Abstract
The acetone and methanol extracts of barks of A. leucophloea, A. ferruginea, A. dealbata, and A. pennata were evaluated for their total phenolics and flavonoid contents and antioxidative activities. Acetone extracts of barks exhibited higher contents of total phenolics and flavonoids. Further, the acetone extracts showed higher activity in DPPH•, ABTS•+, and OH•; FRAP; metal chelation; phosphomolybdenum reduction; and peroxidation inhibition. In conclusion, the results implied that the barks could be considered as health supplements and nutraceuticals/functional foods.
Keywords:
INTRODUCTION
Oxidative stress, the consequence of the imbalance between prooxidants and antioxidants in an organism, is considered to play a very important role in the pathogenesis of several degenerative diseases, such as diabetes, cancer, and cardiovascular diseases. Free radicals are capable of damaging crucial biomolecules such as nucleic acids, lipids, proteins, polyunsaturated fatty acids and carbohydrates and may cause DNA damage that can lead to mutations.[Citation1] Lipid oxidation is of great concern to the food industry because it leads to the development of undesirable off-flavors and potentially toxic reaction products.[Citation2] The products of lipid oxidation are known to be health hazards since they are associated with aging, membrane damage, heart disease and cancer. The addition of antioxidants is effective in retarding the oxidation of lipids and lipid containing foods. Antioxidants are micro-constituents present in the diet that can delay or inhibit lipid oxidation by inhibiting the initiation or propagation of oxidizing chain reactions.[Citation3] Plant-derived antioxidants and their potential to decrease the risk of diseases caused by oxidative stress are of particular interest.[Citation4] Potential sources of natural antioxidants are being searched in different types of plant materials such as vegetables, fruits, leaves, oilseeds, cereal crops, tree barks, roots, spices, and herbs. The antioxidant phenolic compounds are not evenly distributed in plant parts; they are present at elevated amounts in barks and many cultures use it for centuries.
Acacia leucophloea (Roxb.) Willd. (A. alba Willd., Mimosa alba Rottler, M. leucophloea Roxb); Acacia ferruginea DC. (Mimosa ferruginea Roxb.); Acacia dealbata Link. and Acacia pennata (L.) Willd. (A. pluricapitata Steud., A. polycephala Graham, A. tomentella Zippel ex Spanoghe., Mimosa pennata L., M. torta Roxb.) belonging to the family Mimosaceae are known as versatile source of components with bioactive properties. The leaves, twigs, roots, gum, barks, flowers, and pods of Acacia species are widely used as indigenous drugs in various ailments. Especially, the bark is traditionally used for treatment of several diseases in ayurvedic system of medicine. Bark of Acacia species contains tannin and the seeds contain fatty oil. Apart from this, a number of flavonoids have been isolated from the heart wood and bark of A. auriculiformis.[Citation5] Leaves of A. salicinia exhibited significant antioxidant and antimutagenic effects.[Citation6] The free radical scavenging and antioxidant potential of ethyl acetate, acetone and methanol extracts/fractions of bark of A. auriculiformis have been established.[Citation7–9 Citation Citation9 While, A. nilotica leaves and barks are powerful antioxidant,[Citation10, Citation11] the chemoprevention of N-nitrosodiethylamine-induced hepatocarcinogenesis by polyphenolics from A. nilotica bark were examined.[Citation12] The stem bark of A. leucophloea is used to treat inflammation, bronchitis, cough, biliousness, skin diseases, leucoderma, pruritus, erysipelas, vomiting, wounds, ulcers, diarrhoea, dysentery, stomatitis, intermittent fevers, leprosy, and toothache. A. ferruginea bark is considered useful for treating itching, leucoderma, ulcers, stomatitis, and diseases of the blood.[Citation13] A. dealbata yields a gum, resembling gum arabic, used in bronchial troubles,[Citation14] while A. pennata bark is used to treat diseases of the blood, biliousness, bronchitis, and asthma.[Citation13]
In view of the above facts, barks of A. leucophloea, A. ferruginea, A. dealbata, and A. pennata were investigated for the presence of components with antioxidant activity to be applied in food system. The present study is the first comparative report on the in vitro antioxidant properties of barks of Acacia species, which is a unique natural source possessing strong antioxidant substance. Owing to these properties, the above tree barks can be used as natural source of dietary antioxidants and nutraceuticals.
MATERIALS AND METHODS
Chemicals
2,2′-Azinobis(3-ethylbenzothiozoline-6-sulfonic acid) diammonium salt (ABTS•+), 2,2-diphenyl-1-picrylhydrazyl (DPPH•), 2,4,6-tripyridyl-S-triazine (TPTZ), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), 3-(2-pyridyl)-5,6-bis(4-phenyl-sulfonic acid)- 1,2,4-triazine (Ferrozine), and linoleic acid were purchased from Sigma-Aldrich (Bangalore, India). Potassium persulfate, butylated hydroxyanisole (BHA), ferrous chloride, hydrogen peroxide, trichloroacetic acid (TCA), sodium thiosulfate, potassium iodide, Tween 40, and ferric chloride were obtained from Himedia (Mumbai, India). Ethylenediamine tetraacetic acid (EDTA) disodium salt was purchased from Merck (Mumbai, India). All other reagents used were of analytical grade.
Preparation of Extracts
Fresh barks of A. leucophloea, A. ferruginea, A. dealbata, and A. pennata were collected from Coimbatore, Tamil Nadu state, India during the month of June 2009. The plants were authenticated and herbarium specimens deposited in the Botany Herbarium, Bharathiar University with voucher numbers: BUBH-6140, BUBH-6141, BUBH-6142, and BUBH-6143, respectively. The plant materials were washed thoroughly in tap water, shade dried at room temperature (25○C), powdered, and used for solvent extraction.
The plant samples were successively extracted with petroleum ether (for disposing lipid and pigments), acetone and methanol using soxhlet apparatus. Each time before extracting with the next solvent, the material was dried in hot air oven at 40○C. The solvents were evaporated using a rotary vacuum-evaporator (RE300, Yamato, Tokyo, Japan) at 50°C and the remaining water was removed by lyophilization (4KBTXL-75, VirTis Benchtop K, NY, USA). The extract recovery in different solvents was expressed as percent of the plant sample dry matter. The freeze-dried extracts thus obtained were dissolved in the respective solvents at the concentration of 1 mg/ml and used for assessment of antioxidant capacity through various chemical assays.
Determination of Total Phenolic and Flavonoid Contents
The total phenolic content of acetone and methanol extracts of barks of Acacia species was determined by Folin ciocalteu method. The amount of total phenolics and tannins were calculated as gallic acid equivalents (GAE) as described by Siddhuraju and Becker.[Citation15] The total flavonoid content was determined by the method described previously by Zhishen, et al.[Citation16] and expressed as gram of rutin equivalent (RE) per 100 grams of extract.
Ferric-Reducing/Antioxidant Power (FRAP) Assay
The antioxidant capacity of acetone and methanol extracts of Acacia species was estimated according to the method described previously by Pulido et al.[Citation17] FRAP reagent (900 μl), prepared freshly and incubated at 37○C, was mixed with 90 μl of distilled water and 30 μl of test sample or methanol (for the reagent blank). The test samples and reagent blank were incubated at 37○C for 30 min in a water bath. The final dilution of the test sample in the reaction mixture was 1/34. The FRAP reagent contained 2.5 mL of 20 mmol/l TPTZ solution in 40 mmol/l HCl plus 2.5 ml of 20 mmol/l FeCl3.6H2O and 25 ml of 0.3 mol/l acetate buffer (pH 3.6). At the end of incubation the absorbance readings were taken immediately at 593 nm, using a spectrophotometer. Methanolic solutions of known Fe (II) concentration, ranging from 100 to 2000 μmol/l (FeSO4.7H2O), were used for the preparation of the calibration curve. The absorbance of the reaction mixture was read at 593 nm. The values are expressed as mmol Fe (II) per milligram extract.
Antioxidant Activity by the ABTS•+ Assay
The total antioxidant activity of the acetone and methanol extracts of Acacia species was measured by ABTS radical cation decolorization assay according to the method of Re et al.[Citation18] ABTS•+ was produced by reacting 7 mM ABTS aqueous solution with 2.4 mM potassium persulfate in the dark for 12–16 h at room temperature. Prior to assay, this solution was diluted in ethanol (about 1:89 v/v) and equilibrated at 30○C to give an absorbance at 734 nm of 0.700 ± 0.02. The stock solution of the sample extracts were diluted such that after introduction of 10 μl aliquots into the assay, they produced between 20 and 80% inhibition of the blank absorbance. After the addition of 1 ml of diluted ABTS•+ solution to 10 μl of sample or Trolox standards (final concentration 0–15 μM) in ethanol, absorbance was measured at 30○C exactly 30 min after the initial mixing. Appropriate solvent blanks were also run in each assay. Triplicate determinations were made at each dilution of the standard, and the percentage inhibition was calculated of the blank absorbance at 734 nm and then was plotted as a function of Trolox concentration. The unit of total antioxidant activity is defined as the concentration of Trolox having equivalent antioxidant activity expressed as μmol/g extracts.
Metal Chelating Activity
The chelating of ferrous ions by acetone and methanol extracts of bark extracts of Acacia species was estimated by the method described by Dinis et al.[Citation19] Briefly the extract samples (100 μl) were added to a solution of 2 mM FeCl2 (0.05 ml). The reaction was initiated by the addition of 5 mM ferrozine (0.2 ml) and the mixture was shaken vigorously and left standing at room temperature for 10 min. Absorbance of the solution was measured spectrophotometrically at 562 nm. The chelating activity of the extracts was evaluated using EDTA as standard. The results were expressed as mg EDTA equivalent/g extract.
Phosphomolybdenum (AEAC) Assay
The antioxidant activity of acetone and methanol extracts of Acacia species was evaluated by the green phosphomolybdenum complex formation according to the previously described method of Prieto et al.[Citation20] An aliquot of 100 μl of sample solution (in 1 mM dimethyl sulphoxide) was combined with 1 ml of reagent solution (0.6 M sulphuric acid, 28 mM sodium phosphate, and 4 mM ammonium molybdate) in a 4-ml vial. The vials were capped and incubated in a water bath at 95○C for 90 min. After the samples had cooled to room temperature, the absorbance of the mixture was measured at 695 nm. The results reported are mean values expressed as grams of ascorbic acid equivalents/100 g extract.
Free Radical Scavenging Activity on DPPH•
The DPPH • radical scavenging activity of different bark extracts of Acacia species was measured according to the method of Blois.[Citation21] IC50 values of the extract, i.e., concentration of extract necessary to decrease the initial concentration of DPPH by 50%, were calculated.
Hydroxyl Radical Scavenging Activity
The scavenging activity of acetone and methanol extracts of Acacia species on hydroxyl radical activity was measured according to the previously described method.[Citation22] Various concentrations (20, 40, and 60 μg) of extracts were added with 1.0 ml of iron-EDTA solution (0.13% w/v ferrous ammonium sulfate and 0.26% w/v EDTA), 0.5 ml of EDTA solution (0.018% w/v), and 1.0 ml of dimethyl sulphoxide (DMSO) (0.85% w/v in 0.1 M phosphate buffer, pH 7.4). The reaction was initiated by adding 0.5 ml of ascorbic acid (0.22%) and incubated at 80–90°C for 15 min in a water bath. After incubation the reaction was terminated by the addition of 1.0 ml of ice-cold TCA (17.5% w/v). Three milliliters of Nash reagent (75.0 g of ammonium acetate, 3.0 ml of glacial acetic acid, and 2 ml of acetyl acetone were mixed and raised to 1 L with distilled water) were added and left at room temperature for 15 min. The reaction mixture without sample was used as control. The intensity of the color formed was measured spectroscopically at 412 nm against reagent blank. The hydroxyl radical scavenging activity of the sample extracts was evaluated as % of antioxidant activity.
The β-Carotene/Linoleic Acid Antioxidant Activity
The β-carotene/linoleic acid antioxidant activity of the antioxidant (bark extracts, BHA, or α-tocopherol, 200 μl) solution was measured according to the previously described method.[Citation23] One milliliter of a β-carotene solution in chloroform (1 mg/10 ml) was pipetted into a flask containing 20 mg of linoleic acid and 200 mg of Tween 40. The chloroform was removed by rotary vacuum evaporator at 45○C for 4 min, and 50 ml of oxygenated distilled water was added slowly to the semi-solid residue with vigorous agitation to form an emulsion. A 5-ml aliquot of the emulsion was added to a tube containing 200 μl of the antioxidant (extracts, BHA, or α-toc) solution at 1 mg/ml concentration and the absorbance was measured at 470 nm immediately against a blank consisting of the emulsion without β-carotene. The tubes were placed in a water bath at 50○C and the absorbance was monitored at 15 min intervals until 180 min. The antioxidant activity of the bark extracts and standard was evaluated as % of antioxidant activity
Statistical Analysis
The data were subjected to a one-way analysis of variance (ANOVA) and the significance of the difference between means was determined by Duncan's multiple range test (P < 0.05) using Statistica software (Statsoft Inc., Tulsa, OK, USA). Values expressed are means of three replicate determinations ± standard deviation.
RESULTS AND DISCUSSION
Recovery Percent, Total Phenolics, and Flavonoid Contents
In the present study, the percent yield, total phenolics and flavonoid contents obtained from acetone and methanol extracts of barks of four Acacia species are shown in . The maximum recovery percentage was recorded in acetone extract of A. leucophloea (11.6%), while A. dealbata gave the least extractive value (2.6%) in the same solvent. Among the different bark extracts, the acetone extract of A. leucophloea and A. ferruginea were found to contain a comparable and a higher amount of total phenolics (78.9 g GAE/100 g extract and 78.0 g GAE/100 g extract, respectively). The methanol extract of A. dealbata contained a lower amount of total phenolics (46.5 g GAE/100 g extract) when compared to other extracts. Whereas, in the level of flavonoid content, the acetone extracts of A. leucophloea (13.40 g RE/100 g extract) followed by A. ferruginea (11.70 g RE/100 g extract) were higher than those of other extracts of barks. Accordingly, the highest yields of total phenolics and flavonoids per 100 g dry powder were realized for the acetone extract of A. leucophloea. Similarly, high total phenolics and flavonoid contents of different Acacia species, such as A. confusa, A. auriculiformis, A. salicina, and A. nilotica, have been reported.[Citation7,Citation10,Citation11,Citation24] Some reports also indicated that the Acacia species contains a variety of bioactive components, such as gallic acid, ellagic acid, isoquercitin, leucocyanadin kaempferol-7-diglucoside, naringenin-glucopyranoside, rutin, derivatives of (+)-catechin-5-gallate, apigenin-6,8-bis-C-glucopyranoside, m-catechol, and their derivatives.[Citation7] The results of the present study clearly indicate that phenolics and flavonoids are important components of Acacia species, and their free radical scavenging and antioxidant effects could be attributed to the presence of these valuable constituents.
Table 1 Extract yield percentage, total phenolics, and flavonoid contents of acetone and methanol extracts of barks of A. leucophloea, A. ferruginea, A. dealbata, and A. pennata
Ferric-Reducing/Antioxidant Power (FRAP) Assay
The FRAP assay measures the ability of antioxidants to reduce the TPTZ–Fe (III) complex to the intensely blue colored TPTZ–Fe (II) complex in acidic medium. Antioxidant potential of the acetone and methanol extracts of barks of Acacia species are expressed as concentration of substances having ferric-TPTZ reducing ability equivalent to that of 1 mmol concentration of Fe (II). The data on the ferric reducing potential of the extracts of Acacia species presented in indicate that acetone extract of A. leucophloea resulted in the highest FRAP activity (2953 mmol Fe (II)/g extract). Methanol extract of A. pennata bark (1812.8 mmol Fe (II)/g extract) was found to have the least ferric reducing/antioxidant power among all extracts. All the other extracts registered moderate FRAP activity. Further, a significant correlation exists between total phenolic content and FRAP (R 2 = 0.680) and between total flavonoid content and FRAP (R 2 = 0.752) (). These results suggest that the acetone and methanol extracts of Acacia species have the ability to donate electrons and thus could scavenge free radicals. Since the extracts from various barks have the ability to scavenge free radicals thereby preventing lipid oxidation via a chain breaking reaction, they could serve as potential nutraceuticals when ingested along with nutrients. Extensive investigations on the antiradical and antioxidant activities of small phenolics, including flavonoids and phenolic acids, have been reported.[Citation25]
Table 2 FRAP, ABTS, metal chelating, and phosphomolybdenum activities of acetone and methanol extracts of barks of A. leucophloe a, A. ferruginea, A. dealbata, and A. pennata
Table 3 Correlation between total phenolics and flavonoid contents and antioxidant capacities
ABTS•+ Radical Scavenging Activity
ABTS •+ , a protonated radical, has characteristic absorbance maxima at 734 nm, which decreases with the scavenging of the proton radicals. ABTS•+ was generated by incubating ABTS with potassium persulfate. The efficacy of acetone and methanol extracts of Acacia species on ABTS radical cation scavenging activity is presented in . Though all the samples exhibited strong ABTS radical scavenging activity, the acetone extract of A. leucophloea bark registered higher activity (63,359 μmol/g) followed by A. ferruginea (57,914 μmol/g). Further, a significant (P < 0.05) correlation exists between total phenolics and the total antioxidant capacity and (R 2 = 0.703) and between total flavonoids and total antioxidant capacity (R 2 = 0.901) (). Rice-Evans et al.[Citation26] have made a similar observation that phenolic compounds play a major role in scavenging the free radicals.
Metal Chelating Activity
Presence of transition metal ions in a biological system could catalyze the Haber–Weiss and Fenton-type reactions, resulting in generation of hydroxyl radicals (OH•). However, these transition metal ions could form chelates with the antioxidants, which result in the suppression of OH• generation and inhibition of peroxidation processes of biological molecules. In this assay, acetone and methanol extracts of barks of Acacia species interfered with the formation of ferrous and ferrozine complex, suggesting that they have chelating activity and can capture ferrous ion before ferrozine. The relative chelating effects on the ferrous ions by acetone and methanol extracts of barks of Acacia species are shown in . The metal scavenging capacity of 100 μg/ml doses of the tested extracts of Acacia species were found to be in the range of 3.2–8.0 mg EDTA/g extract. Generally, the acetone extracts of bark possessed higher metal chelating ability when compared to methanol extracts of bark. In the present study, the Acacia extracts showed significant correlations between total phenolics and metal chelating activity (R 2 = 0.806) and flavonoids and metal chelating activity (R 2 = 0.695) (). Grinberg et al.[Citation27] also suggested that the protective activity of tea polyphenols against OH• dependent salicylate hydroxylation was as a result of iron chelation. It is reported that chelating agents are effective as secondary antioxidants because they reduce the redox potential, thereby stabilizing the oxidized form of the metal ion. On the other hand, literature on the adverse effects of polyphenols on iron bioavailability have emphasized that the binding of iron to the flavonoid antioxidant can suppress the accessibility of the iron to the oxygen molecules.
Phosphomolybdenum Assay
Acetone and methanol extracts of Acacia species were also used to determine their antioxidant capacities by the formation of green phosphomolybdenum complex. The formation of the complex at 95°C was measured by the intensity of absorbance of the reaction mixture at the extract concentration of 100 μg/ml as shown in . The phosphomolybdenum method is based on the reduction of Mo(VI) to Mo(V) by the antioxidant compound and the formation of green phosphate/Mo(V) complex with the maximal absorption at 695 nm. The assay was successfully used to quantify vitamin E in seeds,[Citation20] and being simple and independent of other antioxidant measurements commonly employed, the assay was extended to plant polyphenols. The experimental data reveals that all these extracts are likely to have the potential of phosphomolybdenum reduction. The phosphomolybdenum reduction potential of various sample extracts were in the order of: ALA > AFA > APA > ADA > ALM > ADM > AFM > APM and the respective values were found to be 95.8 > 93.0 > 92.5 > 91.7 > 90.1 > 84.1 > 75.9 > 74.8 g ascorbic acid equivalents/100 g of the extract. A moderate level of correlation exists between total phenolic content and phosphomolybdenum reduction (R 2 = 0.573) and total flavonoid content and phosphomolybdenum reduction (R 2 = 0.485) (). In the ranking of the antioxidant capacity obtained by this method, acetone extract of the studied Acacia species showed better and comparable antioxidant capacity. The differential response of extracts in various antioxidant assay systems may be explained by the fact that the transfer of electrons/hydrogen from antioxidants occur at different redox potential in various assay systems and the transfer also depends on the structure of the antioxidants.
DPPH• Radical Scavenging Activity
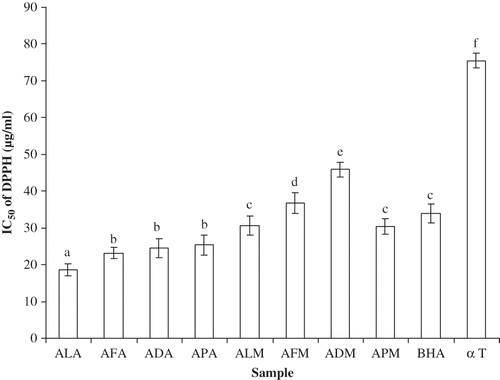
DPPH• radical scavenging activity has been widely used to evaluate the antioxidant activity of plant extracts and foods.[Citation28] The antioxidants in the sample extracts react with DPPH, which is a stable free radical, and convert it to 1,1-diphenyl-2-(2,4,6- trinitrophenyl) hydrazine. The degree of discoloration indicates the scavenging potentials of the antioxidant compounds which can be detected spectrophotometrically at 517 nm. Concentration of the sample necessary to decrease initial concentration of DPPH• by 50% (IC50) under the experimental condition was calculated. Therefore, a lower value of IC50 indicates a higher antioxidant activity. shows the DPPH radicals scavenging capacity of the extracts along with BHA and α-tocopherol as references. All the bark extracts exhibited appreciable DPPH radical scavenging activity ranging from IC50 18.6 μg/ml to IC50 45.9 μg/ml. When compared to the standard BHA (IC50 33.9 μg/ml), all extracts showed higher radical scavenging activity with the exception of methanol extract of A. ferruginea and A. dealbata (IC50 36.7 μg/ml and IC50 45.9 μg/ml, respectively). In the present test also, a significant correlation exists between total phenolics and DPPH• scavenging activity (R 2 = 0.735) and between total flavonoids and DPPH• scavenging activity (R 2 = 0.538) (). This suggests that the barks contain more phenolics that may be attributed to the antioxidant properties of Acacia species. Manian et al.[Citation29] also reported that the strong DPPH scavenging activity of tea could be attributed in part to the tea catechins and some low molecular polyphenols.
Figure 1 DPPH• radical scavenging activity of acetone and methanol extracts of barks of A. leucophloea, A. ferruginea, A. dealbata, and A. pennata. ALA: A. leucophloea acetone extract; AFA: A. ferruginea acetone extract; ADA: A. dealbata acetone extract; APA: A. pennata acetone extract; ALM: A. leucophloea methanol extract; AFM: A. ferruginea methanol extract; ADM: A. dealbata methanol extract; APM: A. pennata methanol extract; BHA: butylated hydroxyanisole; α T: α-tocopherol. Values are means of three replicate determinations (n = 3) ± standard deviation. Bars having different letters are significantly different (P < 0.05).
Hydroxyl Radical Scavenging Activity
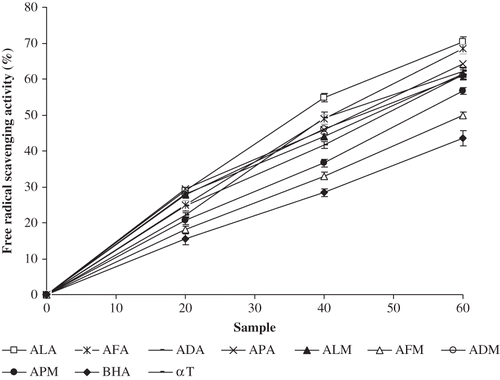
The hydroxyl radical is an extremely reactive free radical formed in biological systems and has been implicated as a highly damaging species in free radical pathology, capable of damaging almost every molecule found in living cells.[Citation30] This radical has the capacity to cause strand breakage in DNA, which contributes to carcinogenesis, mutagenesis, and cytotoxicity. Thus, removing OH • is very important for the protection of living systems. In the present study, the hydroxyl radical scavenging activity is estimated by generating the hydroxyl radicals using an ascorbic acid-iron EDTA system. The scavenging abilities of the bark extracts of Acacia species are shown in . All the extracts exhibited hydroxyl radical scavenging activity in a dose-dependent manner (20–60 μg). However, among them, acetone extract of A. leucophloea (70.3%) registered the highest hydroxyl radical scavenging activity at 60 μg concentration in the reaction mixture, which is higher than the standard BHA (43.6%) and α-tocopherol (62.1%). The hydroxyl radical scavenging activity by the extracts shows significant correlation (P < 0.05) with their total phenolics (R 2 = 0.531) and flavonoid contents (R 2 = 0.679) (). The ability of the above mentioned extracts to quench hydroxyl radicals seems to be directly related to the prevention of propagation of lipid peroxidation and seems to be a good scavenger of active oxygen species.
Figure 2 Hydroxyl radical scavenging activity of acetone and methanol extracts of barks of A. leucophloea, A. ferruginea, A. dealbata, and A. pennata. ALA: A. leucophloea acetone extract; AFA: A. ferruginea acetone extract; ADA: A. dealbata acetone extract; APA: A. pennata acetone extract; ALM: A. leucophloea methanol extract; AFM: A. ferruginea methanol extract; ADM: A. dealbata methanol extract; APM: A. pennata methanol extract; BHA: butylated hydroxyanisole; α T: α-tocopherol. Values are means of three replicate determinations (n = 3) ± standard deviation.
β-Carotene Bleaching Assay
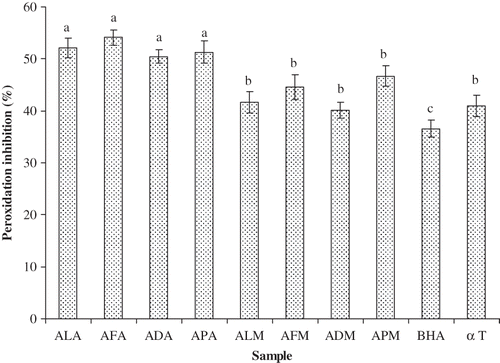
In the β-carotene bleaching assay, oxidation of linoleic acid releases linoleic acid peroxide as free radicals that oxidize β-carotene resulting in discoloration, thus decreasing absorbance value.[Citation31] A linear relationship was found between the ability of the sample extract to inhibit oxidation and antioxidant capacity. In the present study, antioxidant capacity of acetone and methanol extracts of Acacia species were in the range of 40.1–54.1% () at a concentration of 200 μg in the final reaction mixture. Interestingly, all the bark extracts registered significantly higher antioxidant capacity than positive controls like BHA and α-tocopherol. The peroxidation inhibition by the bark extracts shows a moderate level of correlation to their total phenolics (R 2 = 0.401) and flavonoid (R 2 = 0.494) contents (). This indicates that the presence of antioxidant extracts may hinder the extent of β-carotene bleaching by acting on the free radicals formed in the system.
Figure 3 β-carotene/linoleic acid peroxidation inhibition activity of acetone and methanol extracts of barks of A. leucophloea, A. ferruginea, A. dealbata, and A. pennata. ALA: A. leucophloea acetone extract; AFA: A. ferruginea acetone extract; ADA: A. dealbata acetone extract; APA: A. pennata acetone extract; ALM: A. leucophloea methanol extract; AFM: A. ferruginea methanol extract; ADM: A. dealbata methanol extract; APM: A. pennata methanol extract; BHA: butylated hydroxyanisole; α T: α-tocopherol. Values are means of three replicate determinations (n = 3) ± standard deviation. Bars having different letters are significantly different (P < 0.05).
CONCLUSION
The present study demonstrated for the first time that the acetone and methanol extracts from barks of investigated Acacia sp.: A. leucophloea, A. ferruginea, A. dealbata, and A. pennata, exhibited outstanding antioxidant activity as measured by various antioxidant assays. Appreciable amounts of total phenolics and flavonoids were found in the analyzed bark extracts. Among the all bark extracts, antioxidant and free radical scavenging activity was well established in acetone extract, comparable with, or in some cases better than, that of the widely used antioxidants BHT and α-tocopherol. The results of the present study would certainly help to ascertain the potency of the crude extracts of different barks as a potential source of natural antioxidants. However, further research is needed to identify individual components forming an antioxidative system and develop their application for food and pharmaceutical industries.
REFERENCES
- Tohma , H.S. and Gulç , I. 2010 . Antioxidant and radical scavenging activity of aerial parts and roots of turkish liquorice (Glycyrrhiza glabra l.) . International Journal of Food Properties , 13 : 657 – 671 .
- Maillard , M.N. , Soum , M.H. , Boivin , P. and Berset , C. 1996 . Antioxidant activity of barley and malt: Relationship with phenolics content . Journal of Food Science and Techology , 29 : 238 – 244 .
- Tyug , T.S. , Johar , M.H. and Ismail , A. 2010 . Antioxidant properties of fresh, powder, and fiber products of mango (Mangifera foetida) fruit . International Journal of Food Properties , 13 : 682 – 691 .
- Okmen , B. , Sigva , H.O. , Mutlu , S. , Doganlar , S. , Yemenicioglu , A. and Frary , A. 2009 . Total antioxidant activity and total phenolic contents in different Turkish eggplant (Solanum melongena L.) . cultivars. International Journal of Food Properties , 12 : 616 – 624 .
- Uniyal , S.K. , Badoni , V. and Sati , O.P. 1992 . A new triterpenoidal saponin from Acacia auriculiformis . Journal of Natural Products , 55 : 500 – 502 .
- Bouhlel , I. , Mansour , H.B. , Limema , I. , Sghaier , M.B. , Mahmouda , A. , Chibani , J.B. , Ghedira , K. and Ghedira , L.C. 2007 . Screening of antimutagenicity via antioxidant activity in different extracts from the leaves of Acacia salicina from the center of Tunisia . Environmental Toxicology and Pharmacology , 23 : 56 – 63 .
- Singh , R. , Singh , S. , Kumar , S. and Arora , S. 2007 . Evaluation of antioxidant potential of ethyl acetate extract/fractions of Acacia auriculiformis A. Cunn . Food and Chemical Toxicology , 45 : 1216 – 1223 .
- Singh , R. , Singh , S. , Kumar , S. and Arora , S. 2007 . Studies on antioxidant potential of methanol extract/fractions of Acacia auriculiformis A . Cunn. Food Chemistry , 103 : 505 – 511 .
- Singh , R. , Singh , S. , Kumar , S. and Arora , S. 2007 . Free radical-scavenging activity of acetone extract/fractions of Acacia auriculiformis A . Cunn. Food Chemistry , 103 : 1403 – 1410 .
- Sultana , B. , Anwar , F. and Przybylski , R. 2007 . Antioxidant activity of phenolic components present in barks of Azadirachta indica, Terminalia arjuna, Acacia nilotica, and Eugenia jambolana Lam. trees . Food Chemistry , 104 : 1106 – 1114 .
- Kalaivani , T. and Mathew , L. 2010 . Free radical scavenging activity from leaves of Acacia nilotica (L.) Wild. ex Delile, an Indian medicinal tree . Food Chemical Toxicology , 48 : 298 – 305 .
- Singh , B.N. , Singh , B.R. , Sarma , B.K. and Singh , H.B. 2009 . Potential chemoprevention of N-nitrosodiethylamine-induced hepatocarcinogenesis by polyphenolics from Acacia nilotica bark . Chemico Biological Interactions , 181 : 20 – 28 .
- Parrotta , J.A. 2001 . Healing Plants of Peninsular India , 323 – 324 . New York, NY, USA : CABI Publishing .
- Prajapati , N.D. 2005 . Agro's Dictionary of Medicinal Plants , 3 Jodhpur , , India : Agrobios, Agro House .
- Siddhuraju , R. and Becker , K. 2003 . Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic orgins of Drumstick tree (Moringa olifera Lam.) leaves . Journal of Agricultural and Food Chemistry , 51 : 2144 – 2155 .
- Zhishen , J. , Mengcheng , T. and Jianming , W. 1999 . The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals . Food Chemistry , 64 : 555 – 559 .
- Pulido , R. , Bravo , L. and Saura-Calixto , F. 2000 . Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay . Journal of Agricultural and Food Chemistry , 48 : 3396 – 3402 .
- Re , R. , Pellegrini , N. , Proteggente , A. , Pannala , A. , Yang , M. and Rice-Evans , C. 1999 . Antioxidant activity applying an improved ABTS radical cation decolorization assay . Free Radical Biology and Medicine , 26 : 1231 – 1237 .
- Dinis , T.C.P. , Madeira , V.M.C. and Almeida , L.M. 1994 . Action of phenolic derivatives (acetoaminophen, salycilate and 5-aminosalycilate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers . Archives of Biochemistry and Biophysics , 315 : 161 – 169 .
- Prieto , P. , Pineda , M. and Aguilar , M. 1999 . Spectrophotometric quantitative of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E . Analytical Biochemistry , 269 : 337 – 341 .
- Blois , M.S. 1958 . Antioxidant determinations by the use of a stable free radical . Nature , 26 : 1199 – 1200 .
- Klein , S.M. , Cohen , G. and Cederbaum , A.I. 1991 . Production of formaldehyde during metabolism of dimethyl sulphoxide by hydroxyl radical generating system . Biochemistry , 20 : 6006 – 6012 .
- Taga , M.S. , Miller , E.E. and Pratt , D.E. 1984 . Chia seeds as a source of natural lipid antioxidants . Journal of American Oil Chemist's Society , 61 : 928 – 931 .
- Tung , Y.T. , Wub , J.H. , Huang , C.Y. , Kuo , Y.H. and Chang , S.T. 2009 . Antioxidant activities and phytochemical characteristics of extracts from Acacia confusa bark . Bioresource Technology , 100 : 509 – 514 .
- Heim , K.E. , Tagliaferro , A.R. and Bobilya , D.J. 2002 . Flavonoid antioxidants: Chemistry, metabolism, and structure-activity relationships . Journal of Nutritional Biochemistry , 13 : 572 – 584 .
- Rice-Evans , C.A. , Miller , N.M. and Paganda , G. 1996 . Structure–antioxidant activity relationships of flavonoids and phenolic acids . Free Radical Biology and Medicine , 20 : 933 – 956 .
- Grinberg , L.N. , Newmark , H. , Kitrossky , N. , Rahamim , E. , Chevion , M. and Rachmilewitz , E. 1997 . Protective effects of tea polyphenols against oxidative damage to red blood cells . Biochemical Pharmacology , 54 : 973 – 975 .
- Soares , J.R. , Dinis , T.C.P. , Cunha , A.P. and Almedia , L.M. 1997 . Antioxidant activities of some extracts of Thumus zygis . Free Radical Research , 26 : 469 – 478 .
- Manian , R. , Anusuya , N. , Siddhuraju , P. and Manian , S. 2008 . The antioxidant and free radical scavenging potential of two different solvent extracts of Camellia sinensis (L.) O. Kuntz, Ficus bengalensis L. and Ficus racemosa L . Food Chemistry , 107 : 1000 – 1007 .
- Lee , J.C. , Kim , H.R. , Kim , J. and Jang , Y.S. 2002 . Antioxidant property of an ethanol extract of the stem of Opuntia ficus-indica var. Saboten . Journal of Agricultural and Food Chemistry , 50 : 6490 – 6496 .
- Talcott , S.T. , Howard , L.R. and Brenes , C.H. 2000 . Antioxidant changes and sensory properties of carrot puree processed with or without periderm tissue . Journal of Agricultural and Food Chemistry , 48 : 1315 – 1321 .