Abstract
In prevalent conditions, fresh-caught fish were held on ice until storage at optimal temperatures. The aim of this study was to investigate and confirm biogenic amines formation and microbiological quality of crayfish during 2 days post-catch icing and 90 days frozen storage. Of the considered biogenic amines in fresh crayfish, puterscine and cadaverine were detected and initial concentrations of them were 5.33 and 50.57 μg/g of flesh, respectively. Psychrotrophs and cadaverine were the major bacteria and biogenic amines detected in crayfish at all sampling stages, respectively. At the end of ice storage, samples had higher biogenic amines and bacterial load when compared with fresh samples (P < 0.05). During the first 30 days of frozen storage, simultaneous with slight changes of biogenic amines, bacterial load significantly decreased (P < 0.05), but as frozen storage time lengthened, progressive development of biogenic amines and microbial load (except for Pseudomonas spp.) was observed. The best correlation was for psychrotrophic with histamine (r = 0.82). At the end of storage, although final values of bacterial load were very negligible, total BAs (487.03 μg/g), especially histamine (110.22 μg/g) exceeded the proposed tolerable maximum levels for total biogenic amines (300 μg/g) and histamine (50 μg/g). It could be concluded that crayfish can be hazardous after 60 days.
INTRODUCTION
Seafood is a rich source for a large number of nutritive and important components that are necessary for human. The high amount of long-chain polyunsaturated fatty acids of the n-3 series, the well-balanced content of essential amino acids, and the good digestibility of fish protein due to low amounts of connective tissue, are some examples of the many benefits that seafood offers.[Citation1,Citation2]
On the other hand, seafood products are extremely perishable food commodities and it is well established that during storage, several factors contribute to post-mortem deterioration of seafood products’ quality.[Citation3,Citation4] Deterioration of fish (either for marine or freshwater products), occurs mainly because of enzymatic and microbial activities, which lead to loss of quality and spoilage.[Citation5,Citation6]
Biogenic amines (BAs), such as putrescine, cadaverine, histamine, and tyramine, are biosynthesized at low level in fish and crustaceans cells as nonvolatile compounds. These compounds function mainly as neurotransmitters and neuromodulators in the nervous system, where in some occasions they serve as circulating neurohormones as well. However, in larger quantities biogenic amines are likely to arise via microbial decarboxylation of amino acids. Presence of these compounds in fish and fish products could be related to spoilage and might result in health problems.[Citation7−Citation11] The amount and type of produced amines are influenced by the food composition, microbial flora, and several other parameters, which might promote bacterial growth during storage, including temperature, ripening, and packaging.[Citation12]
The importance of biogenic amines analysis has been well established due to their potential toxicity and effects on fish flavor,[Citation13] fishmeal production,[Citation14] and possible use of them as quality markers in fish and fish products.[Citation15−Citation17] Biogenic amines, such as histamine and tyramine, are considered as antinutritional compounds and one of the more common reasons for food intoxication.[Citation18] In sensitive individuals, they represent a health risk, especially if their effects are enhanced by other substances.[Citation19,Citation20] Hygienic condition during handling and cold storage during transportation could affect the final quality of seafood.[Citation21,Citation22]
Biogenic amines production have been reviewed extensively in some fish species, such as rainbow trout,[Citation9,Citation23,Citation24] carp,[Citation2] Atlantic herring,[Citation17] wild European eel,[Citation25] seer fish,[Citation26] cod,[Citation27] wild white grouper,[Citation28] and sea bass.[Citation25] After harvesting, it is common to hold fishes on ice until storage at optimal temperature and the time elapsing between fish harvesting and arrival at technological process (consumption, frozen storage, etc.) may vary from 8 h up to 2 days (personal communication). To the best of our knowledge, there is yet insufficient data on the biogenic amines production and bacterial changes of fish and crustaceans during short-term post-catch icing (transportation with ice) and frozen storage. In this study, producing biogenic amines and bacterial changes of crayfish (Astacus leptodactylus) during 2 days post-catch icing and 90 days frozen storage were investigated with an emphasis on a potential quality index and the correlation of such changes under the same conditions with each other.
MATERIALS AND METHODS
Preparation of Fish and Experimental Procedure
Adult crayfish (A. leptodactylus) were caught in October–November 2010 from the Aras Dam Lake (West Azerbaijan, Iran). Live crayfish were immediately immersed into ice water to be killed. Individuals with similar weight of 45 ± 5 g were kept in ice during transportation to the laboratory of Artemia and Aquatic Animals Research Institute (West Azerbaijan, Urmia, Iran). Less than 6 h post-harvesting six numbers of ice-stored crayfish were selected and analyzed immediately at day 0 (zero). The remaining ones were covered with ice and stored for 2 days. The ice-to-crayfish ratio was 3:1 (w/w).[Citation9] After 2 days, ice-stored samples were separately packed in Zip-Lok plastic bags, then transferred to a freezer and kept at −24°C for 90 days. Samples were randomly dissected by hand with a sterile scalpel to get their tail muscles and analyzed in triplicate at time intervals of 0 (or fresh), 1 and 2 days of ice storage, and 30, 60, and 90 days of freezing storage. Before analysis, thawing of samples was performed in a refrigerator (Electro-Steel, Tehran, Iran) at 4 ± 1°C for approximately 7 h.
Standards and Reagents
All used chemicals and solvents were of analytical and chromatographic grade. Standard amines, namely, tryptamine hydrochloride, putrescine dihydrochloride, and cadaverine dihydrochloride were obtained from Sigma (St. Louis, MO, USA) and histamine dihydrochloride was purchased from Merck (Darmstadt, Germany). Benzoyl chloride (for synthesis), HCl, 7-diaminoheptane, NaCl, NaHCO3, dansyl chloride and NaOH were extra pure and all of them have been purchased from Merck (Darmstadt, Germany).
Preparation of Sample Extracts
Samples for analyses were prepared by acid extraction and derivatization was done by slightly modifying the method of Moret and Conte.[Citation29] Initially, 20 mL of 0.1 M hydrochloric acid containing a known amount of 1, 7-diaminoheptane (internal standard) were added to approximately 10 g of crayfish tail muscle in a centrifuge tube and homogenized in a Politron homogenizer (Kinematica, Lucerne, Switzerland) for 2 min. The suspension was centrifuged at 12,000× g for 20 min at 4°C. Then, the supernatant was recovered and the residue was extracted for the second time as mentioned above. Both aqueous extracts were mixed and diluted up to 50 mL with 0.1 m HCl. Following the dilution, in order to an extraction with organic solvent (butanol), immediately 5 mL of acid extract, with three portions of 5 mL butanol were mixed in a test tube. The organic extracts were saturated with NaCl and pH was adjusted to 11.5 with NaOH.
Derivatization of Sample Extracts and Standards
Derivatization of the samples was done by mixing two drops of 1 M hydrochloric acid and 1 mL of organic extract. Then samples evaporated under vacuum (LABOROTA 4003 Heidolph instruments) and1 mL of 0.1 M hydrochloric acid, 500 μL saturated solution of NaHCO3, and 1 mL dansyl chloride solution (5 mg mL−1) were added to the residue. The reaction vessel was transferred to an incubator and kept at 40°C under agitation for 60 min. Immediately following the incubation, the solution was dried under vacuum. The residue was dissolved in 500 μl of acetonitrile. The solution was filtered (VARIAN, Bond Elut C18) and injected into the high-performance liquid chromatography (HPLC). A gradient elution program shown in was used. The mobile phase consisted of two solvent reservoirs: (i) acetonitrile and (ii) water, and its flow-rate was 0.8 mL min−1. The peaks were detected at 254 nm. Samples were analyzed in triplicate and reported values were expressed in μg g−1 of crayfish flesh.
Table 1 HPLC program elution for the quantification of biogenic amines analysis in crayfish (A. leptodactylus)
Biogenic amines were analyzed by a high performance liquid chromatography (HPLC) equipped with a Wellchrom HPLC pump, K-1001 (KNAVER Germany), dynamic mixing chamber, degasser (KNAVER), Wellchrom solvent organizer K-1500 (KNAVER), UV-detector K-2501 (KNAVER), Autosampler Triathlon type 900, and a personal computer running the software Chromegate. Separation and quantification of biogenic amines was performed using an EC 150 × 4.6 NUCLEODUR C18 Gravity 5 μm HPLC column (Silica for powerful LC separation, MN, Germany).
Biogenic Amine Index (BAI) Calculation
The biogenic amine index was calculated according to the procedures proposed by Veciana-Nogues et al.[Citation19] The biogenic amine index was obtained by the following formula:
Microbiological Analyses
Five grams of tail flesh of sampled crayfish were taken aseptically and transferred to 45 ml sterile physiological saline solution (0.8% sodium chloride) and then homogenized for 3 min with a Politron homogenizer (Kinematica, Lucerne, Switzerland). The homogenized sample was decimally diluted using the same diluent (1:10, by vol.). For microbial enumeration, 0.1 ml of each dilution was spread on the surface of the sterile plates. King agar medium was used for enumeration of psychrotrophic bacteria, incubated at 21°C for 48 h.[Citation30] Pseudomonas spp. was enumerated on cetrimide agar, after incubation for 48 h at 37°C.[Citation31] For the mesophilic viable count enumeration, a 1.0-mL sample was inoculated into 10 mL of molten (45°C) nutrient agar. After setting, a 10-mL overlay of molten medium was added, and then incubated for 48 h at 37°C.[Citation32] All data were expressed as a multiple of 103 CFU/g and performed in triplicate.
Statistical Analysis
All data are represented as the mean ± standard deviation. Before the statistical analysis, all data were tested for normality and homogeneity of variances by Kolmogorov–Smirnov and Leven tests, respectively, and then subjected to one-way analysis of variance (ANOVA). Tukey’s test was used to determine the differences in the mean values. Differences were considered significant at the P < 0.05 level. Linear regression was used to determine correlation of biogenic amines with bacterial counts. All statistical tests were done using the statistical software for Windows SPSS, version 18.0.
RESULTS AND DISCUSSION
Biogenic Amines Concentration
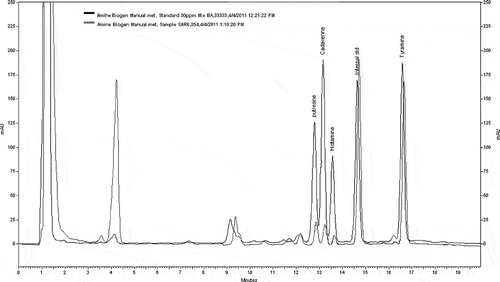
The reliability was evaluated by a recovery test on six samples, and indicates the results of the recovery test. Meanwhile, the representative HPLC chromatogram and peak identification recorded for mixer of standard biogenic amines and an extract of crayfish sample is shown in . All of the biogenic amines had recovery mean values ranging from 65 to 88%. Our results are in agreement with Moret and Conte[Citation29] who reported that recovery results in salmon and tuna were in the range from 65–84%, and 68–84%, respectively.
Table 2 Mean recoveries of biogenic amines from crayfish (A. leptodactylus)
Figure 1 HPLC chromatogram and peak identification recorded for mixer of standard biogenic amines and an extract of crayfish (A. leptodactylus).
The changes of biogenic amines during post-catch icing and frozen storage of crayfish are shown in . Out of the studied biogenic amines in fresh crayfish, puterscine and cadaverine were detected and initial concentrations of them were 5.33 and 50.57 μg/g of flesh, respectively. Meanwhile, histamine and tyramine were detected at the 2nd day of ice storage (). In most of the previous investigations, tyramine and histamine were not detected in fresh fishes.[Citation9,Citation26,Citation33] According to Martínez-Álvarez et al.[Citation34] and Mietz and Karmas,[Citation33] putrescine and cadaverine were the main biogenic amines formed during chilled storage of shrimp and lobster. Similar low histamine and tyramine levels have been detected by Martínez-Álvarez et al.[Citation34] and López-Caballero et al.[Citation35] in Norwegian lobsters and pink shrimp.
Table 3 Biogenic amines concentration (μg/g flesh)A in crayfish (A. leptodactylus) during post-catch icing and frozen storage
At the end of the ice storage, samples had higher BAs when compared with fresh samples (P < 0.05; ). In agreement with our results, in trout,[Citation9] wild white grouper,[Citation28] European hake,[Citation36] seer fish,[Citation26] European catfish,[Citation37] barramundi,[Citation38] and wild sea bass,[Citation25] all of the biogenic amines showed an increasing trend during ice storage, under the same conditions. López-Caballero et al.[Citation35] also reported that during shrimp storage, increasing of biogenic amines was observed.
Although, in general, progressive development of biogenic amines was observed during frozen storage, biogenic amines concentration did not undergo significant changes until the end of the first 30 days of frozen storage and no significant change in tyramine level was observed until the 60th day of frozen storage (P > 0.05; ). Our results are in agreement with those reported by Ben-Gigirey et al.[Citation39] for albacore flesh, who reported that during the 90 days of frozen storage (−25°C) of albacore flesh, concentration of biogenic amines were increased.
Among the studied biogenic amines, histamine is potentially hazardous.[Citation40] It is worth noting that in this study the final amount of histamine was 110.22 mg/kg, which is two times higher than the allowable limit (50 mg/kg) suggested by the US Food and Drug Administration for scombroid fish and related products.[Citation41] It is important to note that the histamine level for the crayfish samples reached this level before 90 days of frozen storage (). Meanwhile, crayfish samples had lower levels of tyramine (57.37 mg/kg) below the safe level (100 mg/kg) for human health suggested by ten Brink et al.[Citation42] and Santos.[Citation43]
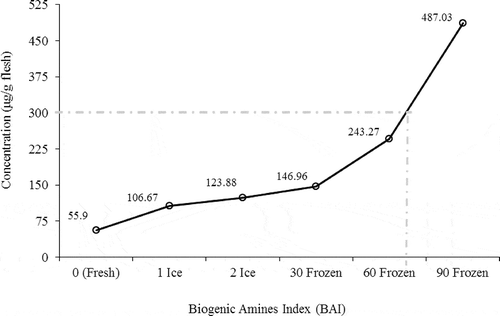
The relative content of the biogenic amines index (BAI) in crayfish muscle during post-catch icing and frozen storage are shown in . The biogenic amines content of fish samples depends on fish species, free amino acid content, gut contents at death, and also with the season of harvesting.[Citation26] As shown in Fig. 2, a remarkable increase of BAI value was observed at the 60th day of frozen storage, where counts of psychrotrophic, Pseudomonas spp. bacteria, and total mesophilic count were 4.73, 2.22, and 3.09 × 103 CFU/g, respectively (). Increasing of total biogenic amines was probably due to the action of previously released decarboxylating enzymes.[Citation39] The European Community (EC) has suggested that a maximum of 300 μg/g for total biogenic amines in fish and fish products may be an appropriate legal limit.[Citation44] In the present study, the total biogenic amines level for the crayfish samples exceeded from this level after the 60th day of frozen storage ().
Table 4 Bacterial changes (103 CFU/g flesh)A of crayfish (A. leptodactylus) during post-catch icing and frozen storage
Microbiological Characteristics
indicates the bacterial changes of crayfish flesh during post-catch icing and frozen storage. The initial counts of psychrotrophic and total mesophilic were about 4.71 and 3.19 × 103 CFU/g flesh, respectively (). According to the results, psychrotrophic bacteria were the dominant bacteria in fresh crayfish and this was followed by mesophilic bacteria, whereas Pseudomonas spp. was not detected (). Our results support the hypothesis of Cann,[Citation45] who reported that the bacterial flora of temperate shrimp is largely psychrotrophic. It is widely accepted that the initial microbial load of freshwater fish varies depending on water conditions and temperature.[Citation24] According to the ICMSF,[46] most aquatic animals at the time of harvesting have in the region of 102–105 CFU/g bacterial count. Moreover, initial microbial load of mainly freshwater prawn was at range of 102–106 CFU/g[Citation47–Citation49] and these results were in line with our observation.
As shown in , although during ice storage, a significant increase was only found for Pseudomonas spp., at the end of ice storage, samples had a higher total bacterial count in comparison with fresh samples (P < 0.05). Our results were in agreement with Chen et al.[Citation50] who reported that both aerobic and facultative bacteria were increased rapidly within the first 2 days during storage of red claw crayfish at 2°C. Also, according to Lalitha and Surendran,[Citation49] a slow rise in bacterial counts noticed between 0 and 3 days in ice stored freshwater prawn.
On the other hand, during the first 30th days of frozen storage, reduction of bacterial load was observed (P < 0.05; ) which might be due to the cold shock during storage under freezing condition. After this time, slightly increasing was observed in psychrotrophic and mesophilic bacteria until the 60th day (P > 0.05; ). Then, although Pseudomonas spp. was not detected, mesophilic and psychrotrophic increased and reached to 3.89 and 5.59 × 103 CFU/g on the 90th day of frozen storage, respectively (P < 0.05; ). Emire and Gebremariam[Citation51] reported that the total bacterial load and total coliforms of Nile Tilapia during frozen storage decreased in the fish samples until the 60th day of storage, but started to increase on the 75th day of storage.
Microbiological spoilage reactions in seafood depend on the initial composition or fish species, original environment, the waters (marine or fresh waters) that those organisms live, storage conditions, and molting stage in crustacean.[Citation52−Citation56] It has been reported that the maximum bacteria counts that indicate good quality of fish during storage period, are 7 log CFU/g.[46] It can be noticed that based on the results of the present study, final bacterial counts were not reached to critical maximum counts of total bacteria.
It is well established that free amino acids play a very important role in bacterial growth and spoilage of fish.[Citation57,Citation58] The best linear regression relationship for psychrotrophic and histamine and tyramine values was found as following (r ∼ 0.82):
CONCLUSIONS
Based on the results of this study, biogenic amines levels and bacterial loads (mesophilic and specially psychrotrophic counts) increased during storage of crayfish. At the end of ice storage, samples had higher total biogenic amines and bacterial counts in comparison with fresh samples, so complementary techniques must be incorporated during the post-catch chilled storage of crayfish. To sum up, although final values of bacterial load were very negligible and did not represent any warning of crayfish decomposition, the total biogenic amines content, especially histamine, were exceeded from the acceptable level proposed by EU after the 60th day of frozen storage. Therefore, after this time, samples were not considered as safe. Results might indicate that there is a need for procedures to reduce biogenic amines of crayfish during harvesting, icing, and frozen storage periods. Although biogenic amines data of the study might suggest that the shelf-life of crayfish during 2 days post-catch icing and 90 days frozen storage was approximately a few days more than the 60th day, it seems that to evaluate the death time of crayfish, the combination of lipid oxidation changes with the organoleptic and nutritional quality needs more investigation.
ACKNOWLEDGMENTS
This work has been carried out in the Artemia and Aquatic Animals Research Institute with the financial support of the Urmia University as part of a research project (Grant No: 010/A/87). The authors wish to thank Messrs. Dr. Zakaria Vahabzade, Dr. Porya Malek Khatabiand, and Mrs. Maryam Rohi for their excellent technical assistance.
REFERENCES
- Hyldig, G. Sensory descriptors. In: Handbook of Seafood and Seafood Products Analysis; Nollet, L.M.L.; Toldra, F.; Eds.; Taylor & Francis: New York, NY, 2009; 481–498.
- Křížek, M.; Vacha, F.; Vorlova, L.; Lukasova, J.; Cupakova, S. Biogenic amines in vacuum-packed and non-vacuum packed flesh of Carp (Cyprinus carpio) stored at different temperatures. Food Chemistry 2004, 88, 185–191.
- Pirini, M.; Gatta, P.P.; Testi, S.; Trigari, G.; Monetti, P.G. Effect of refrigerated storage on muscle lipid quality of sea bass (Dicentrarchus labrax) fed on diets containing different levels of vitamin E. Food Chemistry 2000, 68 (3), 289–293.
- Abedian-Kenari, A.; Regenstein, J.M.; Hosseini, S.V.; Rezaei, M.; Tahergorabi, R.; Nazari, R.M.; Mogaddasi, M.; Kaboli, S.A. Amino acid and fatty acid composition of Cultured Beluga (Huso huso) of different ages. Journal of Aquatic Food Product Technology 2009, 18 (3), 245–265.
- Arashisar, S.; Hisar, O.; Kaya, M.; Yanik, T. Effects of modified atmosphere and vacuum packaging on microbiological and chemical properties of rainbow trout (Oncorhynchus mykiss) fillets. International Journal of Food Microbiology 2004, 97, 209–214.
- Liston, J. Microbiology in fishery science. In: Advances in Fish Science and Technology; Connell, J.J.; Eds.; Fishing News Books Ltd: Farnham, Surrey, England, 1980; 138–157.
- Joosten, H.M.L.J. The biogenic amine contents of Dutch cheese and their toxicological significance. Netherlands Milk and Dairy Journal 1988, 42 (1), 25–42.
- Sneddon, L.U.; Taylor, L.C.; Huntingford, F.A.; Watson, D.G. Agonistic behaviour and biogenic amines in shore crabs Carcinus maenas. Journal of Experimental Biology 2000, 203, 537–545.
- Rezaei, M.; Montazeri, N.; Langrudi, H.E.; Mokhayer, B.; Parviz, M.; Nazarinia, A. The biogenic amines and bacterial changes of farmed rainbow trout (Oncorhynchus mykiss) stored in ice. Food Chemistry 2007, 103, 150–154.
- Huss, H.H. Quality and Quality Changes in Freshwater Fish; Fisheries Technical Paper No. 348. Food and Agriculture Organization (FAO) of United Nations Press: Rome, 1995; 1–195.
- Rodrígueza, C.J.; Besteiro, I.; Pascual, C. Biochemical changes in freshwater rainbow trout (Oncorhynchus mykiss) during chilled storage. Journal of the Science of Food and Agriculture 1999, 79, 1473–1480.
- Halasz, A.; Barath, A.; Sarkadi, L.S.; Holzapfel, W. Biogenic amines and their production by microorganisms in food. Trends in Food Science and Technology 1994, 5, 42–49.
- Yuko, M.; Henmi, H.; Nishioka, F. Extractive components in the skeletal muscle from ten different species of scombroid fishes. Fisheries Science 1994, 60 (4), 473–478.
- Espe, M.; Haaland, H.; Njaa, L.R. Substitution of fish silage protein and a free amino acid mixture for fish meal protein in a chicken diet. Journal of the Science of Food and Agriculture 1992, 58 (3), 315–319.
- Lehane, L. Update on histamine fish poisoning. Medical Journal of Australia 2000, 173, 149–152.
- Eliassen, K.A.; Reistad, R.; Risoen, U.; Ronning, H.F. Dietary polyamines. Food Chemistry. 2002, 78 (3), 273–280.
- Özogul, F.; Taylor, K.D.A.; Quantick, P.; Ozogul, Y. Biogenic amines formation in Atlantic herring (Clupea harengus) stored under modified atmosphere packaging using a rapid HPLC method. International Journal of Food Science and Technology 2002, 37, 515–522.
- Slorach, S. A. Histamine in food. In: Handbook of Experimental Pharmacology; Veciana Noguęş, B.; Ed.; Berlin: Springer, 1991; 511–519.
- Veciana-Nogues, M.T.; Marine-Font, A.; Vidal-Carou, M.C. Biogenic amines as hygienic quality indicators of tuna. Relationships with microbial counts, ATP-related compounds, volatile amines, and organoleptic changes. Journal of Agricultural and Food Chemistry 1997, 45 (6), 2036–2041.
- Wu, M.L.; Yang, C.C.; Yang, G.Y.; Ger, J.; Deng, J.F. Scombroid fish poisoning: An overlooked marine food poisoning. Veterinary and Human Toxicology 1997, 39, 236–241.
- Silva, T.M.; Sabaini, P.S.; Evangelista, W.P.; Maria Beatriz, A.; Gloria, M.B.A. Occurrence of histamine in Brazilian fresh and canned tuna. Food Control 2011, 22, 323–327.
- Křǐžek, M.; Vacha, F.; Pelikánova, T. Biogenic amines in carp roe (Cyprinus carpio) preserved by four different methods. Food Chemistry 2011, 126 (3), 1493–1497.
- Dawood, A.A.; Karkalas, J.; Roy, R.N.; Williams, C.S. The occurrence of non-volatile amines in chilled-stored rainbow trout (Salmoirideus). Food Chemistry 1988, 27, 33–45.
- Chytiri, S.; Paleologos, E.; Savvaidis, I.; Kontominas, M.G. Relation of biogenic amines with microbial and sensory changes of whole and filleted freshwater rainbow trout (Oncorhynchus mykiss) stored on ice. Journal of Food Protection 2004, 67, 960–965.
- Özogul, F.; Özogul, Y. Biogenic amine content and biogenic amine quality indices of sardines (Sardina pilchardus) stored in modified atmosphere packaging and vacuum packaging. Food Chemistry 2006, 99, 574–578.
- Mohan, C.O.; Ravishankar, C.N.; Srinivasa Gopal, T.K.; Ashok Kumar, K.; Lalitha, K.V. Biogenic amines formation in seer fish (Scomberomorus commerson) steaks packed with O2 scavenger during chilled storage. Food Research International 2009, 42, 411–416.
- Lapa-Guimarães, J.; Trattner, S.; Pickova, J. Effect of processing on amine formation and the lipid profile of cod (Gadusmo rhua) roe. Food Chemistry 2011, 129, 716–723.
- Özogul, F.; Özogul, Y.; Kuley, E. Nucleotide degradation and biogenic amine formation of wild white grouper (Epinephelus aeneus) stored in ice and at chill temperature (4°C). Food Chemistry 2008, 108, 933–941.
- Moret, S.; Conte, L.S. High-performance liquid chromatographic evaluation of biogenic amines in foods. An analysis of different methods of sample preparation in relation to food characteristics. Journal of Chromatography 1996, 729, 363–369.
- Institute of Standards and Industrial Research of Iran (ISIRI). Enumeration of psychrotroph and psychrophilic microorganism in food. No. 2629. ISIRI: Karaj, Iran, 1991 (in Persian).
- Institute of Standards and Industrial Research of Iran (ISIRI). Meat and meat products- enumeration of Pseudomonas spp. No. 4791. ISIRI: Karaj, Iran, 2000 (in Persian).
- Institute of Standards and Industrial Research of Iran (ISIRI). Standard methods for preparation of food samples and enumeration of microorganisms in food. No. 356. ISIRI: Karaj, Iran, 1997 (in Persian).
- Mietz, J.L.; Karmas, E. Polyamine and histamine content of rockfish, salmon, lobster and shrimp as an indicator of decomposition. Journal of the Association of Official Analytical Chemists 1978, 61, 139–145.
- Martínez-Álvarez, Ó.; Gómez-Guillén, M.D.C.; Montero, P. Chemical and microbial quality indexes of Norwegian lobsters (Nephrops norvegicus) dusted with sulphites. International Journal of Food Science and Technology 2008, 43, 1099–1110.
- López-Caballero, M.E.; Huidobro, A.; Pastor, A.; Tejada, M. Microflora of gilthead seabream (Sparus aurata) stored in ice. Effect of Washing. European Food Research and Technology 2002, 215, 396–400.
- Rodrígueza, O.; Losada, V.; Aubourg, S.; Barros-Velázquez, J. Enhanced shelf-life of chilled European hake (Merluccius merluccius) stored in slurry ice as determined by sensory analysis and assessment of microbiological activity. Food Research International 2004, 37, 749–757.
- Özogul, F.; Kamari, N.; Kuley, E.; Özogul, Y. The effects of ice storage on inosine monophosphate, inosine, hypoxanthine, and biogenic amine formation in European catfish (Silurus glanis) fillets. International Journal of Food Science and Technology 2009, 44, 1966–1972.
- Bakar, J.; Yassoralipour, A.; Bakar, F.A.; Rahman, R.A. Biogenic amine changes in barramundi (Latescalcarifer) slices stored at 0°C and 4°C. Food Chemistry 2010, 119, 467–470.
- Ben-Gigirey, B; Vieites Baptista De Sousa, J.M.; Villa, T.G.; Barros-Velázquez, J. Changes in biogenic amines and microbiological analysis in albacore (Thunnus alalunga) muscle during frozen storage. Journal of Food Protection 1998, 61, 608–615.
- Arnold, S.H.; Brown, W.D. Histamine toxicity from fish products. Advances in Food Research 1978, 24, 113–154.
- USFDA (US Food and Drug Administration). Scombrotoxin (histamine) formation, fish and fishery products hazards and controls guide. Department of Health and Human Services, Public Health Service, Food and Drug Administration, Center for Food Safety and Applied Nutrition, Office of Seafood: Washington, 2002; 73–93.
- ten Brink, B.; Damink, C.; Joosten, H.M. Occurrence and formation of biological active amines in foods. International Journal of Food Microbiology 1990, 11 (1), 73–84.
- Santos, M.H.S. Biogenic amines: Their importance in foods. International Journal of Food Microbiology 1996, 29 (2–3), 213–231.
- European Commission. Council Directive 91/493/EC of 22 July 1991 laying down the health conditions for the production and the placing on the market of fishery products. Official Journal L 1991, 268, 15–34.
- Cann, D.C. Proceedings of tropical products institute conference on handling processing and marketing of tropical fish. In: Bacteriology of Shellfish with Reference to International Trade; Torry Research Station: London, U.K., 1977; 511 pp.
- International Commission on Microbiological Specifications for Foods (ICMSF). Sampling plans for fish and shellfish. In: Microorganisms in Foods. Sampling for Microbiological Analysis: Principles and Scientific Applications; ICMSF; Ed.; University of Toronto Press: Canada, 1986; 181–196.
- Angel, S.; Weinberg, Z.G.; Juven, B.J.; Lindner, P. Quality changes in freshwater prawn, Macrobrachium rosenbergii during storage in ice. Journal of Food Technology 1985, 20, 553–560.
- Leitào, M.F.F.; Rios, D.P.A. Microbiological and chemical changes in freshwater Prawn (Macrobrachium rosenbergii) stored under refrigeration. Brazilian Journal of Microbiology 2000, 31, 178–183.
- Lalitha, K.V.; Surendran, P.K. Microbiological changes in farm reared freshwater prawn (Macrobrachium rosenbergiide Man) in ice. Food Control 2006, 17, 802–807.
- Chen, G.; Xiong, Y.L.; Kong, B.; Newman, M.C.; Thompson, K.R.; Metts, L.S.; Webster, C.D. Microbiological and physicochemical properties of red claw crayfish (Cherax quadricarinatus) stored in different package systems at 2°C. Journal of Food Science 2007, 72 (8), 442–449.
- Emire, S.A.; Gebremariam, M.M. Influence of frozen period on the proximate composition and microbiological quality of Nile tilapia fish (Oreochromis niloticus). Journal of Food Processing and Preservation 2010, 34, 743–757.
- Huss, H.H. Control of indigenous pathogenic bacteria in seafood. Food Control. 1997, 8, 91–98.
- Gram, L.; Dalgaard, P. Fish spoilage bacteria-problems and solutions. Current Opinion in Biotechnology 2002, 13, 262–266.
- De Albuquerque-Cavalcanti, C.; García-Carreño, F.L.; Del Toro, M.A.N. Trypsin and trypsin inhibitors from Peaneid shrimp. Journal of Food Biochemistry 2001, 26, 233–251.
- Sharifian, S.; Zakipour, E.; Mortazavi, M.S.; Arshadi, A. Quality assessment of tiger tooth Croaker (Otolithes ruber) during ice storage. International Journal of Food Properties 2011, 14 (2), 309–318.
- Erkan, N. Sensory, chemical, and microbiological attributes of sea bream (Sparus aurata): Effect of washing and ice storage. International Journal of Food Properties 2007, 10, 421–434.
- Jay, J.M.; Kontou, K.S. Fate of free amino acids and nucleotides in spoiling beef. Applied Microbiology 1967, 15 (4), 759–764.
- Ingram, M.; Dainty, R.H. Changes caused by microbes in spoilage of meats. Journal of Applied Bacteriology 1971, 34 (1), 21–39.