Abstract
The present study was carried out to determine the antioxidant and different free radical scavenging activities of 70% methanolic extract of Diplazium esculentum. Total antioxidant activity of the extract was evaluated as trolox equivalent antioxidant capacity value. The IC50 values for scavenging of different free radicals indicated its efficient free radical scavenging properties. The extract acted as an iron chelator and also possessed reducing power. It also inhibited lipid peroxidation. Moreover, the extract yielded high phenolic and flavonoid content. Therefore, the results indicated that 70% methanolic extract of D. esculentum acted as a potential antioxidant and free radical scavenger.
INTRODUCTION
It is now well known that free radicals, the byproducts of aerobic metabolism, are fundamental in modulating various physiological functions.[Citation1] Free radicals are produced by endogenous sources, such as mitochondrial leak, respiratory burst, enzyme reactions, and auto-oxidant reactions, and environmental sources, such as smoking, pollutants, ultraviolet and ionizing radiations, and xenobiotics.[Citation2] They have unpaired electrons and seek stability through electron pairing with biological molecules, such as protein, lipid, and DNA in healthy cells, and cause protein and DNA damage along with lipid peroxidation. All these factors result in the disruption of the antioxidant defense mechanism of the body, which may lead to ‘oxidative stress', bringing about a variety of pathophysiological conditions, such as coronary heart disease, neurodegenerative disorders, diabetes, arthritis, inflammatory diseases, lung damage, aging, and cancer.[Citation3–5 Citation Citation5
The body has effective antioxidant defense systems, which constitute enzymes, such as superoxide dismutase (SOD), and catalase and compounds, such as ascorbic acid, tocopherol, and glutathione.[Citation6] But all these endogenous antioxidants are not sufficient in protecting the body against oxidative stress. Therefore, dietary supplementation through natural antioxidants in place of synthetic antioxidants is necessary in strengthening the antioxidant system of the body by inhibiting free radical generation and thus preventing chronic diseases. Recently, much attention has been directed towards exploring natural antioxidants because they are natural products that are considered to be a safe source.
Diplazium esculentum (Koenig ex Retz.) Sw. (Family—Athyriaceae) is an edible fern distributed throughout Asia and Oceania. It is known as paco in the Philippines, linguda in northern India referring to the curled fronds, and dheki shak in the northern region of West Bengal, India. The young fronds are stir-fried as a vegetable or used in salads. The genus Diplazium includes 400 known species. Diplazium esculentum is one of the most common varieties and perhaps the most commonly consumed fern. Several studies have been performed to determine the antioxidant activity of this plant using different methodologies,[Citation7–10 Citation Citation Citation10 but there have been no reports on various endogenously produced free radicals scavenging activities by this plant.
Therefore, the objective of the present study was not just to evaluate the total antioxidant potential but also the scavenging activities of different endogenously produced free radicals that comprise various reactive oxygen species (ROS) and reactive nitrogen species (RNS), including hydroxyl, superoxide, nitric oxide, hydrogen peroxide, peroxynitrite, singlet oxygen, and hypochlorous acid, using 70% methanolic extract of D. esculentum. The extract was also examined for iron chelating capacity, measurement of reduction power, lipid peroxidation inhibition ability, and for total phenolic and flavonoid content.
MATERIALS AND METHODS
Chemicals
2,2′-Azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) was obtained from Roche Diagnostics, Mannheim, Germany. 6-Hydroxy-2,5,7,8-tetramethychroman-2-carboxylic acid (Trolox) was obtained from Fluka, Buchs, Switzerland. Potassium persulfate (K2S2O8), ethylenediamine tetraacetic acid (EDTA), ascorbic acid, 2-deoxy-2-ribose, trichloroacetic acid (TCA), mannitol, nitro blue tetrazolium (NBT), reduced nicotinamide adenine dinucleotide (NADH), phenazine methosulfate (PMS), sodium nitroprusside (SNP), sulfanilamide, naphthylethylenediamine dihydrochloride (NED), L-histidine, lipoic acid, sodium pyruvate, quercetin, and ferrozine were obtained from Sisco Research Laboratories Pvt. Ltd., Mumbai, India. Hydrogen peroxide, potassium hexacyanoferrate, Folin-Ciocalteu reagent, sodium carbonate (Na2CO3), butylated hydroxytoluene (BHT), sodium hypochloride (NaOCl), aluminium chloride (AlCl3), ammonium iron (II), sulfate hexahydrate ((NH4)2Fe (SO4)26H2O), potassium nitrite (KNO2), N,N-dimethyl-4-nitrosoaniline, and xylenol orange were obtained from Merck, Mumbai, India. Gallic acid and curcumin were obtained from MP Biomedicals, Illkirch, France. Ferrous sulfate and catalase were obtained from HiMedia Laboratories Pvt. Ltd., Mumbai, India. Evans Blue was purchased from BDH, London, England. Mangese dioxide was obtained from SD Fine Chemicals, Mumbai, India. Diethylene-triamine-pentaacetic acid (DTPA) was obtained from Spectrochem Pvt. Ltd., Mumbai, India. Thiobarbituric acid (TBA) was obtained from Loba Chemie, Mumbai, India. Sodium nitrite was obtained from Qualigens Fine Chemicals, Mumbai, India.
Plant Material
Young D. esculentum plants were collected from different areas of North Bengal University campus, Darjeeling, India. These were identified by Professor A.P. Das, Plant Taxonomy Laboratory, Department of Botany, University of North Bengal and a voucher specimen (Accession No. 9601) was submitted to him.
Animals
Inbred adult Swiss albino mice (20 ± 2 g) were used for lipid peroxidation inhibition assay. They were maintained in the animal house, Department of Zoology, University of North Bengal with food and water ad libitum under a constant 12-h dark/light cycle at an environmental temperature of 25 ± 2°C. The experiment was performed after obtaining approval from the Animal Ethical Committee (Registration No. 840/ac/04/CPCSEA).
Sample Preparation
Samples were prepared according to a previously described method.[Citation11] The young fronds of D. esculentum were dried at room temperature for 7 days, finely powdered, and used for extraction. The powder (100 g) was mixed with 500 ml methanol:water (7:3) using a shaker for 15 h; then the mixture was centrifuged at 2850× g and the supernatant was decanted. The pellet was mixed again with 500 ml methanol-water and the entire process was repeated once again, i.e., the extraction procedure was done twice. The supernatants collected from the two phases were mixed in a round-bottom flask and concentrated under reduced pressure in a rotary evaporator. The concentrated extract was then lyophilized. The residue was kept at -20°C for future use. Double-distilled water (MilliQ grade) was used as the solvent for the lyophilized extract in all the experiments.
Total Antioxidant Activity
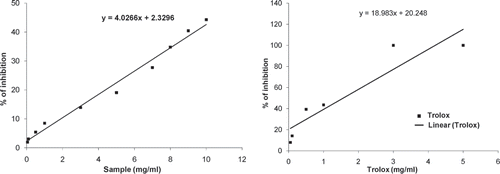
The antioxidant activity of the D. esculentum extract was assayed depending on the ability to scavenge ABTS•+ radical cation compared to the trolox standard.[Citation12] The ABTS•+ radical cation was pregenerated by mixing 7 mM ABTS stock solution with 2.45 mM potassium persulfate (final concentration) and incubating for 12–16 h in the dark at room temperature until the reaction was complete and the absorbance was stable. The absorbance of the ABTS•+ was equilibrated to 0.70 (±0.02) by diluting with water at room temperature. Then 1 ml of ABTS•+ was mixed with 10 μl of the test sample (0.05–10 mg/ml) and the absorbance was measured at 734 nm after 6 min. All experiments were repeated six times. The percentage inhibition of absorbance was calculated and plotted as a function of the concentration of standard and sample to determine the trolox equivalent antioxidant capacity (TEAC), which was calculated from dividing the gradient of the plot for the sample by the gradient of the plot for trolox.
Hydroxyl Radical Scavenging Activity
This was assayed according to a standard method with a slight modification.[Citation13] This assay is based on quantification of the degradation product of 2-deoxyribose by condensation with TBA. Hydroxyl radical was generated by the Fe3+-ascorbate-EDTA-H2O2 system (Fenton reaction). The reaction mixture contained, in a final volume of 1 ml, 2-deoxy-2-ribose (2.8 mM); KH2PO4-KOH buffer (20 mM, pH 7.4); FeCl3 (100 μM); EDTA (100 μM); H2O2 (1.0 mM); ascorbic acid (100 μM); and various concentrations (0–200 μg/ml) of the test sample or reference compound. After incubation for 1 h at 37°C, 0.5 ml of the reaction mixture was added to 1 ml 2.8% TCA, then 1 ml 1% aqueous TBA was added and the mixture was incubated at 90°C for 15 min to develop the color. After cooling, the absorbance was measured at 532 nm against an appropriate blank solution. All tests were performed six times. Mannitol, a classical OH• scavenger, was used as a positive control. Percentage inhibition was evaluated by comparing the test and blank solutions.
Superoxide Radical Scavenging Activity
This activity was determined based on the reduction of NBT according to a previously reported method.[Citation14] The non-enzymatic phenazine methosulfate-nicotinamide adenine dinucleotide (PMS/NADH) system generates superoxide radicals that reduce nitro blue tetrazolium (NBT) into a purple-colored formazan. The 1-ml reaction mixture contained phosphate buffer (20 mM, pH 7.4), NADH (73 μM), NBT (50 μM), PMS (15 μM), and various concentrations (0–20 μg/ml) of sample solution. After incubation for 5 min at ambient temperature, the absorbance was taken at 562 nm against an appropriate blank solution. All tests were performed six times. Quercetin was used as the positive control.
Nitric Oxide Radical Scavenging Activity
At physiological pH, nitric oxide generated from aqueous sodium nitroprusside (SNP) solution interacts with oxygen to produce nitrite ions, which may be quantified by the Griess Illisvoy reaction.[Citation15] The reaction mixture contained 10 mM SNP, phosphate buffered saline (pH 7.4), and various concentrations (0–70 μg/ml) of the test solution in a final volume of 3 ml. After incubation for 150 min at 25°C, 1 ml sulfanilamide (0.33% in 20% glacial acetic acid) was added to 0.5 ml of the incubated solution and allowed to stand for 5 min. Then 1 ml naphthylethylenediamine dihydrochloride (NED) (0.1% w/v) was added and the mixture was incubated for 30 min at 25°C. The pink chromophore generated during diazotization of nitrite ions with sulfanilamide and subsequent coupling with NED was measured spectrophotometrically at 540 nm against a blank sample. All tests were performed six times. Curcumin was used as a standard.
Hydrogen Peroxide Scavenging Activity
This activity was determined according to a previously described method[Citation16] with minor changes. An aliquot of 50 mM H2O2 and various concentrations (0–2 mg/ml) of samples were mixed (1:1 v/v) and incubated for 30 min at room temperature. After incubation, 90 μl of the H2O2 sample solution was mixed with 10 μl of HPLC-grade methanol and 0.9 ml FOX reagent was added (prepared in advance by mixing 9 volumes of 1 mM xylenol orange and 2.56 mM ammonium ferrous sulfate in 0.25 M H2SO4). The reaction mixture was then vortexed and incubated at room temperature for 30 min. The absorbance of the ferric-xylenol orange complex was measured at 560 nm. All tests were performed six times. Sodium pyruvate was used as the reference compound.[Citation17]
Peroxynitrite Scavenging Activity
Peroxynitrite (ONOO−) was synthesized by a previously described method.[Citation18] An acidic solution (0.6 M HCl) of 5 ml H2O2 (0.7 M) was mixed with 5 ml of 0.6 M KNO2 on an ice bath for 1 s and 5 ml of ice-cold 1.2 M NaOH was added. Excess H2O2 was removed by treatment with granular MnO2 prewashed with 1.2 M NaOH and the reaction mixture was left overnight at -20°C. Peroxynitrite solution was collected from the top of the frozen mixture and the concentration was measured spectrophotometrically at 302 nm (ϵ = 1670 M−1 cm−1).
The Evans Blue bleaching assay[Citation19] was used to measure the peroxynitrite scavenging assay, with slight modification. The reaction mixture contained 50 mM phosphate buffer (pH 7.4), 0.1 mM DTPA, 90 mM NaCl, 5 mM KCl, 12.5 μM Evans Blue, various doses of plant extract (0–200 μg/ml), and 1 mM peroxynitrite in a final volume of 1 ml. After incubation at 25°C for 30 min, the absorbance was measured at 611 nm. The percentage scavenging of ONOO− was calculated by comparing the results of the test and blank samples. All tests were performed six times. Gallic acid was used as the reference compound.
Singlet Oxygen Scavenging Activity
The production of singlet oxygen (1O2) was determined by monitoring N,N-dimethyl-4-nitrosoaniline (RNO) bleaching, using a previously reported spectrophotometric method.[Citation20] Singlet oxygen was generated by a reaction between NaOCl and H2O2, and the bleaching of RNO was monitored at 440 nm. The reaction mixture contained 45 mM phosphate buffer (pH 7.1), 50 mM NaOCl, 50 mM H2O2, 50 mM histidine, 10 μM RNO, and various concentrations (0–200 μg/ml) of sample in a final volume of 2 ml. It was incubated at 30°C for 40 min and the decrease in RNO absorbance was measured at 440 nm. The scavenging activity of the sample was compared with that of lipoic acid, which was used as a reference compound. All tests were performed six times.
Hypochlorous Acid Scavenging Activity
Hypochlorous acid was prepared immediately before the experiment by adjusting the pH of a 10% (v/v) solution of NaOCl to 6.2 with 0.6 M H2SO4, and the concentration of HOCl was determined by measuring the absorbance at 235 nm using the molar extinction coefficient of 100 M−1 cm−1. The assay was carried out according to a previously described method[Citation21] with minor changes. The scavenging activity was evaluated by measuring the decrease in absorbance of catalase at 404 nm. The reaction mixture contained a final volume of 1 ml, 50 mM phosphate buffer (pH 6.8), catalase (7.2 μM), HOCl (8.4 mM), and increasing concentrations (0–100 μg/ml) of plant extract. The assay mixture was incubated at 25°C for 20 min and the absorbance was measured against an appropriate blank. All tests were performed six times. Ascorbic acid, a potent HOCl scavenger, was used as a reference compound.[Citation22]
Fe2+ Chelation
The ferrous ion chelating activity was evaluated by a standard method[Citation23] with minor changes. The reaction was carried out in HEPES buffer (20 mM, pH 7.2). Briefly, various concentrations (0–120 μg/ml) of plant extract were added to 12.5 μM ferrous sulfate solution and the reaction was initiated by the addition of ferrozine (75 μM). The mixture was shaken vigorously and incubated for 20 min at room temperature, and then the absorbance was measured at 562 nm. All tests were performed six times. EDTA was used as a positive control.
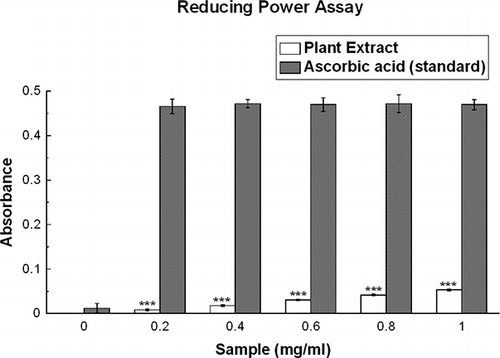
Reducing Power
The Fe3+-reducing power of the extract was determined by a previously described method[Citation24] with slight modification. Different concentrations (0–1 mg/ml) of the extract (0.5 ml) were mixed with 0.5 ml phosphate buffer (0.2 M, pH 6.6) and 0.5 ml potassium hexacyanoferrate (0.1%), followed by incubation at 50°C in a water bath for 20 min. After incubation, 0.5 ml of TCA (10%) was added to terminate the reaction. The upper portion of the solution (1 ml) was mixed with 1 ml of distilled water, and 0.1 ml of FeCl3 solution (0.01%) was added. The reaction mixture was left over for 10 min at room temperature and the absorbance was measured at 700 nm against an appropriate blank solution. All tests were performed six times. A higher absorbance of the reaction mixture indicated greater reducing power. Ascorbic acid was used as a positive control.
Lipid Peroxidation Inhibition Assay
This assay was carried out according to a previously described method,[Citation25] with slight modification. Brain homogenate was prepared by centrifuging Swiss albino mouse brain (20 ± 2 g) with 50 mM phosphate buffer (pH 7.4) and 120 mM KCl, at 3000 rpm for 10 min. A 100 μl aliquot of the supernatant homogenate was mixed with the plant extract of various concentrations (0–25 μg/ml), followed by the addition of 0.1 mM FeSO4 and 0.1 mM ascorbic acid, and incubated for 1 h at 37°C. Next, 500 μl 28% TCA was used to stop the reaction and then 380 μl 2% TBA was added with heating at 95°C for 30 min to generate the color. Then the samples were cooled on ice, centrifuged at 8000 rpm for 2 min, and the absorbance of the supernatant was taken at 532 nm. All tests were performed six times. Trolox was used as the standard.
Determination of Total Phenolic Content
Total phenolic content was determined using Folin-Ciocalteu (FC) reagent according to a previously described method[Citation26] with a slight modification. Briefly, the plant extract (0.1 ml) was mixed with 0.75 ml of FC reagent (previously diluted 1000-fold with distilled water) and incubated for 5 min at 22°C, then 0.75 ml of 0.06% Na2CO3 solution was added. After incubation at 22°C for 90 min, the absorbance was measured at 725 nm. All tests were performed six times. The phenolic content was evaluated using gallic acid as a standard. The result was expressed as mg of gallic acid equivalent phenolic content present in 1 g sample plant material.
Determination of Total Flavonoid Content
The total flavonoid content was determined with aluminium chloride (AlCl3) according to a known method[Citation27] using quercetin as a standard. The plant extract (0.1 ml) was added to 0.3 ml of distilled water followed by NaNO2 (0.03 ml, 5%). After 5 min at 25°C, AlCl3 (0.03 ml, 10%) was added. After further 5 min, the reaction mixture was treated with 0.2 ml 1 mM NaOH. Finally, the reaction mixture was diluted to 1 ml with water and the absorbance was measured at 510 nm. All tests were performed six times. The flavonoid content was evaluated using quercetin as a standard. The result was expressed as mg of quercetin equivalent flavonoid present in 1 g of sample plant material.
Statistical Analysis
All data are given as the mean ± SD of six measurements. Statistical analysis was performed using KyPlot version 2.0 beta 15 (32 bit). The IC50 values were calculated by the formula Y = 100*A1/(X + A1), where A1 = IC50, Y = response (Y = 100% when X = 0), X = inhibitory concentration. The IC50 values were compared by paired t tests. p < 0.05 was considered significant.
RESULTS
Total Antioxidant Activity
The total antioxidant activity of the extract was calculated from the decolorization of ABTS•+, which was measured spectrophotometrically at 734 nm. Interaction with the plant extract or standard trolox suppressed the absorbance of the ABTS•+ radical cation and the results, expressed as percentage inhibition of absorbance, are shown in Figs. and , respectively. The TEAC value of the extract was 0.21 ± 0.02.
Figure 2 Hydroxyl radical scavenging assay. Hydroxyl radical scavenging activities of the D. esculentum extract and the reference compound mannitol. The data represent the percentage inhibition of deoxyribose degradation. The results are mean ± S.D. of six parallel measurements. *p < 0.05 and ***p < 0.001 vs. 0 μg/ml. IC50 values of the plant extract and standard are 811 ± 23.73 and 571.45 ± 20.12 μg/ml, respectively.
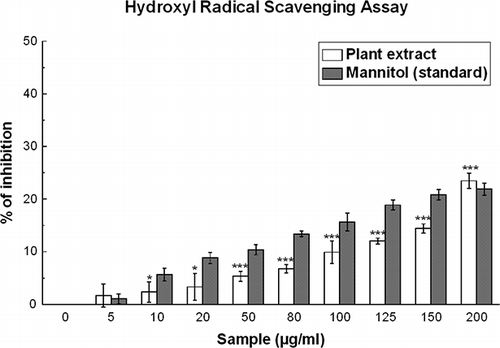
Figure 1 (a) Total antioxidant activity of D. esculentum extract on decolorization of ABTS radical cation. The percentage inhibition was plotted against the concentration of the sample. All data are expressed as mean ± S.D. (n = 6). (b) Total antioxidant activity of reference compound trolox on decolorization of ABTS radical cation. The percentage inhibition was plotted against the concentration of the sample. All data are expressed as mean ± S.D. (n = 6).
Hydroxyl Radical Scavenging Activity
This assay shows the abilities of the extract and standard mannitol to inhibit hydroxyl radical-mediated deoxyribose degradation in a Fe3+-EDTA-ascorbic acid and H2O2 reaction mixture. The results are shown in . The IC50 values () of the extract and the standard in this assay were 811.00 ± 23.73 and 571.45 ± 20.12 μg/ml, respectively. Though the IC50 value of the extract was greater than that of the standard, at 200 μg/ml, the percentages of inhibition were 23.4 and 21.9% for D. esculentum and mannitol, respectively.
Table 1 Scavenging of reactive oxygen species, iron chelating, and lipid peroxidation inhibition activity (IC50 values) of Diplazium esculentum and reference compounds
Figure 3 Superoxide radical scavenging assay. Scavenging effect of D. esculentum plant extract and the standard quercetin on superoxide radical. All data are expressed as mean ± S.D. (n = 6). ***p < 0.001 vs. 0 μg/ml. IC50 values of the plant extract and standard are 90.39 ± 2.22 and 42.06 ± 1.35 μg/ml, respectively.
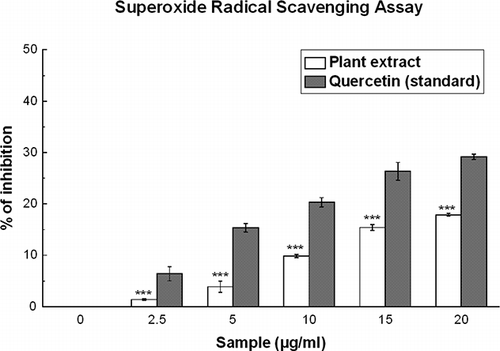
Superoxide Radical Scavenging Activity
Superoxide radicals, generated from the PMS-NADH coupling, can be measured by their ability to reduce NBT. The decrease in absorbance at 560 nm with the plant extract and the reference compound quercetin indicates their abilities to quench superoxide radicals in the reaction mixture. As shown in , the IC50 values () of the plant extract and quercetin on superoxide scavenging activity were 90.39 ± 2.22 and 42.06 ± 1.35 μg/ml, respectively. At 20 μg/ml, the percentage of inhibition of the plant extract was 17.8%, whereas that of quercetin was 29.1%.
Figure 4 The nitric oxide radical scavenging activity of D. esculentum extract and the standard curcumin. The data represent the percentage nitric oxide inhibition. Each value represents mean ± S.D. (n = 6). *p < 0.05, **p < 0.01, and ***p < 0.001 vs. 0 μg/ml. IC50 values of the plant extract and standard are 204.28 ± 18.31 and 90.82 ± 4.75 μg/ml, respectively.
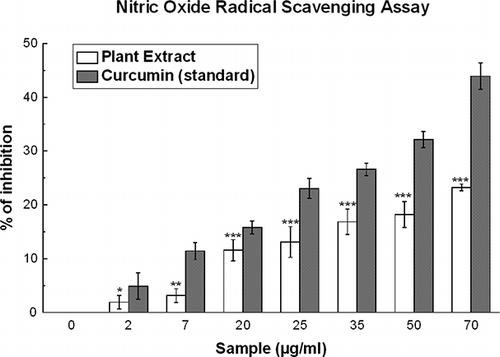
Nitric Oxide Radical Scavenging
As shown in , D. esculentum extract also caused a moderate dose-dependent inhibition of nitric oxide with an IC50 value () of 204.28 ± 18.31 μg/ml. Curcumin was used as a reference compound and 90.82 ± 4.75 μg/ml curcumin was needed for 50% inhibition. At 70 μg/ml, the percentage of inhibition of the plant extract was 23.2% whereas that of curcumin was 43.9%.
Figure 5 Hydrogen peroxide scavenging assay. Effect of D. esculentum plant extract and the sodium pyruvate on the scavenging of H2O2. The data represent the percentage H2O2 scavenging. All data are expressed as mean ± S.D. (n = 6). ***p < 0.001 vs. 0 mg/ml. IC50 values of the plant extract and standard are 4.17 ± 0.86 and 3.24 ± 0.30 mg/ml, respectively.
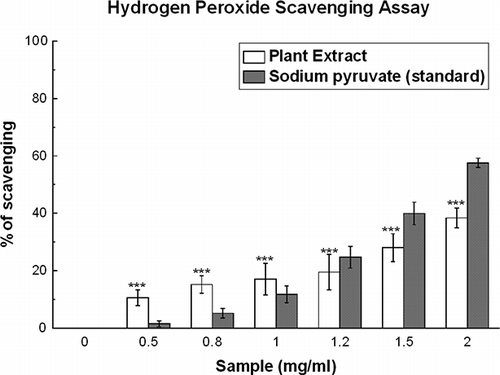
Hydrogen Peroxide Scavenging Activity
Hydrogen peroxide scavenging activity was assayed by the FOX reagent method. shows that the plant extract has good H2O2 scavenging activity (IC50 = 4.17 ± 0.86 mg/ml) when compared with the standard sodium pyruvate (IC50 = 3.24 ± 0.30 mg/ml) (). At 2 mg/ml, the percentage of scavenging was 38.4 and 57.5% for D. esculentum and sodium pyruvate, respectively. At lower doses, viz. 0.5, 0.8, and 1 mg/ml, the percentages of scavenging of the plant extract were 10.5, 15.2, and 17.0%, respectively, which were much higher than that of the sodium pyruvate (1.5, 5.2, and 11.7% for 0.5, 0.8, and 1 mg/ml, respectively).
Figure 6 Peroxynitrite anion scavenging assay. The peroxynitrite anion scavenging activity of D. esculentum plant extract and the standard gallic acid. Each value represents mean ± S.D. (n = 6). *p < 0.05 and ***p < 0.001 vs. 0 μg/ml. IC50 values of the plant extract and standard are 3.35 ± 3.33 and 0.87 ± 0.05 mg/ml, respectively.
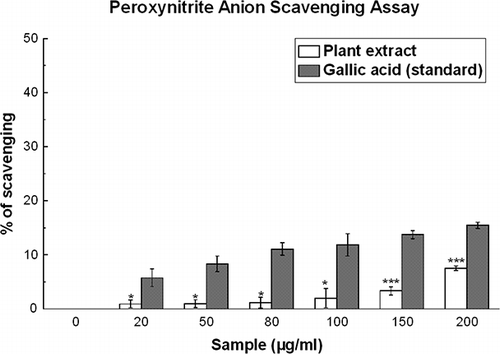
Peroxynitrite Scavenging Activity
shows that the peroxynitrite scavenging activity of the plant extract is concentration dependent. The calculated IC50 value was 3.35 ± 0.33 mg/ml, which was much greater than that of the reference compound gallic acid (IC50 = 0.87 ± 0.05 mg/ml) (), indicating that the plant extract is not a good peroxynitrite scavenger when compared to gallic acid. At 200 μg/ml, the scavenging percentages were 7.5 and 15.4% for D. esculentum and gallic acid, respectively.
Figure 7 Singlet oxygen scavenging assay. Effects of D. esculentum plant extract and the standard lipoic acid on the scavenging of singlet oxygen. The results are mean ± S.D. of six parallel measurements. ***p < 0.001 vs. μg/ml. IC50 values of the plant extract and standard are 278.88 ± 6.02 and 46.15 ± 1.16 μg/ml, respectively.
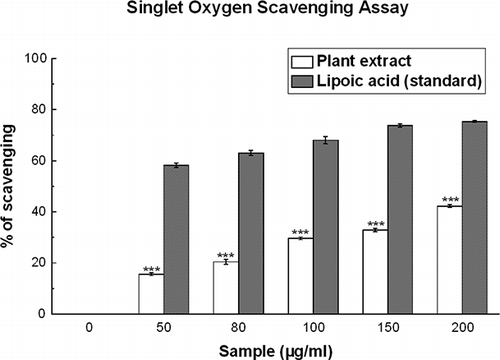
Singlet Oxygen Scavenging Activity
D. esculentum extract has moderate singlet oxygen scavenging activity when compared to that of lipoic acid (). The IC50 value () of the test sample was 278.88 ± 6.02 μg/ml, whereas that of lipoic acid was 46.15 ± 1.16 μg/ml. At 200 μg/ml, the percentage scavenging of plant extract was 42.2%, whereas that of lipoic acid was 75.3%.
Figure 8 Hypochlorous acid scavenging activities of D. esculentum plant extract and the standard ascorbic acid. All data are expressed as mean ± S.D. (n = 6). **p < 0.01 and ***p < 0.001 vs. 0 μg/ml. IC50 values of the plant extract and standard are 338.96 ± 11.60 and 235.95 ± 5.75 μg/ml, respectively.
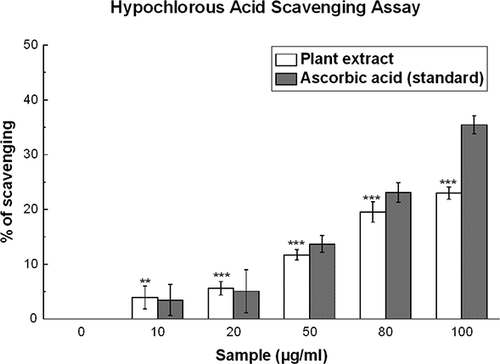
Hypochlorous Acid Scavenging Activity
shows that D. esculentum possesses an efficient hypochlorous acid scavenging activity (IC50 = 338.96 ± 11.60 μg/ml) when compared to that of ascorbic acid (IC50 = 235.95 ± 5.75 μg/ml) (). At 100 μg/ml, the percentage scavenging of the plant extract was 22.9% whereas that of ascorbic acid was 35.4%. At lower doses, viz. 10 and 20 μg/ml, the percentages scavenging of the plant extract were 3.9 and 5.6%, respectively, which were higher than that of ascorbic acid (3.4 and 5.0% for 10 and 20 μg/ml, respectively).
Figure 9 (a) Fe2+ chelation assay of D. esculentum. Effects of D. esculentum plant extract on Fe2+-ferrozine complex formation is shown. The data are expressed as percentage inhibition of chromogen formation. The results are mean ± S.D. of six parallel measurements. *p < 0.05 and **p < 0.01 vs. 0 μg/ml. IC50 value of the plant extract was 1.33 ± 1.13 mg/ml. (b) Fe2+ chelation assay of standard EDTA. Effects of EDTA on Fe2+-ferrozine complex formation is shown. The data are expressed as percentage inhibition of chromogen formation. The results are mean ± S.D. of six parallel measurements. IC50 value of the standard was 0.001 ± 0.00005 mg/ml.
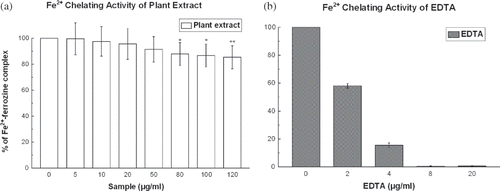
Fe2+ Chelation
Ferrozine produces a violet complex with Fe2+. In the presence of a chelating agent, complex formation is interrupted, and as a result, the violet color of the complex is decreased. The results (Figs. and ) demonstrate that the formation of the ferrozine-Fe2+ complex is inhibited in the presence of the test and reference compounds. The IC50 values () of the plant extract and EDTA were 1.33 ± 1.13 and 0.001 ± 0.000 mg/ml, respectively. At 120 μg/ml, the percentage inhibition of the plant extract was 14.72%, whereas at 20 μg/ml that of EDTA was 99.34%.
Reducing Power
As illustrated in , Fe3+ was transformed to Fe2+ in the presence of D. esculentum extract and the reference compound (ascorbic acid) to measure the reductive capability. Although the activity of ascorbic acid was better than the sample with absorbance values of 0.05 and 0.47 at 1 mg/ml for the plant extract and reference compound, respectively, still the plant extract showed somewhat moderate reducing capability.
Figure 11 Inhibition of lipid peroxidation by D. esculentum extract and the standard trolox. The data is expressed as the percentage of lipid peroxidation inhibition of brain homogenate, induced by Fe2+/ascorbic acid. Each value represents mean ± S.D. (n = 6). ***p < 0.001 vs. 0 μg/ml. IC50 values of the plant extract and standard are 141.67 and 6.76 μg/ml, respectively.
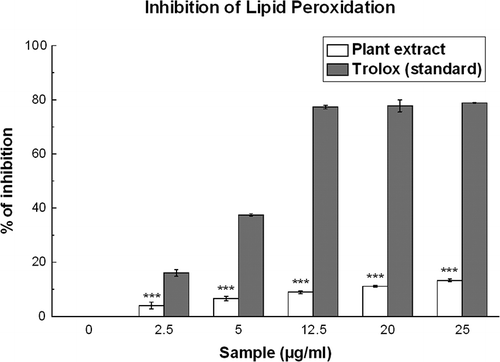
Lipid Peroxidation Inhibition Assay
The IC50 values () of the sample (141.67 ± 4.19 μg/ml) and the standard (6.76 ± 0.17 μg/ml) support the fact that the inhibitory efficiency of the plant extract is poor compared to standard trolox. As shown in , the increase in lipid peroxidation inhibition with increasing concentration of the plant extract reflects its antioxidant property.
Determination of Total Phenolic Content
The 70% methanolic extract of D. esculentum showed 126.67 ± 8.16 mg gallic acid equivalent phenolic content in 1 g of dried plant extract.
Determination of Total Flavonoid Content
The 70% methanolic extract of D. esculentum showed 94.33 ± 6.12 mg quercetin equivalent flavonoid content in 1 g of dried plant.
DISCUSSION
Free radicals play a crucial role in the pathogenesis of various diseases, especially degenerative diseases, and extensive lysis.[Citation28] They are constantly generated in the living system and responsible for the damage of cellular biomolecules, such as proteins, enzymes, nucleic acids, lipids, and carbohydrates, and adversely affect the immune function.[Citation29] Antioxidants inhibit the production of free radicals and also play a key role to inactivate them. All human cells protect themselves against oxidative damage by various antioxidant mechanisms, but sometimes these are not sufficient to prevent the damage caused by free radicals. Therefore, many synthetic drugs are used to protect against oxidative damage caused by free radicals but they have adverse side effects. For this reason, natural antioxidants in the form of common food supplements from plant origin have drawn much attention in recent years, as several investigators have reported that the plant-derived antioxidants have beneficial effects on human health.[Citation30, Citation31]
In the present study, the total antioxidant capacity of the young frond extract of D. esculentum was measured by an ABTS radical cation decolorization assay that has been commonly used because of its good reproducibility and easy quality control.[Citation32] The reaction between ABTS and potassium persulfate results in the production of a blue colored chromophore, ABTS•+. After addition of the plant extract this preformed radical cation was converted to ABTS in a dose dependent manner. The result is compared with trolox and the TEAC value demonstrates the extract as a potent antioxidant.
Hydroxyl radical is one of the major reactive oxygen species that causes lipid peroxidation and DNA damage.[Citation33, Citation34] It was produced by incubating Fe3+-EDTA with ascorbic acid and H2O2 at pH 7.4. Hydroxyl radicals formed in this reaction were then reacted with 2-deoxy-2-ribose to generate a malondialdehyde (MDA)-like product. This compound formed a pink chromogen upon heating with TBA at low pH. Additions of D. esculentum extract to the reaction mixture removed hydroxyl radicals from the sugar and prevented further damage. The dose dependent increase in the scavenging activity indicates its potential as a good hydroxyl radical scavenger. Observation also showed that at a higher dose, the plant extract works as a better hydroxyl radical scavenger than the standard mannitol.
Superoxide anion radical (O2 •-) originates from the one-electron reduction of free molecular oxygen by nicotinamide adenine dinucleotide phosphate oxidase, which is the membrane-bound enzyme.[Citation35, Citation36] In a cell, it changes to other harmful reactive oxygen species like hydrogen peroxide and hydroxyl radical.[Citation37] It indirectly initiates lipid oxidation by generating singlet oxygen. On excessive production, this radical causes tissue injury and organ dysfunction during sepsis and septic shock.[Citation38–40 Citation Citation40 Xanthine oxidase-derived superoxide radical has been linked to the postischemic tissue injury and generation of neutrophil chemotoxins. Elimination of superoxide radical anion generated by this enzymatic pathway would be beneficial in the case of ischemia.[Citation41] The present study indicates the dose dependent increasing superoxide radical scavenging activity of the plant extract. Therefore, superoxide radical scavenging activity of D. esculentum extract could play a therapeutic role in superoxide radical mediated ischemia.
Nitric oxide (NO), an important bioregulatory molecule, plays an important role in diverse physiological conditions, including neural signal transduction, control of blood pressure, platelet function, and antitumor and antimicrobial activity.[Citation42] But sustained levels of production of NO are directly toxic to tissues and contribute to the vascular collapse associated with septic shock, whereas chronic expression of nitric oxide radical is associated with various carcinomas and inflammatory conditions, including diabetes, multiple sclerosis, arthritis, and ulcerative colitis.[Citation43] The toxicity increases greatly when NO reacts with a superoxide radical and forms highly reactive peroxynitrite anion (ONOO−) implicated in the pathogenesis of diseases, such as heart diseases, Alzheimer's disease, and atherosclerosis.[Citation44] The methanolic extract of D. esculentum shows significant nitric oxide scavenging activity in a dose dependent manner. The nitric oxide generated from sodium nitroprusside reacts with oxygen to form nitrite. The extract directly competes with oxygen to react with nitric oxide, thus inhibiting nitrite formation. Reducing the generation of nitric oxide in the digestive tract was found to be effective in preventing the reactions of nitrate with amines and amides to form carcinogenic nitrosoamines and nitrosoamides.[Citation45] Hence, the NO scavenging activity of the plant extract could play a preventive role against nitrosoamine-mediated carcinogenesis.
Hydrogen peroxide (H2O2) is a weak oxidizing agent that belongs to a non-radical form of ROS. The H2O2, formed by the two-electron reduction of O2, is not a free radical but is an oxidizing agent. In the presence of O2 and transition metal ions, H2O2 can generate hydroxyl ion via the Fenton reaction. H2O2 at micromolar levels is poorly reactive. However, higher levels of H2O2 can attack some energy-producing systems. H2O2 inactivates the glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase. In addition, H2O2 also forms hydroxyl ion in the presence of metal ions and oxygen facilitates this reaction. Hence, metal chelating and H2O2 scavenging processes are important for living organisms.[Citation46] The present study clearly shows the dose dependent increasing H2O2 scavenging activity of the D. esculentum extract. It is interesting to note that at lower concentrations, the plant extract possesses much higher H2O2 scavenging activity than that of the standard sodium pyruvate, thus indicating its efficiency as a potent H2O2 scavenger.
Peroxynitrite is relatively stable compared to other free radicals but once protonated it forms the highly reactive peroxynitrous acid (ONOOH).[Citation47] It has strong oxidizing properties toward various cellular constituents, including sulphydryls, lipids, amino acids, and nucleotides, and causes cell death, lipid peroxidation, carcinogenesis, and aging.[Citation48] It has been shown that the systemic inflammatory response and lung injury occur following intestinal ischemia-reperfusion (IIR) with an increase of nitric oxide, inducible isoform of NO synthase (iNOS) expression, and the formation of peroxynitrite in the lung. Inhibition of iNOS prevented the lung injury, suggesting that excessive NO production and peroxynitrite formation are cytotoxic to the cell and tissue and are involved in the secondary lung injury induced by the IIR.[Citation49] Therefore, inhibiting peroxynitrite may be a novel pharmacological approach to prevent cell injury and multiple organ failure. The methanolic extract of D. esculentum exhibits significant dose dependent increasing peroxynitrite scavenging activity indicating its effectiveness as a good peroxynitrite scavenger for the prevention of peroxynitrite mediated diseases.
Singlet oxygen, a high energy form of oxygen, is generated in the skin by ultraviolet radiation. Singlet oxygen induces hyperoxidation, oxygen cytotoxicity, and decreases the antioxidative activity.[Citation50] Results indicate that D. esculentum extract has good singlet oxygen scavenging activity but is not as efficient as the standard lipoic acid.
Hypochlorous acid (HOCl) is a major oxidant produced by neutrophils and monocytes, via the myeloperoxidase-catalyzed oxidation of chloride by hydrogen peroxide.[Citation51] HOCl is a potent oxidant capable of damaging host tissue during inflammation. Inappropriate and/or excessive activation of neutrophils leads to oxidative stress and collateral damage of surrounding tissues. Cysteine and methionine residues in proteins and reduced glutathione (GSH) appear to be the main targets for HOCl, thereby altering the structure and function of proteins and lowering the antioxidant status in the cell. HOCl has the ability to inactivate the antioxidant enzyme catalase through breakdown of the heme prosthetic group. In the present study, we observed that catalase inactivation is inhibited in the presence of the D. esculentum extract, signifying its HOCl scavenging activity. The result also suggests that at lower doses the extract is more efficient in HOCl scavenging than the standard ascorbic acid.
In excess, iron can react with superoxide anion and hydrogen peroxide and convert them in to hydroxyl radical (OH•) (Haber-Weiss reaction) that cause severe injury to membranes, proteins, and DNA. It decomposes lipid hydro-peroxides into peroxyl and alkoxyl radicals responsible for the chain reaction of lipid peroxidation. To date, there are no definitive examples in the literature of D. esculentum acting to bind heavy metal ions. According to the result of the present study, though the plant extract is not as good as the standard EDTA in iron chelating, but the decrease in concentration dependent color formation in the presence of the extract indicate that it has iron chelating activity to some extent.
The reducing capacity of a compound may serve as a significant indicator of its potential antioxidant activity. The reducing properties are generally associated with the presence of reductones, which have been shown to exhibit antioxidant action by breaking the chain reactions by donating a hydrogen atom.[Citation52] Reductones are also reported to react with certain precursors of peroxide, thus preventing peroxide formation.[Citation53] The result obtained from the experiment indicates that the plant extract has some reducing capacity, thus justifying its antioxidant activity.
In the process of lipid peroxidation, the iron catalyzed generation of ferry-perferryl complex or hydroxyl radicals accelerates peroxidation by decomposing lipid hydroperoxides in to peroxyl and alkoxyl radicals. The highly reactive hydroxyl radicals react with polyunsaturated fatty acid moieties of cell membrane and yield carbonyl products like malondialdehyde (MDA), which generate a pink chromogen with TBA.[Citation54] With the addition of the sample extract or standard, the generation of MDA is reduced, thus indicating the ability of the sample, although less than the standard, to inhibit lipid peroxidation.
The results indicate that D. esculentum plant extract contains significant amounts of flavonoids and phenolic compounds. Both these classes of compounds have good antioxidant potential and their effects on human nutrition and health are considerable. The mechanism of action of flavonoids is through scavenging or chelation.[Citation55] Phenolic compounds are also very important plant constituents because their hydroxyl groups confer scavenging ability.[Citation56]
CONCLUSION
On the basis of the results obtained in the present study, it can be concluded that the 70% methanolic extract of D. esculentum contained a high amount of flavonoids and phenolic compounds, exhibited good antioxidant and free radical scavenging activities, and also possessed reducing power, iron chelating capacity, and lipid peroxidation inhibition ability. These in vitro assays indicated that this plant extract was a significant source of natural antioxidants, which might be helpful in preventing the progression of various oxidative stress associated diseases. Therefore, further studies should be carried out to isolate the bioactive components of this plant that confer the antioxidant property. Furthermore, the in vivo antioxidant activity of this extract needs to be studied thoroughly prior to clinical use.
ACKNOWLEDGMENTS
University Grants Commission (UGC), New Delhi, India has provided the financial support to carry out this study (Vide Letter F. No. 37-464/2009 (SR) dt.11.01.2010). The authors would also like to extend thanks to Mr. Rhitajit Sarkar, Division of Molecular Medicine, Bose Institute, Kolkata, for his assistance in operating the instruments throughout the study.
REFERENCES
- Halliwell , B. 1991 . Reactive oxygen species in living systems: Source, biochemistry, and role in human disease . American Journal of Medicine , 91 : S14 – S22 .
- Young , I. and Woodside , J. 2001 . Antioxidants in health and disease . British Medical Journal , 54 : 176
- Braca , A. , Sortino , C. , Politi , M. , Morelli , I. and Mendez , J. 2002 . Antioxidant activity of flavonoids from Licania licaniaeflora . Journal of Ethnopharmacology , 79 : 379 – 381 .
- Maxwell , S.R. 1995 . Prospects for the use of antioxidant therapies . Drugs , 49 : 345 – 361 .
- Stadtman , E.R. 1992 . Protein oxidation and aging . Science , 257 : 1220 – 1224 .
- Niki , E. , Shimaski , H. and Mino , M. 1994 . Antioxidantism-Free Radical and Biological Defense , 3 – 16 . Tokyo , Japan : Gakkai Syuppn Center .
- Phomkaivon , N. and Areekul , V. 2009 . Screening for antioxidant activity in selected Thai wild plants . Asian Journal of Food and Agro-Industry , 2 ( 4 ) : 433 – 440 .
- Wong , S.P. , Leong , L.P. and Koh , J.H.W. 2006 . Antioxidant activities of aqueous extracts of selected plants . Food Chemistry , 99 ( 4 ) : 775 – 783 .
- Nanasombat , S. and Teckchuen , N. Antimicrobial . 2009 . antioxidant and anticancer activities of Thai local vegetables . Journal of Medicinal Plants Research , 3 ( 5 ) : 443 – 449 .
- Rahmat , A. , Kumar , V. , Fong , L.M. , Endrini , S. and Sani , H.A. 2003 . Determination of total antioxidant activity in three types of local vegetables shoots and the cytotoxic effect of their ethanslic extracts against different cancer cell lines. Asia Pacific Journal of Clinical Nutrition , 12 ( 3 ) : 308 – 311 .
- Hazra , B. , Biswas , S. and Mandal , N. 2008 . Antioxidant and free radical scavenging activity of Spondias pinnata . BMC Complementary and Alternative Medicine , 8 : 63
- Re , R. , Pellegrini , N. , Proteggente , A. , Pannala , A. , Yang , M. and Rice-Evans , C. 1999 . Antioxidant activity applying an improved ABTS radical cation decolorization assay . Free Radical Biology and Medicine , 26 : 1231 – 1237 .
- Elizabeth , K. and Rao , M.N.A. 1990 . Oxygen radical scavenging activity of curcumin . International Journal of Pharmaceutics , 58 : 237 – 240 .
- Fontana , M. , Mosca , L. and Rosei , M.A. 2001 . Interaction of enkephalines with oxyradicals . Biochemical Pharmacology , 61 : 1253 – 1257 .
- Garratt , D.C. 1964 . The Quantitative Analysis of Drugs , Vol. 3 , 456 – 458 . Japan : Chapman and Hall Ltd . Volume
- Long , L.H. , Evans , P.J. and Halliwell , B. 1999 . Hydrogen peroxide in human urine: Implications for antioxidant defense and redox regulation . Biochemical Biophysical Research Communications , 262 : 605 – 609 .
- Floriano-Sánchez , E. , Villanueva , C. , Medina-Campos , O.N. , Rocha , D. , Sánchez-González , D.J. , Cárdenas-Rodríguez , N. and Pedraza-Chaverrí , J. 2006 . Nordihydroguaiaretic acid is a potent in vitro scavenger of peroxynitrite, singlet oxygen, hydroxyl radical, superoxide anion, and hypochlorous acid and prevents in vivo tyrosine nitration in lung . Free Radical Research , 40 : 523 – 533 .
- Beckman , J.S. , Chen , H. , Ischiropulos , H. and Crow , J.P. 1994 . Oxidative chemistry of peroxynitrite . Methods in Enzymology , 233 : 229 – 240 .
- Bailly , F. , Zoete , V. , Vamecq , J. , Catteu , J.P. and Bernier , J.L. 2000 . Antioxidant actions of ovothiol-derived 4-mercaptoimidazoles: Glutathione peroxidase activity and protection against peroxynitrite- induced damage . FEBS Letters , 486 : 19 – 22 .
- Pedraza-Chaverrí , J. , Barrera , D. , Maldonado , P.D. , Chirino , Y.I. , Macías-Ruvalcaba , N.A. , Medina-Campos , O.N. , Castro , L. , Salcedo , M.I. and Hernández-Pando , R. 2004 . S-Allylmercaptocysteine scavenges hydroxyl radical and singlet oxygen in vitro and attenuates gentamicin induced oxidative and nitrosative stress and renal damage in vivo . BMC Clinical Pharmacology , 4 : 5
- Aruoma , O.I. and Halliwell , B. 1987 . Action of hypochlorous acid on the antioxidant protective enzymes superoxide dismutase, catalase and glutathione peroxidase . Biochemical Journal , 248 : 973 – 976 .
- Pedraza-Chaverrí , J. , Arriaga-Noblecía , G. and Medina-Campos , O.N. 2007 . Hypochlorous acid scavenging capacity of garlic . Phytotherapy Research , 21 : 884 – 888 .
- Haro-Vicente , J.F. , Martinez-Gracia , C. and Ros , G. 2006 . Optimization of in vitro measurement of available iron from different fortificants in citric fruit juices . Food Chemistry , 98 : 639 – 648 .
- Oyaizu , M. 1986 . Studies on products of browning reactions: Antioxidant activities of products of browning reaction prepared from glucose amine . Japanese Journal of Nutrition , 44 : 307 – 315 .
- Kizil , G. , Kizil , M. , Yavuz , M. , Emen , S. and Hakimoglu , F. 2008 . Antioxidant activities of ethanol extracts of Hypericum triquetrifolium and Hypericum scabroides . Pharmaceutical Biology , 46 : 231 – 242 .
- Singleton , V.L. and Rossi , J.A. 1965 . Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents . American Journal of Enology and Viticulture , 16 : 144 – 158 .
- Zhishen , J. , Mengcheng , T. and Jianming , W. 1999 . The determination of flavonoid content in mulberry and their scavenging effects on superoxide radicals . Food Chemistry , 64 : 555 – 559 .
- Halliwell , B. and Gutteridge , J.M. 1998 . Free Radicals in Biology and Medicine , Oxford : Oxford University Press .
- Nilsson , J. , Stegmark , R. and Akesson , B. 2004 . Total antioxidant capacity in different pea (Pisum sativum) varieties after blanching and freezing . Food Chemistry , 86 : 501 – 507 .
- Gungor , N. and Sengul , M. 2008 . Antioxidant activity, total phenolic content and selected physicochemical properties of white mulberry (Morus alba L.) fruits . International Journal of Food Properties , 11 : 44 – 52 .
- Okmen , B. , Sigva , H.O. , Mutlu , S. , Doganlar , S. , Yemenicioglu , A. and Frary , A. 2009 . Total antioxidant activity and total phenolic contents in different Turkish eggplant (Solanum melongena L.) cultivars . International Journal of Food Properties , 12 : 616 – 624 .
- Lee , B.W. , Lee , J.H. , Gal , S.W. , Moon , Y.H. and Park , K.H. 2006 . Selective ABTS radical-scavenging activity of prenylated flavonoids from Cudrania tricuspidata . Bioscience, Biotechnology, and Biochemistry , 70 ( 2 ) : 427 – 432 .
- Aurand , L.W. , Boone , N.H. and Giddings , G.G. 1977 . Superoxide and singlet oxygen in milk lipid peroxidation . Journal of Dairy Science , 60 : 363 – 369 .
- Packer , L. and Ong , A.S.H. 1997 . Biological Oxidants and Antioxidants: Molecular Mechanisms and Health Effects , Champaign , IL : AOCS Press . Eds
- Badwey , J.A. and Karnovsky , M.L. 1980 . Active oxygen species and the functions of phagocytic leucocytes . Annals of Internal Medicine , 93 : 480 – 489 .
- Babior , B.M. 2000 . Phagocytes and oxidative stress . American Journal of Medicine , 109 : 33 – 44 .
- Al-Mamun , M. , Yamaki , K. , Masumizu , T. , Nakai , Y. , Saito , K. , Sano , H. and Tamura , Y. 2007 . Superoxide anion radical scavenging activities of herbs and pastures in northern Japan determined using electron spin resonance spectrometry . International Journal of Biological Sciences , 3 ( 6 ) : 349 – 355 .
- Brigham , K.L. 1991 . Oxygen radicals—An important mediator of sepsis and septic shock . Klinische Wochen-Schrift , 69 : 1004 – 1008 .
- Takeda , K. , Shimada , Y. , Okada , T. , Amano , M. , Sakai , T. and Yoshiya , I. 1986 . Lipid peroxidation in experimental septic rats . Critical Care Medicine , 14 : 719 – 723 .
- Walker , P.D. and Shah , S.V. 1990 . Reactive oxygen metabolites in endotoxin induced acute renal failure in rats . Kidney International , 38 : 1125 – 1132 .
- Selloum , L. , Reichl , S. , Müler , M. , Sebihi , L. and Arnhold , J. 2001 . Effects of flavonols on the generation of superoxide anion radicals by xanthine oxidase and stimulated neutrophils . Archives of Biochemistry and Biophysics , 395 : 49 – 56 .
- Kumaran , A. and Karunakaran , J. 2006 . Nitric oxide radical scavenging active components from Phyllanthus emblica L . Plant Foods for Human Nutrition , 61 : 1 – 5 .
- Tylor , B.S. , Kion , Y.M. , Wang , Q.I. , Sharpio , R.A. , Billiar , T.R. and Geller , D.A. 1997 . Nitric oxide down-regulates hepatocyte-inducible nitric oxide synthase gene expression . Archives of Surgery , 132 : 1177 – 1183 .
- Ischiropoulos , H. , al-Mehdi , A.B. and Fisher , A.B. 1995 . Reactive species in ischemic rat lung injury: Contribution of peroxynitrite . American Journal of Physiology , 269 : 158 – 164 .
- Boone , C.W. , Kelloff , G.J. and Malone , W.E. 1990 . Identification of candidate cancer chemopreventive agents and their evaluation in animal models and human clinical trials: A review . Cancer Research , 50 : 2 – 9 .
- Aruoma , O.I. 1998 . Free radicals, oxidative stress, and antioxidants in human health and disease . Journal of Americal Oil Chemists' Society , 75 : 199 – 212 .
- Balavoine , G.G. and Geletti , Y.V. 1999 . Peroxynitrite scavenging by different antioxidants. Part1: Convenient study. Nitric Oxide , 3 : 40 – 54 .
- Choi , H.R. , Choi , J.S. , Han , Y.N. , Bae , S.J. and Chung , H.Y. 2002 . Peroxynitrite scavenging activity of herb extracts . Phytotherapy Research , 16 ( 4 ) : 364 – 367 .
- Zhou , J.L. , Jin , G.H. , Yi , Y.L. , Zhang , J.L. and Huang , X.L. 2003 . Role of nitric oxide and peroxynitrite anion in lung injury induced by intestinal ischemia-reperfusion in rats . World Journal of Gastroenterology , 9 ( 6 ) : 1318 – 1322 .
- Kochevar , E.I. and Redmond , W.R. 2000 . Photosensitized production of singlet oxygen . Methods in Enzymology , 319 : 20 – 28 .
- Winterbourn , C.C. , Vissers , M.C. and Kettle , A.J. Myeloperoxidase. 2000 . Current Opinion in Hematology , 7 : 53 – 58 .
- Geckil , H. , Ates , B. , Durmaz , G. , Erdogan , S. and Yilmaz , I. Antioxidant . 2005 . free radical scavenging and metal chelating characteristics of propolis . American Journal of Biochemistry and Biotechnology , 1 ( 1 ) : 27 – 31 .
- Matsusighe , K. , Basnet , P. , Kadota , S. and Namba , T. 1996 . Potent free radical scavenging activity of dicaffeoyl quinic acid derivatives from propolis . Journal of Traditional Medicine , 13 : 217 – 228 .
- Hazra , B. , Sarkar , S. , Mandal , S. , Biswas , S. and Mandal , N. 2009 . Studies on antioxidant and antiradical activities of dolichos biflorus seed extract . African Journal of Biotechnology , 8 ( 16 ) : 3927 – 3933 .
- Cook , N.C. and Samman , S. 1996 . Flavonoids—Chemistry, metabolism, cardioprotective effects, and dietary sources . Journal of Nutritional Biochemistry , 7 : 66 – 76 .
- Diplock , A.T. 1997 . Will the good fairies please prove to us that vitamin E lessens human degenerative disease? . Free Radical Research , 27 : 511 – 532 .