Abstract
The present study includes macroscopical as well as microscopical description of the plant Melothria maderaspatana Linn., along with determination of various physicochemical parameters, such as different ash values, extractive values, and fluorescence drug analysis. Preliminary phytochemical screening along with quantification of total phenolic and flavonoid content were also determined in the whole plant extract. The presence of ergosterol, which was isolated and characterized by performing thin layer chromatography and spectral techniques like Fourier transform infrared spectroscopy, 1HNMR, and mass spectrometry from chloroform fraction, was also reported for the first time. The other phytochemical isolated from the same fraction was found to be β–sitosterol. Thus, the present study will provide referential information to the researchers having interest in the relevant field for correct identification and authentication of the plant, which will minimize the adulteration of that particular crude drug and its formulations.
INTRODUCTION
The importance of medicinal plants from antiquity to date has led pharmacognosy, over the years, to become one of the pillars of areas like pharmacy, medicine, natural product chemistry, and others. All these scientific disciplines now recognize the importance of plants as sources of medicines and have initiated active research programs either to isolate new lead compounds or to produce standardized extracts.[Citation1,Citation2] Considering the importance of medicinal plants is very essential to evaluate various qualitative and quantitative parameters that will prevent the adulteration of a drug and its formulation.[Citation3]
The plant Melothria maderaspatana Linn. (Cucurbitaceae) is commonly known as Agumaki in Hindi and is mainly distributed in China, Brazil, South Africa, Madagascar, and India. In India, the plant is used traditionally for treatment of various ailments.[Citation4] The people from southern parts of India prepare a food preparation (Aadai/Paratha) from fresh leaves of M. maderaspatana mixed with rice powder for the treatment of cough and phlegm. Leaves of this plant are eaten as such by the males of this area, which is considered to promote fertility in males. The powdered root is boiled with water and taken in the form of herbal tea as a remedy for vomiting and chest pain.[Citation5] Some local tribes of Orissa (India) use this herb for the treatment of diabetes mellitus.[Citation6] The tender shoots and bitter leaves are used as gentle aperients and are prescribed in vertigo and biliousness.[Citation7] Many pharmacological activities, such as hepatoprotective,[Citation8] anti-oxidant,[Citation9] immunomodulatory,[Citation10] anti-arthiritic,[Citation11] anti-inflammatory,[Citation6] anti-microbial,[Citation12] anti-hyperglycemic, free radical scavenging,[Citation13] and hypolipidemic[Citation14] activities, have been reported from this plant. The ethyl acetate, hexane, and methanol extracts of M. maderaspatana were reported to have anti-platelet activity in an in vitro model.[Citation15] A recent study has shown that a consumption of M. maderaspatana leaf tea decreased the blood pressure and had beneficial effects on lipid profile, fibrinogen, bilirubin, and body mass index in human volunteers.[Citation16] Even though the plant has been successfully evaluated for various pharmacological activities, there still is no relevant data available regarding the various qualitative and quantitative parameters ensuring the quality control profile of the plant. Therefore, the present study was conducted to obtain a proper pharmacognostical profile of the plant to maintain its quality and purity.
MATERIALS AND METHODS
Macroscopic and Microscopic Analysis
M. maderaspatana plants were collected from the Ayurveda garden of TAMPCOL, Chennai, India and were identified by Mr. Narayanappa, Chief Botanist TAMPCOL (Chennai). A voucher specimen (SH/MM/02) has been deposited in the Department of Pharmaceutics, Institute of Technology, Banaras Hindu University. Macroscopy of the plant was studied according to the methods described by Trease and Evans.[Citation17] Hand sections of the roots, stem, leaf, petiole, and tendril of M. maderaspatana were taken, stained, and mounted following usual micro-techniques.[Citation18] Plant parts were than macerated separately in Schultz's fluid, washed with water, teased, and mounted in glycerin for studying the characteristics of individual cells and tissues.
Physicochemical Standards
The obtained plant material was air dried and powdered, which was further evaluated for various physicochemical parameters, such as total ash, acid insoluble ash, water, and alcohol soluble extractive values, as per the procedure mentioned in Indian Pharmacopoeia.[Citation19]
Fluorescence Characteristics
The fluorescence drug analysis of the air-dried coarse powdered drug was done according to the method described by Kokaski et al.[Citation20] under ultraviolet light.
Preliminary Phytochemical Screening
The coarsely powdered plant material (500 g) was extracted with methanol (1.5 L) using Soxhlet apparatus, which was then concentrated and fractionated using different solvents, such as hexane, chloroform, and ethyl acetate, depending on polarity. These fractions were than concentrated and dried in a Rota evaporator (IKA, Germany) initially and then in a vacuum desiccator. Further preliminary phytochemical screenings of these fractions were done for the presence of various phytoconstituents by using standard procedure.[Citation21]
Estimation of Total Phenolic and Flavonoid Content
The total phenolic content of methanolic extract of M. maderaspatana was determined using Folin-Ciocalteau reagent (FCR). This method depends on the reduction of FCR by phenols to a mixture of blue oxides, which have a maximal absorption in the region of 765 nm.[Citation22] The flavonoids content was determined by aluminum chloride method using rutin as a reference compound. This method is based on the formation of a complex flavonoid-aluminum having the absorbance maximum at 415 nm.[Citation23]
Isolation and Standardization of Phytoconstituents
Thin layer chromatography (TLC) was performed for all the fractions of the extracts to determine the solvent system showing the best separation of phytoconstituents. On the basis of results obtained from the TLC study, chloroform fraction was subjected to column chromatography on silica gel and was eluded with different solvents starting with n-hexane and further increasing of the polarity.
RESULTS
Macroscopical Analysis
The leaves are simple, petiolate, green, and deltoid in shape, 3–10 cm in width, 5–8 cm in length, and slightly bitter in taste. Petioles are 3–7 cm long, slender, and pubescent. Stems are cylindrical, 1–6 mm in diameter. The surface of fresh stems are hairy with a greenish tinge but light brown in colour when dry, showing faint longitudinal ridges and is soft to touch due to trichomes. Roots are 10–20 cm long with lateral branches and 1–5 mm in diameter, light brown in color, with smooth surface bearing scars of rootlets ().
Microscopical Analysis
Transverse section of petiole
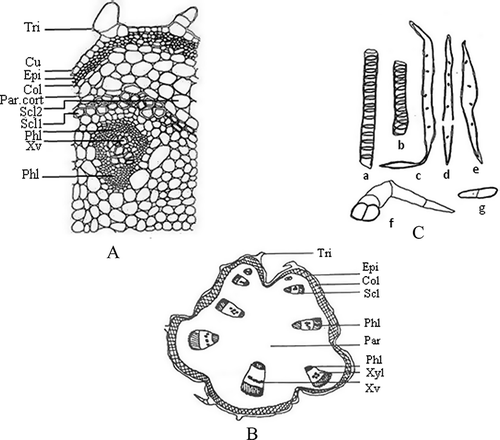
The transverse section of a petiole shows a circular outline in the basal region, but develops ridges and furrows as well as two arch-like bulges in the middle and apical region. The epidermis is single layered, covered with a moderately thick cuticle and a few uniserriate covering trichomes, 2–3 cells long. Epidermis is followed by a wide zone of cortex, comprised of collenchymatous cells (2–6 layers) on the outer side and the parenchymatous cells (2–6 layers) on the inner side of the basal region of the petiole. It is followed by pericycle showing a more or less continuous ring of sclerenchyma with intervening parenchyma at 2–3 places in the basal region. The pericyclic sclerenchyma is developed only as capping of phloem in the middle region and in the apical region it is absent. The pericycle is followed by ground tissues, in which vascular bundles (VB) are scattered (seven in the basal region arranged in a circle, seven in the middle region arranged in the form of a discontinuous ring along with two (one each) below the bulging arms. Another five larger size VB are arranged in the discontinuous ring, with two smaller ones arranged one each below the bulging arms). Pith is made up of parenchymatous cells ().
Figure 2 Transverse section of the basal region of petiole (a), schematic diagram of basal region of petiole (b), and isolated elements of the petiole (c). In Fig. 2a: Col: collenchyma; Cu: cuticle; Epi: epidermis; Par: parenchyma; Par.cort: parenchymatous cortex; Phl: phloem; Scl: sclerenchyma; Scl1: sclerenchymatous pericycle; Scl2: cortical parenchyma tendency of thickening; Tri: trichome; Xv: xylem vessel; Xyl: xylem. In Fig. 2c: a–b: xylem vessels; c–e: fibres; f: trichome; g: parenchyma.
Transverse section of midrib
The midrib shows a distinct biconvex outline in the basal and middle region, but planoconvex in the apical region. The transverse section of midrib shows single layer epidermis on both sides. The upper epidermal cells are larger than the lower epidermal cells. Both of the epidermises consist of simple, uniserriate covering trichomes of 1–4 cells long with pointed or blunt ends. In addition to this, trichomes having bi to tetra seriate base with apical cells similar to uniseriate trichomes are also present. The upper and lower epidermises are followed by 3–7 and 2–4 layers of collenchymatous cells. Below the collenchyma on the dorsal side a wide zone of parenchymatous cells are present, but on the ventral side, bicollateral vascular bundles are found. The central portion shows a large bicollateral vascular bundle (). Numerical values of different leaf constants of M. maderaspatana are given in
Figure 3 Transverse section of midrib region of leaf (a), schematic diagram of tendril (b), and trichomes and xylem vessel in isolated elements of leaf (c). In Fig. 3a: A1: midrib and part of lamina; A2: lamina; A3: margin of lamina; Col: collenchyma; Epi: epidermis; L.Epi: lower epidermis; Pal: palisade cells; Par: parenchyma; Phl: phloem; Pi: pith; S.P: spongy parenchyma; Scl: sclerenchyma; St: stomata; Tri: trichome; U.Epi: upper epidermis; V: vessel; Xyl: xylem. In Fig. 3c: a–c: trichomes of basal region of midrib; d–e: trichomes of middle region of midrib; f–h: trichomes of apical region of midrib; i–j: trichomes and xylem vessel in isolated elements.
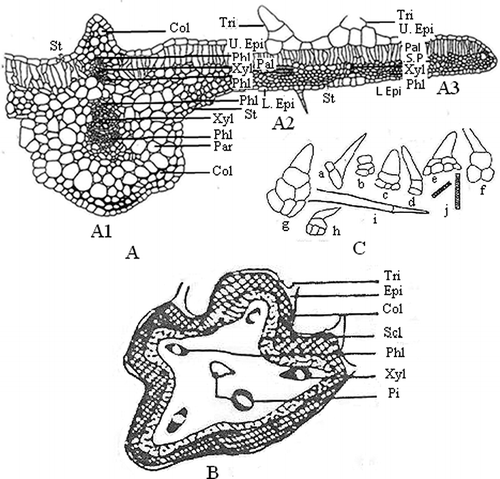
Table 1 Numerical values of the leaf of M. maderaspatana
Transverse section of lamina
The lamina of leaf presents a dorsiventral structure, covering trichomes and ranunculaceous type of stomata, which are present in both the epidermises. The upper epidermis is followed by a single layer of palisade cells, except at a few places where they are two-layered. The palisade is followed by three to four layers of spongy parenchymatous cells, which is traversed by a large number of veins. The palisade cells at the extreme margin are replaced by spongy parenchymatous cells ().
Transverse section of tendril
The transverse section of tendril shows an asymmetrical ridged and furrowed outline. The epidermis is single layered with covering trichomes, which are a miniature replica of the trichomes of petiole, except that they are thin walled. The epidermis is followed by a wide zone of collenchyma. It is followed by a continuous ring of sclerenchyma. Beneath the sclerenchyma ring there is a wide zone of ground tissue in which five conjoint bicollateral vascular bundles are distributed, one below each ridge. The centre region is represented by hollow pith surrounded by ground tissue ().
Transverse section of stem
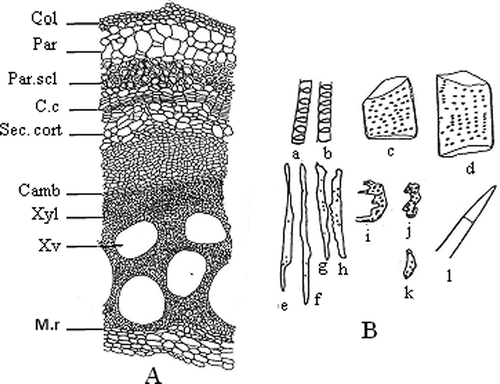
In the transverse section of the mature stem, ridges and furrows are gradually vanished. The epidermis peels off at places. The transverse section of the mature stem shows only a few ruptured patches of epidermal cells because of formation of cork as the growth proceeds. The outermost layer of the stem consists of 3–4 layers of collenchymatous cells which are tangentially flattened. It is followed by a wide zone of cortex made up of parenchymatous cells (4–6 layers), which are round to oval or polyhedral in shape. Beneath the cortex, 4–7 layers of a continuous ring of pericyclic fibers are present, representing the pericycle. Nine bicollateral vascular bundles are arranged in the ring and the xylem vessels are drum or barrel shaped ().
Figure 4 Transverse section of mature stem (a) and isolated elements of mature stem (b). In Fig. 3b: Camb: cambium; C.c: cork cambium; Col: collenchyma; M.r: medullary rays; Par: parenchyma; Par.scl: parenchymatous sclerenchyma; Sec.cort: secondary cortex; Xv: xylem vessel; Xyl: xylem. In Fig. 3b: a–d: xylem vessels; e–h: fibres; i–j: tracheids; k: xylem parenchyma; l: trichome.
Transverse section of root
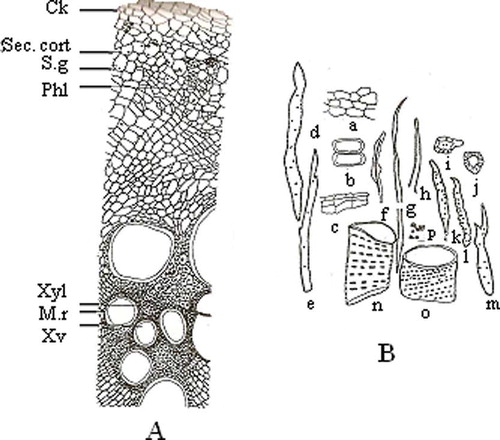
The transverse section shows a ridged and furrowed outline in the early stage of mature root and a circular outline in the fully mature root. The early stage mature root shows a single layer of epidermis followed by a narrow zone of parenchymatous cortex. In the mature root cork there are 3–5 layers of brick shaped cork cells with heavy suberisation. It is followed by 5–8 layers of secondary parenchymatous cortex, and stone cells are present in the form of a discontinuous ring in the outer region of the secondary cortex. Simple and compound starch grains are present in some of the cells of the secondary cortex. The central region is represented by a wide zone of highly ridged and furrowed xylem occupying about 2/3 of the thickness of the root traversed by 5–10 cells-wide medullary rays ().
Figure 5 Transverse section of the mature root (a) and isolated elements of the mature root (b). In Fig. 4a: Ck: cork; Phl: phloem; M.r: medullary rays; Sec.cort: secondary cortex; S.g: starch grain; Xv: xylem vessel; Xyl: xylem. In Fig. 4b: a: part of cortex; b: xylem parenchyma; c: cork fragments; d–h: fibres; i–j: stone cells; k–m: tracheids; n–o: xylem vessels; p: starch grain.
Physicochemical Standards
All the physicochemical standards, such as total ash, acid insoluble ash, water soluble extractive, and alcohol soluble extractive values, are reported in .
Table 2 Physicochemical standards
Fluorescence Characteristics
Fluorescence analysis of powdered drug was studied under ultraviolet light on treatment with various reagents, which are expressed in .
Table 3 Fluorescence characteristics
Preliminary Phytochemical Screening
The preliminary phytochemical screening of different fractions of extract of M. maderaspatana was carried out using different reagents, which are demonstrated in .
Table 4 Preliminary phytochemical screening
Quantitative Analysis of Total Phenolic and Flavonoid Content
The total phenolic content of methanolic extract of M. maderaspatana was carried out using gallic acid as a standard, which was found to be 22.37 mg/g equivalent to gallic acid, whereas total flavonoid content in methanolic extract was determined using rutin as a standard and was found to be 17 mg/g equivalent to rutin.
Isolation and Standardization of Phytoconstituents
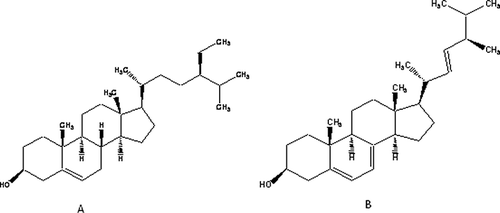
The compounds were obtained from the chloroform fraction when the column was run through 5% hexane:ethyl acetate as a solvent system. The compounds were soluble in chloroform and confirmed to be β-sitosterol and ergosterol by recording melting point, performing TLC and spectral techniques like Fourier transform infrared spectroscopy, 1HNMR, and mass spectrometry. The melting point was recorded by using Sonar melting point apparatus ISO 9001-2000 (Associate Scientific Technologies, New Delhi, India) in open capillary tubes. The IR spectra study was performed in Shimadzu FT/IR 8400 infrared spectrophotometer (Shimadzu, Japan) using KBr disc method for sample preparation. 1HNMR spectroscopy was performed on a Jeol FX 300Q FT NMR spectrophotometer (Germany). The ESI-MS was recorded on a Micromass Quattro II triple quadrupole mass spectrometer (Waters, USA) having a Jasco PU-980HPLC pump connected to it and acetonitrile was used as a solvent at 80°C source temperature.
Ergosterol
Recrystallized from ethanol—melting point: 159–161°C, Rf: 0.67 (hexane:ethyl acetate (9:1)); IR(KBr) [ν cm−1]: 1464 (C–Hbend), 2854 (CH3), 2956 (CH2), 3421 (OH); 1HNMR (CDCl3) [δ ppm]: 0.5–1.01 (m, 18H, CH3), 1.25–1.75 (m, 14H, CH2), 3.5 (m, 5H, CH), 4.35 (m, 4H, =CH), 5.31 (s, 1H, OH); ESI-MS m/z: 396 (M+, 95), 363 (100), 337 (15), 253 (40), 211 (20), 157 (30), 105 (15), 69 (25).
β–Sitosterol
Recrystallized from ethanol—melting point: 134–136°C, Rf: 0.63 (hexane:ethyl acetate (8:2)); IR(KBr) [ν cm−1]: 1458 (C–Hbend), 2866 (CH3), 2910 (CH2), 3417 (OH); 1HNMR (CDCl3) [δ ppm]: 0.93 (m, 18H, CH3), 1.14 (m, 22H, CH2), 4.99 (s, 1H, OH), 3.03 (m, 8H, CH), 5.54 (s, 1H, OH) ().
DISCUSSION
Even though the importance of numerous medicinal advantages of M. maderaspatana have been reported in the literature, there is so far no pharmacognostical report on the microscopical and other physicochemical parameters justifying the quality control profile of that particular plant. Therefore, the present study was conducted for obtaining quality control standards of the plant. According to the World Health Organisation (WHO), pharmacognostical standards are considered to be the primary step for diagnosis of the herbal drug, which includes macroscopic and microscopic evaluation of the particular plant/plant parts. Further, the histochemical studies of plant tissues tends to broaden views about the type of compounds and their occurrence in plant tissues, which helps in proper selection of a plant/plant parts.[Citation24] The special features of M. maderaspatana from its histochemical studies showed multicellular uniserriate covering trichomes and ranunculaceous type of stomata in the epidermis of the petiole, midrib, lamina, and tendril. Nine bicollateral vascular bundles arranged in a ring with drum- or barrel-shaped xylem vessels are some special features of the stem, while mature root showed heavy suberised brick-shaped cork cells with simple and compound starch grains present in the cells of the secondary cortex.
Physicochemical standards evaluated in the present study, such as total ash, helps in determining both physiological and non-physiological ash, while acid insoluble ash gives an idea regarding the amount of silica present, especially as sand and siliceous earth. The extractive value of a plant material enables us to know the amount of active constituents present in the plant/plant parts using different solvents.[Citation25] The phytoconstituents, i.e., total phenolics, and flavonoids evaluated in the present study have been evidently reported for many pharmacological activities. Phenolic compounds are strong antioxidants and free radical scavengers with anticarcinogenic, antibacterial, anti-inflammatory activities and are also used in coronary heart disease, some types of tumors, and coronary artery disease.[Citation26,Citation27] Flavonoids, by increasing the capillary permeability, have been shown to possess antioxidant and anti-inflammatory activity and are also associated in the treatment of various cardiovascular diseases.[Citation28,Citation29]
In the present study, the presence of ergosterol isolated from the chloroform fraction has been reported for the first time. Another phytosterol isolated from the same fraction was found to be β-sitosterol. Both of the compounds were initially confirmed with the help of co-TLC by comparing them with reference standards. Further, the melting points of both the compounds were taken, which was found to be 159–161°C for ergosterol and 134–136°C for β-sitosterol, respectively. All of the FTIR, 1HNMR, and mass analysis data supported the molecular structure of ergosterol showing the presence of methyl, methylene, carbon-carbon double bonds, and alcoholic hydroxyl groups. It also confirmed the presence of a total number of protons (43) and molecular weight (396) from the molecular peaks. The FTIR data supporting to the molecular structure of β-sitosterol showed the presence of methyl, methylene, carbon-carbon double bonds, and alcoholic groups. Again, from 1HNMR spectra, the δ values confirmed the presence of all of the above functional groups and total number of protons (51). Thus, the above isolated compounds may act as a chemical marker for the standardization of M. maderaspatana.
In conclusion, the qualitative and quantitative parameters evaluated in the present study will help us in maintaining a standard quality control profile of the plant. This will help researchers from relevant fields in proper identification and authentication of M. maderaspatana. These quality control standards will help in minimizing the adulteration and, therefore, will assist in maintaining the medicinal importance of the plant.
REFERENCES
- Prasad , S.K. , Singh , P.N. , Wahi , A.K. and Hemalatha , S. 2010 . Pharmacognostical standardization of Withania coagulans Dunal . Pharmacognosy Journal , 2 : 386 – 394 .
- Gurib-Fakim , A. 2006 . Review medicinal plants: Traditions of yesterday and drugs of tomorrow . Molecular Aspects of Medicine , 27 : 1 – 93 .
- Shanbhag , D.A. and Jayaraman , S. 2008 . Application of HPTLC in standardization of homoeopathic mother tincture . Pharmacognosy , 4 : 155 – 159 .
- Anonymous . 1969 . The Wealth of India , 336 C.S.I.R. Publications : New Delhi, India .
- Rathna , V. and Mudaliar , K.S.M. 2008 . Gunapadam, Siddha Meteria Medica , 2nd , Vol. 1 , 766 Indian Medicine , Homeopathy Division : Chennai, India .
- Sinha , B.N. , Sasnal , D. and Basu , S.P. 1997 . Pharmacological studies on Melothria maderaspatana . Fitoterapia , 68 : 75 – 78 .
- Kirthikar , K.R. and Basu , B.D. 1933 . Indian Medical Plants, III; M/s Bishen Singh Mahendra Pal Singh. Dehradun, India 1160 – 1160 .
- Thabrew , M.I.R.A. , Jayatilaka , P.A.P.W. and Perera , D.J.B. 1988 . Evaluation of efficacy of Melothira madaraspatama in the alleviation of carbon tetrachloride induced liver dysfunction. Journal of Ethnopharmacology , 23 : 305 – 312 .
- Jayatilaka , K.A.P.W. , Thabrew , A.I. and Perera , D.J.B. 1990 . Effects of Melothria maderaspatana on CCl4-induced changes in rats’ hepatic microsomal drug metabolism enzyme activity . Journal of Ethnopharmacology , 30 : 97 – 105 .
- Thabrew , M.I. , De Silva , K.T.D. , Labadio , R.P. and De Bio , P.A.F. 1991 . Vander Berg, B . Immunomodulatory activity of three Sri Lankan medicinal plants used in hepatic disorders. Journal of Ethanopharmacology , 33 : 63 – 66 .
- Ramakrishnamacharya, A.; Krishnaswamy , M.K. , Rao , R.R. and Vishwanathan , S. 1995 . Clinical studies on anti rhuematic activity of Melothria maderaspatana. Seminar on research in Ayurveda and Siddha , 36 CCRAS, New Delhi .
- Hemamalini , K. and Varma , VK. 2007 . Antimicrobial activity of methanolic leaves extract of Melothria maderaspatana Linn . Pharmacologyonline , 3 : 323 – 326 .
- Hemalatha , S. , Wahi , A.K. , Singh , P.N. and Chansouria , J.P.N. 2010 . Evaluation of anti-hyperglycemic and free radical scavenging activity of Melothria maderaspatana Linn. in streptozotocin-induced diabetic rats. African Journal of Pharmacy and Pharmacology , 4 : 817 – 822 .
- Pandey , D. , Pandey , S. and Hemalatha , S. 2010 . Hypolipidemic activity of aqueous extract of Melothria maderaspatana . Pharmacologyonline , 3 : 76 – 83 .
- Iman , R.A. , Lakshmi Priya , B. , Chithra , R. , Shalini , K. , Sharon , V. , Chamundeeswari , D. and Vasantha , J. 2006 . In vitro antiplatelet activity-guided fractionation of aerial parts of Melothria maderaspatana . Indian Journal of Pharmaceutical Sciences , 68 : 668 – 670 .
- Raja , B. , Kaviarasan , K. , Arjunan , M.M. and Pugalendi , K.V. 2007 . Effect of Melothria maderaspatana leaf-tea consumption on blood pressure, lipid profile, anthropometry, ibrinogen, bilirubin, and albumin levels in patients with hypertension . Journal of Alternative and Complementary Medicine , 13 : 349 – 354 .
- Trease , G.E. and Evans , W.C. 1983 . Pharmacognosy, 14th Bailiere Tindall and Co , London .
- Johansen , D.A. 1940 . Plant Microtechnique , 1st , 182 – 203 . New York , London : McGraw Hill Book Co .
- Anonymous . 1996 . Pharmacopoeia of India, 2 , 4th , A-53 – A-55 . New Delhi , India : Government of India, Ministry of Health, Controller of Publication .
- Kokaski , C.J. , Kokaski , R.J. and Slama , F.J. 1949 . Fluorescence of powdered vegetable drug under ultraviolet radiation . Journal of American Pharmaceutical Association , 47 : 715 – 717 .
- Khandelwal , K.R. 2007 . Practical Pharmacognosy, Techniques and Experiments , 17th , 149 – 156 . India : Nirali Prakashan: Pune .
- Hagerman , A. , Harvey-Muller , I. and Makkar , H.P.S. 2000b . Quantification of Tannins in Tree Foliage—A Laboratory Manual; , 4 – 7 . Vienna : FAO/IAEA .
- Kumaran , A. and Karunakaran , J. 2007 . In vitro antioxidant activities of methanol extracts of five Phyllanthus species from India . LWT–Food Sciences and Technology , 40 : 344 – 352 .
- Shanmugakumar , S.D. , Amerjothy , S. and Balakrishna , K. Pharmacognostical . 2006 . antibacterial and antifungal potentials of the leaf extracts of Pithecellobium dulce Benth. Pharmacognosy Magazine , 2 : 0973 – 1296 .
- Anonymous . 2002 . Quality Control Methods for Medicinal Plant Materials , 28 – 30 . Delhi-51 : WHO, Geneva; A.I.T.B.S. Publishers & Distributors Regd .
- Yildiz , G. , Vatan , Ö. , Çelikler , S. and Dere , S. 2011 . Determination of the phenolic compounds and antioxidative capacity in Red Algae Gracilaria bursa-pastoris . International Journal of Food Properties , 14 : 496 – 502 .
- Raczkowska , J. , Mielcarz , G. , Howard , A. and Raczkowski , M. 2011 . UPLC and spectrophotometric analysis of polyphenols in wines available in the polish market . International Journal of Food Properties , 14 : 514 – 522 .
- Gabor , M. 1979 . “ Anti-inflammatory drugs ” . In Handbook of Experimental Pharmacology , 68 New York : Springer .
- Crespy , V. , Morand , C. , Besson , C. , Manach , C. , Demigne , C. and Remesy , C. 2002 . “ Quercetin, but not its glycosides, is absorbed from the rat stomach ” . In Journal of Agricultural and Food Chemistry50 618 – 621 .