Abstract
Peroxidases are heme containing enzymes that are produced by a number of organisms. A plant peroxidase was purified from leaves of chard (Beta vulgaris subspecies cicla) by ammonium sulphate precipitation, dialyses, and CM-sephadex cation exchange chromatography. Purification fold was approximately 36 with 14.4% yield. Optimum pH, optimum temperature, optimum ionic strength, and stable pH values of purified enzyme were determined for the guaiacol substrate. Michaelis constant (KM) and maximum rate (Vmax) values were determined from the Lineweaver–Burk graph for pyrogallol and guaiacol substrates. Phenolic and flavonoid contents in chard that connected with antioxidant properties were determined as 17.5 μg (GAE/mg extract) and 11.7 μg (QE/mg extract), respectively. In conclusion, chard is shown to be a novel rich source of phenolic antioxidants and has a high peroxidase activity.
INTRODUCTION
Chard (Beta vulgaris subspecies cicla) is a biennial medicinal vegetable that has large shiny green leaves. Fresh leaves of chard are being used in cookery in many parts of the world, especially in Turkey, the Mediterranean, and Europe. The leaves and stalks of chard contain nutritionally significant concentrations of some vitamins and minerals.[Citation1] Some phenolic[Citation2] and flavonoid[Citation3] compounds have been detected on this plant. The effective antioxidant[Citation1, Citation4] and antimicrobial[Citation5] potential of this plant have been reported in many recent studies.
Antioxidant enzymes are endogenous systems that can scavenge reactive oxygen species (ROS). Superoxide dismutase, catalase, and peroxidases are well known antioxidant enzymes that prevent intracellular ROS formation and lipid peroxidation.[Citation6] Superoxide dismutase converts superoxide radicals into H2O2 and catalase degrades H2O2 by dismutation to water and oxygen.[Citation7]
Peroxidases (EC 1.11.1.7; hydrogen peroxide oxidoreductase) are heme-containing enzymes that catalyze the oxidation of a variety of substrates using hydrogen peroxide.[Citation8] Hydrogen peroxide is a non-radical ROS product of normal aerobic metabolism. However, the high amount of H2O2 could be converted into other more reactive ROS, such as hydroxyl radicals that can oxidize biomolecules, leading to tissue damage, cell death, ageing, cancer, and cardiovascular diseases. Thus, H2O2-scavenging activity becomes a key aspect of total antioxidant activity.[Citation9,Citation10] Peroxidase is specific to the hydrogen acceptors like hydrogen peroxide. Polyphenolic compounds, ascorbate, and several amino acids are hydrogen donors.
Peroxidases are commonly found in many organisms, including animals, bacteria, fungi, and most of green plants.[Citation11] Plant peroxidases have been classified into three families: prokaryotic peroxidases, fungal peroxidases, and higher plant peroxidases.[Citation12] Plant peroxidases contain two calcium ions (Ca2+) and disulfide bridges that have importance for the structural and thermal stabilities of the enzyme.[Citation13] Plant peroxidases play very important physiological roles, such as providing defense against pathogens and stress,[Citation14] participating in the lignification process in cell wall damage or repair,[Citation15] catalyzing the catabolism of phyto-growth hormones, controlling respiration, catalyzing removal of hydrogen peroxide, and conserving food quality and undesirable color changes.[Citation16, Citation17]
Many studies have been done on purification and characterization peroxidases from different plants, including rice,[Citation18] tea leaves,[Citation19] leaves of royal palm tree,[Citation11] turnip,[Citation20] arabidopsis leaves,[Citation21] sweet potatoes,[Citation12] cauliflower,[Citation22] black radish,[Citation23] and spinach leaves.[Citation24]
Purification of peroxidase enzyme from leaves of chard as a new source and its kinetic properties, such as substrate specificity, optimum pH, optimum temperature, optimum ionic strength, and stable pH conditions, were examined in this study. Also, total phenolic and total flavonoid contents of chard connected with antioxidant properties were determined separately.
MATERIALS AND METHODS
Plant Materials
Leaves of chard were obtained freshly from a local garden in Muş, Turkey. Fresh leaves of chard were being used for all steps of study.
Chemicals
CM-Sephadex C-50, sodium phosphate, sodium acetate, Coomassie brilliant blue G-250, tris-trizma, ammonium sulphate, hydrogen peroxide, guaiacol, and pyrogallol were purchased from Sigma Aldrich (St. Louis, MO, USA). Other chemicals were obtained from Merck (Darmstadt, Germany).
Enzyme Extraction
Plant leaves were cut into small pieces and 10-g pieces of chard was powdered with liquid nitrogen. This powder was mixed with 100 mL of phosphate buffer (pH 7.0, 0.1 M). The homogenate was filtered through filter paper and centrifuged at 12,000 × g for 60 min. Supernatant was obtained to be used for the enzyme activity. Several precipitations with ammonium sulphate, between 0–10%, 10–20%, 20–30%, 30–40%, 40–50%, 50–60%, 60–70%, 70–80%, and 80–90%, were examined to find the proper saturation point.[Citation25,Citation26] The precipitate was dissolved in a small quantity of 0.1 M phosphate buffer (pH 7.0). The proper precipitate was suspended in phosphate buffer (pH 7.0, 0.1 M) and dialyzed for 8 h at 4°C against 10 mM phosphate buffer (pH 7.0). All steps in the preparation of the enzyme extract were carried out at 4°C with ice.
Preparation of Ion-Exchange Chromatography
Three grams of dried CM-Sephadex C-50 material was dissolved in 100 mL of distilled water and incubated for 24 h at room temperature. Following incubation time, the material was mixed with 100 mL of NaOH (0.5 N) and was allowed to stand for an hour. Then, that was stirred into 100 mL of HCl (0.5) and was allowed to stand for an hour. The material was then washed with distilled water until the effluent reached a pH of 7. Finally, the exchanger was suspended in 0.05 M phosphate buffer (pH 7.5); then it was packed in a column (3 × 30 cm), washed, and equilibrated with the same buffer.[Citation27,Citation28] The flow rates for washing and equilibration were adjusted by peristaltic pump at 5 mL/h.
Enzyme Purification
For purification of peroxidase from leaves of chard, the dialyzed enzyme extract was loaded onto a CM-Sephadex C-50 ion exchange column and equilibrated with 50 mM phosphate buffer. Then the proteins bound to the column were eluted with a linear gradient of 0–1 M NaCl in 50 mM phosphate buffer (100 mL) at a flow rate of 5 mL/h by using a gradient mixer apparatus (Pharmacia Fine Chemicals, NJ, USA). Fractions of 3 mL were collected for enzyme activity assay.[Citation29]
Enzyme Activity Assay
Peroxidase activity in the leaves of the chard sample was measured by using pyrogallol and guaiacol as the substrate. Temperature was controlled using a circulating water bath with a heater/cooler (Grant LTD 6G −20 to 100°C, Grant Ltd., Cambridge, England). The changes in absorbance at 470 nm were read for 3 min using a spectrophotometer (Shimadzu uv-1800). The mixture contained 25 μL of the enzyme sample, 1 mL 22.5 mM H2O2, 1 mL 45 mM guaiacol or pyrogallol, and 975 μL phosphate buffer (pH 7.0, 0.1 M). The change in the absorbance was measured for 3 min at 25°C and the activities were determined as EU/ml.min.[Citation30,Citation31]
Analysis of Protein
The determination of the qualitative protein amount was measured spectrophotometrically at 280 nm.[Citation32] Similarly, the quantitative protein determination was achieved by absorbance measurements at 595 nm according to Bradford, with bovine serum albumin as a standard.[Citation33]
Effect of pH and Ionic Strength
Optimum pH and stable pH were examined for pH conditions. For optimum pH, peroxidase activity was investigated in the range of pH 3.0–9.0 using the following buffers: acetate buffer (0.01 M) on the range of pH 3.0–4.5, phosphate buffer (0.01 M) on the range of pH 5.0–7.5, and Tris/HCl buffer on the range of pH 7.5–9.0, respectively. All measurements were made with hydrogen peroxide and guaiacol.[Citation22] Also, enzyme activity was observed for 12 days to determine the stable pH value of the enzyme. The process was carried out with buffers that previously used on optimum pH study.
Effect of temperature was determined by measuring enzyme activity at different temperatures. At a certain temperature, enzyme activity was determined by the addition of enzyme to the mixture as rapidly as possible. The process was carried out by a circulatory water bath in a temperature range between 0–90°C. The heated mixture's absorbances were monitored for 3 min at 25°C with a spectrophotometer and the activities were determined as EU/mL min.[Citation34] The effect of ionic strength on the enzyme activity was examined with using different concentrations of buffers (0.0125–0.500 M). Studies were carried out with optimum pH.
Kinetic Studies
Enzyme activities were measured by using five different concentrations of the guaiacol and pyrogallol substrates for determining K M and V max values of substrates. K M and V max values were calculated from the Lineweaver–Burk graphs. K M and V max values were calculated for peroxidase reactions with each of the two substrates, using the Lineweaver–Burk transformation of the Michaelis-Menten equation. The V max/K M ratio is called the “catalytic power” and the value of this ratio determines the more effective substrate.[Citation35,Citation36]
Total Phenolic Content by Folin-Ciocalteau Assay
Total phenolic content in chard leaves was estimated by a colorimetric assay based on the procedure as described previously.[Citation37] Briefly, 1 mg water extract of chard was pipetted into a test tube and the volume was adjusted to 23 ml with distilled water. Folin-Ciocalteu's reagent (0.5 ml) and 2% Na2CO3 (1.5 ml) were added 3 min later. The samples were vortexed and then left to stand at room temperature for 30 min. Absorbance measurements were recorded at 760 nm. Distilled water was used for the blank and also instead of sample distilled water, it was used for the control. A calibration curve of gallic acid was prepared, and phenolic contents were determined from the regression equation of the calibration curve. The results were reported as gallic acid equivalent per mg extract.
Total Flavonoid Content
Total flavonoid content in water extract of chard leaves was estimated by a colorimetric assay based on the procedure as described previously.[Citation38] At first, 1 mg of water extract was pipetted into a test tube. Then, 0.1 ml CH3COOK (1.0 M) and 0.1 ml of 10% Al(NO3)3 in 4.3 ml of ethanol solution was added. The samples were vortexed and then left to stand at room temperature for 40 min. Absorbance measurements were recorded at 415 nm. Distilled water was used as the blank, and also instead of the sample, distilled water was used for the control. A calibration curve of quercetin was prepared, and flavonoid contents were determined from regression equation of the calibration curve. The results were reported as quercetin equivalent per milligram extract.
RESULTS AND DISCUSSION
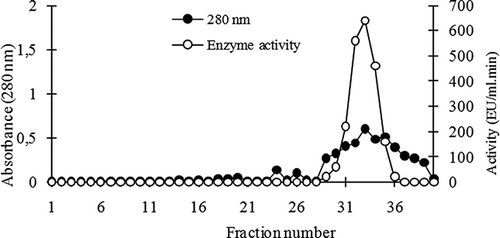
Peroxidase enzyme from leaves of chard was purified by ammonium sulphate precipitation, dialyses, and CM-sephadex cation exchange chromatography steps. Saturation of enzyme was determined between the 50–90% range point. The enzyme precipitation was dissolved in buffer and then dialyzed. The dialyzed enzyme extract was subjected to CM-Sephadex C 50 ion exchange chromatography and bound proteins were eluted with a linear gradient of 0–1 M NaCl in 100 mM phosphate buffer (pH 8.0) at a flow rate of 5 mL/h. Eluates were collected as 3 mL fractions. Enzyme activity of each eluate was measured at 470 nm and the qualitative protein amount was measured at 280 nm with a uv-vis spectrophotometer ().
A peak of enzyme activity with protein was detected by CM-Sephadex C 50 ion exchange chromatography. Fractions of this peak were collected and 18 ml of total volume was obtained. This enzyme sample was being used in following characterization and kinetic studies. The purification steps of enzyme were summarized in . As a result of this process, peroxidase was purified approximately 36-fold with a 14.4% yield. There were similar results on the purification of peroxidases on different plant sources in the literature. Purification fold of wheat bran peroxidase was reported to be 11 with 20% yield.[Citation8] Also, spinach peroxidase was purified at 22.6-fold with 4.2% yield,[Citation24] and cauliflower (Brassica oleracea L.) peroxidase was purified at 19.3-fold and 0.2% efficiency.[Citation22]
Table 1 Purification of chard (Beta vulgaris subspecies cicla) peroxidase
Guaiacol, 2,2′-azino-bis(3-ethyl benzothiazoline-6-sulphonic acid) (ABTS), pyrogallol, and catechol are the most commonly used hydrogen donors in kinetical studies of peroxidases. Guaiacol and pyrogallol substrates were being used in the present study for characterization of peroxidase.
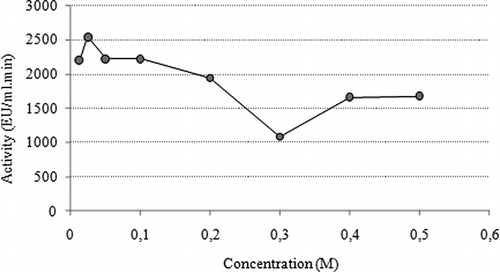
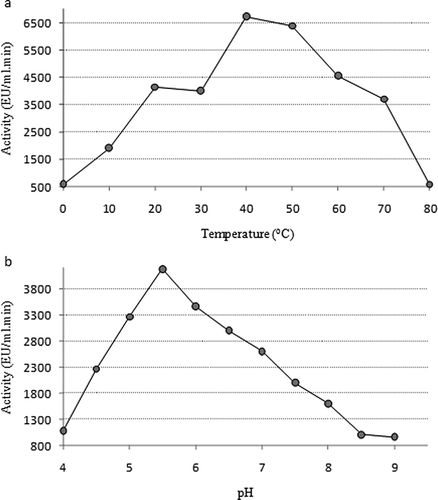
In order to determine the optimum temperature values of the enzyme, peroxidase activity was measured at different temperatures in a range between 0 and 80°C. The optimum temperature of peroxidase enzyme was determined to be 40°C. It was observed that the enzyme activity increased until 40°C and after this point it started to decrease (). The optimum pH value for enzyme activity was determined by measuring the enzyme activity at different pH values.[Citation39] The optimum pH of peroxidase from leaves of chard was determined to be 5.5 using 0.1 M sodium phosphate buffers (). The results of the ionic strength study revealed that the salt concentration affects peroxidase activity. Purified peroxidase activity was measured at 0.025 M of buffer concentration as maximum value. The effect of ionic strength was summarized in .
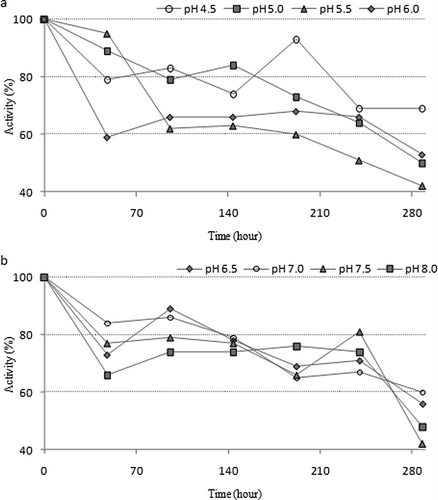
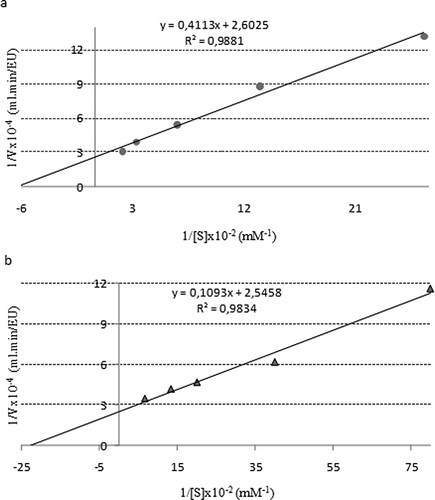
For researching the stable pH value of the purified enzyme, peroxidase activity was monitored in three different buffers for 12 days,[Citation40] and it was demonstrated that peroxidase was more stable at pH 4.5 at the end of this incubation period. The results were shown in and . K M and V max values were determined for guaiacol and pyrogallol substrates. The purified peroxidase enzyme activities were measured at five different concentrations of substrates while at a constant concentration of H2O2. K M and V max values were calculated from the Lineweaver–Burk graphs ( and ). The purified enzyme had K M values of 15.8 and 23.3 mM for guaiacol and pyrogallol substrates, respectively. Also, V max values of purified enzyme were measured as 3840 and 3949 EU/mL.min for guaiacol and pyrogallol substrates, respectively. The value of V max/K m ratio was higher for guaiacol and, therefore, guaiacol was determined as a better substrate than pyrogallol.
Figure 5 Lineweaver-Burk graphs using two different substrates: (a) with guaiacol substrate and (b) with pyrogallol substrate.
Phenolic compounds, which contain at least one hydroxyl group on an aromatic ring, are present ubiquitously in plants. Phenolic compounds can interrupt chain oxidation reactions by donation of a hydrogen atom or chelating metals.[Citation9,Citation41] The plants that contain high amounts of phenolic compounds have efficient antioxidant properties. Also, the structure of the aromatic ring and the number of hydroxyl groups are important associated factors on antioxidant abilities. In a recent study, a series of phenolic acids were tested for their ability to scavenge hydrogen peroxide; caffeic acid is found to be the most efficient H2O2-scavenger among the tested phenolics.[Citation10]
The total phenolic content of water extract of chard was determined by Folin-Ciocalteu method. The results from regression equation of the calibration curve (A = 0.002 × [GAE], R 2 = 0.97), determined as gallic acid equivalents per one mg of extract. The result of water extract was 17.5 μg (GAE/mg extract). Flavonoids are the most common group of polyphenolic compounds in the human diet and are found ubiquitously in plants. Also, they are commonly known for their antioxidant activities.[Citation42, Citation43] The results from the regression equation of the calibration curve (A = 0.012 × [QE] + 0.047, R 2 = 0.979), determined as quercetin equivalents per 1 mg of extract (QE/mg extract). According to the results of this study, total flavonoid contents of chard water extract was 11.7 μg (QE/mg extract). Both total phenolics and total flavonoids were at important levels, which are closely associated with antioxidant activity.
CONCLUSION
A peroxidase enzyme was purified from chard leaves (Beta vulgaris subspecies cicla) by the steps of ammonium sulphate precipitation, dialyses, and CM-sephadex cation exchange chromatography approximately 36-fold with 14.4%. According to the results of the study, the optimum pH and stable pH of the enzyme was determined at the range of acidic pH. Also, guaiacol was found to be a more effective substrate than pyrogallol for this enzyme. According to the results of the study, peroxidase enzyme activity was at high levels, which might be attributed to some antioxidant properties. In addition, total phenolic and total flavonoid contents of chard were determined to be connected with high enzyme activity and antioxidant potential. Finally, chard leaves would be a beneficial food additive or pharmaceutical ingredient.
REFERENCES
- Pyo , Y. , Lee , T. , Logendra , L. and Rosen , R.T. 2004 . Antioxidant activity and phenolic compounds of Swiss chard (Beta vulgaris subspecies cycla) extracts . Food Chemistry , 85 : 19 – 26 .
- Kugler , F. , Stintzing , F.C. and Carle , R. 2004 . Identification of betalains from petioles of differently colored Swiss Chard (Beta vulgaris L. ssp. Cicla [L.] Alef. Cv. bright lights) by high-performance liquid chromatography-electrospray ionization mass spectrometry . Journal of Agricultural and Food Chemistry , 52 : 2975 – 2981 .
- Ninfali , P. , Bacchiocca , M. , Antonelli , A. , Biagiotti , E. , Di Gioacchino , A.M. , Piccoli , G. , Stocchi , V. and Brandi , G. 2007 . Characterization and biological activity of the main flavonoids from Swiss Chard (Beta vulgaris subspecies cycla) . Phytomedicine , 14 : 216 – 221 .
- Saçan , Ö. and Yanardag , R. 2010 . Antioxidant and antiacetylcholinesterase activities of chard (Beta vulgaris L. var. Cicla) . Food and Chemical Toxicology , 48 : 1275 – 1280 .
- Ponce , A.G. , Roura , S.I. , del Valle , C.E. and Fritz , R. 2002 . Characterization of native microbial population of Swiss chard (Beta vulgaris, type Cicla) . Lebensmittel-Wissenschaft & Technology , 35 : 331 – 337 .
- Gülçin , İ. , Beydemir , S. , Hisar , O. , Köksal , E. and Reiter , R.J. 2009 . Melatonin administration increases antioxidant enzymes activities and reduces lipid peroxidation in the rainbow trout (Oncorhynchus mykiss, Walbaum) erythrocytes . Turkish Journal of Veterinary and Animal Sciences , 33 ( 3 ) : 241 – 245 .
- Tejera Garcia , N.A. , Iribarne , C. , Palma , F. and Lluch , C. 2007 . Inhibition of the catalase activity from Phaseolus vulgaris and Medicago sativa by sodium chloride . Plant Physiology and Biochemistry , 45 : 535 – 541 .
- Manu , B.T. and Prasada Rao , U.J.S. 2009 . Calcium modulated activity enhancement and thermal stability study of a cationic peroxidase purified from wheat bran . Food Chemistry , 114 : 66 – 71 .
- Gülçin , İ. , Tel , A.Z. and Kirecci , E. 2008 . Antioxidant, antimicrobial, antifungal, and antiradical activities of Cyclotrichium niveum (Boiss.) Manden and Scheng . International Journal of Food Properties , 11 ( 2 ) : 450 – 471 .
- Ma , X. , Li , H. , Dong , J. and Qian , W. 2011 . Determination of hydrogen peroxide scavenging activity of phenolic acids by employing gold nanoshells precursor composites as nanoprobes . Food Chemistry , 126 : 698 – 704 .
- Sakharov , I.Y. , Castillo , J.A. , Ariza , J.C. and Galaev , I.Y. 2000 . Purification and stability of peroxidase of African oil palm Elaies guineensis . Bioseparation , 9 : 125 – 132 .
- Rompel , A. , Albers , M. , Naseri , J.I. , Gerdemann , C. , Karentzopoulos , K.B. , Jasper , B. and Krebs , B. 2007 . Purification, cloning and characterization of a novel peroxidase isozyme from sweetpotatoes (Ipomoea batatas) . Biochimica et Biophysica Acta , 1774 : 1422 – 1430 .
- O'Brien , P.J. Peroxidases . 2000 . Chemico-Biological Interactions , 129 : 113 – 139 .
- Biles , C.L. and Martyn , R.D. 1993 . Peroxidase, polyphenoloxidase and shikimate dehydrogenase isozymes in relation to the tissue type, maturity and pathogen induction of watermelon seedlings . Plant Physiology and Biochemistry , 31 : 499 – 506 .
- Wakamatsu , K. and Takahama , U. 1993 . Changes in peroxidase activity and in peroxidase isozymes in carrot callus . Physiologia Plantarum , 68 : 167 – 171 .
- Ashie , N.A. , Simpson , B.K. and Smith , J.P. 1996 . Mechanisms for controlling enzymatic reactions in foods . Critical Reviews in Food Science and Nutrition , 36 : 1 – 30 .
- Lopez-Serrano , M. and Ros Barcelo , A. 1996 . Purification and characterization of a basic peroxidase isoenzyme from strawberries . Food Chemistry , 55 : 133 – 137 .
- Ito , H. , Hiraoka , N. , Ohbayashi , A. and Ohashi , Y. 1991 . Purification and characterization of rice peroxidases . Agricultural and Biological Chemistry , 55 : 2445 – 2454 .
- Kvaratskhelia , M. , Winkel , C. and Thorneley , R.N.F. 1997 . Purification and characterization of a novel class III peroxidase isoenzyme from tea leaves . Plant Physiology , 114 : 1237 – 1245 .
- Duarte-Vazquez , M.A. , Garcia-Almendarez , B.E. , Regalado , C. and Whitaker , J.R. 2001 . Purification and properties of a neutral peroxidase isozyme from turnip (Brassica napus L. var. purple top white globe) roots . Journal of Agricultural and Food Chemistry , 49 : 4450 – 4456 .
- Shah , K. , Penel , C. , Gagnon , J. and Dunand , C. 2004 . Purification and identification of a Ca2+-pectate binding peroxidase from Arabidopsis leaves . Phytochemistry , 65 : 307 – 312 .
- Köksal , E. and Gülçin , İ. 2008 . Purification and characterization of peroxidase from cauliflower (Brassica oleracea L.) buds . Protein and Peptide Letter , 15 ( 4 ) : 320 – 326 .
- Sisecioglu , M. , Gülçin , İ. , Cankaya , M. , Atasever , A. , Sehitoglu , M.H. , Kaya , H.B. and Özdemir , H. 2010 . Purification and characterization of peroxidase from Turkish black radish (Raphanus sativus L.) . Journal of Medicinal Plants Research , 4 ( 12 ) : 1187 – 1196 .
- Köksal , E. 2011 . Peroxidase from leaves of spinach (Spinacia oleracea) partial purification and some biochemical properties . International Journal of Pharmacology , 7 : 135 – 139 .
- Yilmaz , H. , Ciftci , M. , Beydemir , S. and Bakan , E. 2002 . Purification of glucose 6-phosphate dehydrogenase from chicken erythrocytes . Investigation of some kinetic properties. Preparative Biochemistry and Biotechnology , 32 ( 3 ) : 287 – 301 .
- Aydemir , T. 2010 . Selected kinetic properties of polyphenol oxidase extracted from Rosmarinus officinalis L . International Journal of Food Properties , 13 ( 3 ) : 475 – 485 .
- Alici , H.A. , Ekinci , D. and Beydemir , S. 2008 . Intravenous anesthetics inhibit human paraoxonase-1 (PON1) activity in vitro and in vivo . Clinical Biochemistry , 41 : 1384 – 1390 .
- Isgor , M.M. and Beydemir , S. 2010 . Some cardiovascular therapeutics inhibit paraoxonase 1 (PON1) from human serum . European Journal of Pharmacology , 645 : 135 – 142 .
- Fujita , S. , Saari , N. , Maegawa , M. , Samura , N. , Hayashi , N. and Tono , T. 1997 . Isolation and characterization of two phloroglucinol oxidases from cabbage (Brassica oleracea L.) . Journal of Agricultural and Food Chemistry , 45 : 59 – 63 .
- Gülçin , İ. , Küfrevioglu , Ö.İ. and Oktay , M. 2005 . Purification and characterization of polyphenol oxidase from nettle (Urtica dioica L.) and inhibitory effects of some chemicals on the enzyme activity . Journal of Enzyme Inhibition and Medicinal Chemistry , 20 : 297 – 302 .
- Söyüt , H. and Beydemir , S. 2008 . Purification and some kinetic properties of carbonic anhydrase from rainbow trout (Oncorhynchus mykiss) liver and metal inhibition . Protein and Peptide Letter , 15 : 528 – 535 .
- Sisecioglu , M. , Cankaya , M. , Gulcin , I. and Ozdemir , H. 2010 . Interactions of melatonin and serotonin with lactoperoxidase enzyme . Journal of Enzyme Inhibition and Medicinal Chemistry , 25 ( 6 ) : 779 – 783 .
- Bradford , M.M. 1976 . A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding . Analytical Biochemistry , 72 : 248 – 251 .
- Köksal , E. , Bursal , E. , Aggül , A.G. and Gülçin , İ. “ Purification and characterization of peroxidase from sweet gourd ” . In International Journal of Food Properties doi: 10.1080/10942912.2010.513216
- Dogan , S. , Turan , P. , Dogan , M. , Arslan , O. and Alkan , M. 2007 . Variations of peroxidase activity among Salvia species . Journal of Food Engineering , 79 : 375 – 382 .
- Söyüt , H. , Beydemir , S. and Hisar , O. 2008 . Effects of some metals on carbonic anhydrase from brains of rainbow trout . Biological Trace Element Research , 123 ( 1–3 ) : 179 – 190 .
- Singleton , V.L. , Orthofer , R. and Lamuela-Raventos , R.M. 1999 . Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent . Methods in Enzymology , 299 : 152 – 178 .
- Gülçin , İ. , Bursal , E. , Sehitoglu , H.M. , Bilsel , M. and Gören , A.C. 2010 . Polyphenol contents and antioxidant activity of lyophilized aqueous extract of propolis from Erzurum, Turkey . Food and Chemical Toxicology , 48 ( 8–9 ) : 2227 – 2238 .
- Beydemir , S. , Gülçin , İ. , Kufrevioglu , Ö.İ. and Çiftçi , M. 2003 . Glucose 6-phosphate dehydrogenase: In vitro and In vivo effects of dantrolene sodium . Polish Journal of Pharmacology , 55 : 787 – 792 .
- Gülçin , İ. and Yıldırım , A. 2005 . Purification and characterization of peroxidase from Brassica oleracea var. acephala . Asian Journal of Chemistry , 17 ( 4 ) : 2175 – 2183 .
- Tohma , H.S. and Gülçin , İ. 2010 . Antioxidant and radical scavenging activity of aerial parts and roots of Turkish liquorice (Glycyrrhiza glabra L.) . International Journal of Food Properties , 13 ( 4 ) : 657 – 671 .
- Spencer , J.P.E. 2008 . Flavonoids: Modulators of brain function? . British Journal of Nutrition , 99 : 60 – 77 .
- Gülçin , İ. , Tel , A.Z. and Kirecci , E. 2008 . Antioxidant, antimicrobial, antifungal and antiradical activities of Cyclotrichium niveum (Boiss.) Manden and Scheng . International Journal of Food Properties , 11 : 450 – 471 .