Anamik , K.S. , Jitender , B. , Suhas , M.S. , Amol , P.K. , Jayshree , R.J. and Ashok , V.B. 2007 . Recent advances in proton pump inhibitors and management of acid-peptic disorders . Bioorganic and Medicinal Chemistry , 15 : 1181 – 1205 .
- Sachs , G. 1997 . Proton pump inhibitors and acid related diseases . Pharmacotherapy , 17 : 22 – 37 .
- Meir , S.J. , Kanner , B. and Akiri , S.P.H. 1995 . Determination and involvement of aqueous reducing compounds in oxidative defense systems of various senescing leaves . Journal of Agricultural and Food Chemistry , 43 : 1813 – 1817 .
- Gordon , M.H. 1990 . “ The mechanism of antioxidant action in vitro ” . In Food Antioxidants , Edited by: Hudson , B. J. F. 1 – 18 . London and New York : Elsevier Applied Science .
- Rehman , Z. , Salariya , A. and Habib , F. 2003 . Antioxidant activity of ginger extract in sunflower oil . Journal of the Science of Food and Agricultural , 83 : 624 – 629 .
- Stoilova , I. , Krastanov , A. , Stoyanova , A. , Denev , P. and Gargova , S. 2007 . Antioxidant activity of a ginger extract (Zingiber officinale) . Food Chemistry , 102 : 764 – 770 .
- Lange , B.M. and Croteau , R. 1999 . Genetic engineering of essential oil production in mint . Current Opinion in Plant Biology , 2 : 139 – 144 .
- Chopra , R.N. , Nayar , S.L. and Chopra , I.C. 1956 . Glossary of Indian Medicinal Plants , 65 – 66 . New Delhi , India : Council of Scientific and Industrial Research .
