Abstract
N, O-double (three methyl silane) trifluoro ethyl amide was used to derivatize polyphenols in apple pomace, and the resultant products were tested. The optimal derivatization conditions were then used in conjunction with the rapid analysis of polyphenols by gas chromatography-mass spectrometry. In the results, the following conditions for derivatization with N, O-double (three methyl silane) trifluoro ethyl amide were obtained : Polyphenols should be derivatized for 12 h at 37.5oC with a mass ratio of derivatization reagent to sample being 10 to 1. After analysis through gas chromatography-mass spectrometry, 24 compounds were identified, 11 of which were phenolic compounds and organic acids. Furthermore, four isomeric compounds of epicatechin were separated out. The method proved to be easy to use and sensitive, and could be used to analyze unknown phenolic compounds.
INTRODUCTION
Apple is one of the most widely consumed fruits and is propagated in global temperate regions,[Citation1] with China being the world’s largest apple producer. Apple pomace is the main by product during the processing of concentrated apple juice, accounting for 25–35% of the weight of dried apple.[Citation2,Citation3] It is a rich in source of carbohydrate, pectin, crude fiber, and minerals,[Citation4] simultaneously, and it can be treated as an excellent example of trash recycling.[Citation5]
In recent years, research has focused on the by-products of concentrated juice processing, in particular the processing and utilization of apple pomace,[Citation6,Citation7] where polyphenols are the main focus of attention.[Citation8−Citation12] Many pharmacological studies show that polyphenols have antioxidant, anticancer, anti-atherosclerosis, anti-hyperlipidemia, bacteriostasis, anticarious, and radiation protective properties.[Citation6,Citation13−Citation19] Phenolic compounds may also help prevent coronary heart disease and strokes as well as having other biological activities. Their antioxidant capacity is 50 times more than vitamin E and 20 times more than vitamin C.[Citation20,Citation21] Therefore, it is important to undertake analysis of the polyphenols found in apple pomace.
Most of the analysis of polyphenols have heavily relied on high-performance liquid chromatography (HPLC), mass spectrometry (MS), and capillary electrophoresis.[Citation22−Citation26] However, the HPLC-MS method is time-consuming which leads to a loss of analytes. The gas chromatography-mass spectrometry (GC-MS) method is simple, fast, and has high detection sensitivity, nevertheless, because of the strong polarity of polyphenols, sample derivatization processing needs to be done first, and the whole process requires complicated sample preparation. During the process, residual moisture in the extract is prone to peak tailing.[Citation27] In this study, with N, O-double (three methyl silane) trifluoro ethyl amide (BSTFA) as a derivatization reagent, combining GC-MC with traditional processing, a rapid method of determining polyphenols in apple pomace through parameter optimization and testing of analytical conditions was established.
MATERIALS AND METHODS
Instruments and Reagents
Instrument: Shimadzu 2010 GC-MS, Rtx-5 capillary column (30m × 0.32mm × 0.5μm). Reagents: derivatization reagent: BSTFA, containing 0.1% of trimethylchlorosilane) was purchased from the Regis Corporation; Ethanol (analytical grade) and macroporous resin NKA-9 were provided by Reagent Co., Ltd (Beijing, China). The apple pomace (Guoguang apple) was obtained from Yuantong Fruit Juice Factory in Yan-tai, Shang dong province, China, and vacuumized before analysis.
Polyphenols Extraction
Ethanol (extraction solvent, 60%) was mixed with apple pomace in a 1:6 solid:liquid ratio of pomace:ethanol(g ml−1). A 250 W ultrasonic processor was used for extraction at 60oC. Extraction time –30min; filtration-rotary evaporation to obtain crude polyphenol extract; purification by NKA-9 macroporous resin at a flow rate of 1.0 ml min−1; elution conditions: 70% ethanol concentration; elution flow rate of 1.0 ml min−1; elution volume – 10 BV; rotary evaporation; sample freeze-dried to obtain the polyphenol powder.[Citation28]
Polyphenols Derivatization
The method used to obtain the polyphenol derivatives: 0.1 g of the powder was put into a sample bottle together with the serum, to make up to a volume of 2–3 ml, after which the sample bottle was closed with a rubber cap. Ten to twenty times the volume BSTFA was added and the sample was shaken and left to stand for 20 min.
The GC-MS Analysis Conditions
Shimadzu 2010 GC-MS, Rtx-5 capillary column (30m × 0.32mm × 0.5μm). Split ratio 20:1; column temperature program settings: Column initial temperature was 100oC, heating rate was 10oC min−1, up to 300oC, maintained for 5 min, making a total analysis time of 25 min; high purity helium was used as a carrier gas flowing at a constant 1 ml min−1; interface temperature was 250oC, inlet temperature was 280oC, EI ion source temperature was 200oC; ionization voltagewas 70 eV with a multiplier voltage of 350 V; scan range was 33–500 m/z; scan speed was 0.5 s/time.
RESULTS AND DISCUSSION
Derivatization Reagent of Choice
In a previous study, polyphenols with high polarities that did not undergo the derivatization process before direct analysis with GC-MS produced fewer peaks and a lower number of peaks than were characteristic of polyphenols.[Citation20] Therefore, if the aim is to analyze polyphenols in apple pomace with GC-MS, then to obtain good peak effects, it is necessary to first use a derivatization treatment to reduce the sample polarity.
Pre-treatment of samples has the following advantages: (a) it increases the number of target samples and samples of volatile impurities and thus helps meet the requirements of GC; (b) it reduces the sample and the adsorption activity of the impurities in the sample (e.g., by removing active hydrogen atoms from the sample, thus changing the coordination properties between the sample and the stationary phase); (c) it allows more qualitative information to be obtained; (d) it improves the separation of compounds such as isomers and opticalisomers, which have similar properties.[Citation29−Citation31]
Commonly used derivative methods include the silane derivative method and the esterification and acylation method. When the samples contain hydroxyl (-OH), amino (-NH2), and thiol (-SH) groups, the silane method is usually used. To reduce errors caused by derivatives, derivatization reaction steps should be minimized in order to improve the speed.[Citation32−Citation35] Therefore, this study increased the testing time of silane as it is considered the strongest of the BSTFA derivatization reagents.
Optimizing BSTFA Derivatization
Choice of derivatization reagent
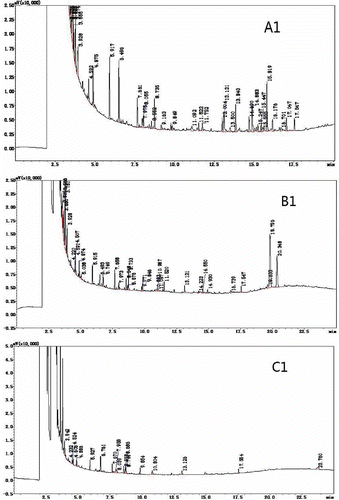
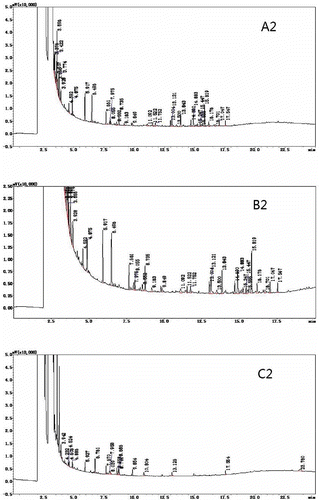
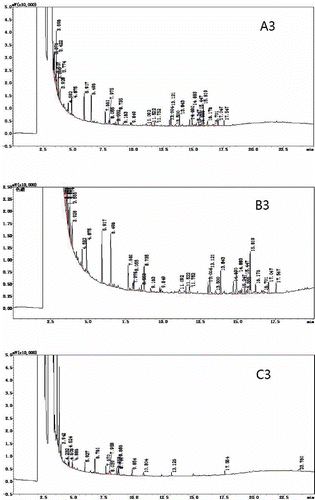
Because the sample matrix consumes some of the derivative agents added, an excess of the chosen derivative agent needs to be added in order to complete the reaction.[Citation36] In this experiment, a sample and derivatization reagent mass ratios of 1:10 and 1:20 were chosen for gas chromatographic analysis. shows that the number of peaks and the peak height in was significantly greater than seen in . The sample and derivatization reagent mass ratio of 1:10, produced superior phenolic derivatives, which indicated that an excess of the derivatization reagent had a certain impact on the peak separation of the sample.
Derivatization Reaction Temperature
The effect of temperature on the derivatization reaction was crucial. If the temperature was too low, the derivatization reaction could not proceed to completion. If the temperature was too high, a small quantity of the sample components would decompose.[Citation37−Citation39] Due to the highly volatile nature of BSTFA, the derivatization reaction temperature was kept relatively low. Therefore, the two temperatures chosen for the derivatization reaction and the gas chromatography analysis were 10 and 37.5°C. The results are shown in . When comparing and , it can be seen that temperature does not have an obvious effect on the BSTFA derivatization reaction. Samples subjected to 37.5°C showed slightly higher maximum peaks than those samples subjected to 10°C. This indicated that at 37.5°C, the BSTFA derivatization reaction was more complete.
Derivatization Reaction Time
Derivatives of the response must be completed, the reaction yield was not necessarily to 100%, but gave better reproducibility of measurements. The derivatives resulting from the reaction should be stable and not break down over time.[Citation40] Therefore, derivatization reaction times of 30 min and 12 h were chosen before the sample underwent GC analysis. The results are shown in , The spectra of the samples analyzed after 12 h were not significantly different from the samples analyzed after 30 min, but the result was slightly better than the 30 min response.
Qualitative Analysis and Composition of Polyphenols in Apple Pomace
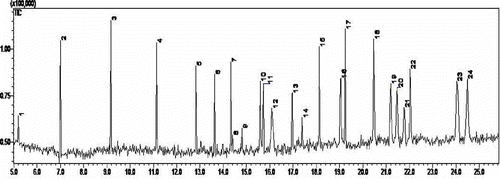
The apple pomace samples contained a large number of polyphenols with chiral carbon atoms, resulting in a large number of isomers. The physical and chemical properties, together with the chromatographic behavior of these isomers, were very similar to the apple isolate, which made it very difficult to identify the polyphenols present.[Citation41] The aim of the derivative pre-treatment was to improve the separation effect between these very similarisomers and optical isomer compounds. In this experiment, after derivatization by BSTFA, the polyphenols were qualitatively analyzed by GC-MS, shows the derivatives observed from the total ion chromatogram. According to Fig. 4, 24 sharp peak shape and intensity high-resolution spectra were observed with a retention time range of 5–25 min. Using MS, the results showed 24 substances including 11 phenols and organic acid species. The peaks numbered 19, 21, 23, and 24 were left as unidentified epicatechins by MS. However, after derivatization pre-treatment, the four epicatechin stereoisomers were separated out. The remaining 13 substances were of the siloxane class, which suggested that they may be derived from the excess reagent added. lists the phenolic compounds and organic acids found by the GC-MS analysis with a matching degree of 85–98%.
CONCLUSIONS
In this article, BSTFA derivatization with GC-MS was used to qualitatively analyze apple pomace polyphenols. It was found that the optimal pre-treatment conditions using BSTFA as derivatization reagent was a mass ratio of phenols to a derivative reagent of 1:10, a reaction derivative temperature of 37.5oC, and a reaction derivative time of 12 h. After using the above derivatization pre-treatment method, followed by GC-MS analysis, 24 sharp peak shape and intensity high-resolution spectra were observed, which included 11 phenolic compounds and organic acid species. Furthermore, four epicatechin isomers were well separated. These results suggest that this BSTFA derivatization method, followed by GC-MS to identify the polyphenols present in the apple pomace, was a simple and straightforward method with a low sample consumption and would therefore be less expensive. This method is ideal to test for polyphenolic compounds of unknown composition.
Table 1 Phenolic compounds and organic acids found in apple pomace
FUNDING
This work was supported by “The Fundamental Research Funds for the Central Universities of China” (No. BLYJ201203), and The National Natural Science Funds of China” (No. 31271981).
REFERENCES
- Kaushal, N.K.; Joshi, V.K.; Sharma, R.C. Effect of stage of apple pomace collection and the treatment on the physical-chemical and sensory qualities of pomacepapad (fruit cloth). International Journal of Food Science and Technology 2002, 39, 388–393.
- Gullon, B.; Falque, E.; Alonso, J.L.; Parajo, J.C. Evaluation of apple pomace as a raw material for alternative applications in food industries. Food Technology and Biotechnology 2007, 45, 426–433.
- Sun, J.; Hu, X.; Zhao, G.; Wu, J.; Wang, Z.; Chen, F.; Liao, X. Characteristics of thin layer infrared drying of apple pomace with and without hot air pre-drying. Food Science and Technology International 2007, 13, 91–97.
- Rachana, S.; Gupta, D.K. Utilization of pomace from apple processing industries: A review. Journal of Food Science and Technology 2010, 47, 365–371.
- Shah, G.H.; Masoodi, F.A. Studies on the utilization of wastes from apple processing plants. Indian Food Packer 1994, 48, 47–52.
- Moure, A.; Cruz, J.M.; Franco, D.; Domínguez, J.M.; Sineiro, J.; Domínguez, H.; Núñez, M.J.; Carlos Parajó, J. Natural antioxidants from residual sources. Food Chemistry 2001, 72, 145–171.
- Schieber, A.; Stintzing, F.C.; Carle, R. By-products of plant food processing as a source of functional compounds – recent developments. Trends in Food Science and Technology 2001, 12, 401–413.
- Schieber, A.; Hilt, P.; Streker, P.; Endress, H.U.; Rentschler, C.; Carle, R. A new process for the combined recovery of pectin and phenolic compounds from apple pomace. Innovative Food Science and Emerging Technologies 2003, 4, 99–107.
- Kammerer, D.R.; Carle, R. Current and emerging methods for the recovery, purification and fractionation of polyphenols from fruit and vegetable by-products. Fruit Processing 2007, 17, 89–94.
- Kammerer, D.; Claus, A.; Carle, R.; Schieber, A. Polyphenol screening of pomace from red and white grape varieties (Vitisvinifera L.) by HPLC-DAD-MS/MS. Journal of Agricultural and Food Chemistry 2004, 52, 4360–4367.
- Maier, T.; Goppert, A.; Kammerer, D.R.; Schieber, A.; Carle, R. Optimization of a process for enzyme-assisted pigment extraction from grape (Vitisvinifera L.) pomace. European Food Research and Technology 2008, 227, 267–275.
- Kammerer, D.R.; Carle, R.; Stanley, R.A.; Saleh, Z.S. Pilot-scale resin adsorption as a means to recover and fractionate apple polyphenols. Journal of Agricultural and Food Chemistry 2010, 58, 6787–6796.
- Kammerer, D.R.; Saleh, Z.S.; Carle, R.; Stanley, R.A. Adsorptive recovery of phenolic compounds from apple juice. European Food Research and Technology 2007, 224, 605–613.
- Wolfe, K.L.; Kang, X.; He, X.; Dong, M.; Zhang, Q.; Liu, R.H. Cellular antioxidant activity of common fruits. Journal of Agricultural and Food Chemistry 2008, 56, 8418–8426.
- Sembries, S.; Dongowski, G.; Mehrländer, K.; Will, F.; Dietrich, H. Dietary fiber–rich colloids from apple pomace extraction juices do not affect food intake and blood serum lipid levels, but enhance fecal excretion of steroids in rats. The Journal of Nutritional Biochemistry 2004, 15, 296–302.
- Ai, Z.L.; Wang, Y.H.; Taximaimaiti, M.; Wang, N.; Li, J.X. Study on effects of apple polyphenols on lead discharging in mice. Chinese Journal of Food Science 2007, 28, 468–470.
- Vidal, R.; Hernandez-Vallejo, S.; Pauquai, T.; Texier, O.; Rousset, M.; Chambaz, J.; Demignot, S.; Lacorte, J.M. Apple procyanidins decrease cholesterol esterification and lipoprotein secretion in Caco-2/TC7 enterocytes. Journal of Lipid Research 2005, 46, 258–268.
- Halliwell, B.; Gutteridge, J.M.; Aruoma, O.I. The deoxyribose method: A simple test-tube assay for determination of rate constants for reactions of hydroxyl radical. Analytical Biochemistry 1987, 165, 215–219.
- Yanagida, A.; Kanda, T.; Tanabe, M.; Matsudaira, F.; Oliveira-Cordeiro, J.G. Inhibitory effects of apple polyphenols and related compounds on cariogenic factors of mutans streptococci. Journal of Agricultural and Food Chemistry 2000, 48, 5666–5671.
- Cheng, C.Y.; Wang, Y.C.; Ding, W.H. Determination of triclosan in aqueous samples using solid-phase extraction followed by on-line derivatization gas chromatography-mass spectrometry. Analytical Sciences 2011, 27, 197–202.
- Bagchi, D.; Garg, A.; Krohn, R.L.; Bagchi, M.; Tran, M.X.; Stohs, S.J. Oxygen free radical scavenging abilities of vitamins C and vitamins E, and a grape seed proanthocyanidins extract in vitro. Research Communications in Molecular Pathology and Pharmacology 1997, 95, 179–189.
- Podsdek, A.; Wilska-Jeszka, J.; Anders, B.; Markowski, J. Compositional characterisation of some apple varieties. European Food Research and Technology 2000, 210, 268–272.
- Chinnici, F.; Gaiani, A.; Natali, N.; Riponi, C.; Galassi, S. Improved HPLC determination of phenolic compounds in Cv. Golden Delicious apples using a monolithic column. Journal of Agricultural and Food Chemistry 2004, 52, 3–7.
- Picinelli, A.; Sua, B.; Mangas, J.J. Analysis of polyphenols in apple products. European Food Research and Technology 1997, 204, 48–51.
- David, M.G.; Joe, Y.; Eric, N.; Eleftherios, P.D.; Alex, K.; George, S.; Andrew, L.W. A global survey of trans-resveratrol concentrations in commercial wines. American Journal of Enology and Viticulture 1995, 46, 159–165.
- Arce, L.; Tena, M.T.; Rios, A.; Valcarcel, M. Determination of trans-resveratrol and other polyphenols in wines by a continuous flow sample clean-up system followed by capillary electrophoresis separation. AnalyticaChimicaActa 1998, 359, 27–38.
- Garnier, N.; Richardin, P.; Cheynier, V.; Regert, M. Characterization of thermally assisted hydrolysis and methylation products of polyphenols from modern and archaeological vine derivatives using gas chromatography–mass spectrometry. Analytica Chimica Acta 2003, 493, 137–157.
- Luan, T.G.; Li, G.K.; Zhang, Z.X. Gas-phase post-derivatization following solid-phase micro extraction for rapid analysis of polyphenols in wine by gas chromatography-mass spectrometry. Acta Scientiarum Naturalium Universitatis Sunyatseni 2001, 40, 62–66.
- Thomas, W.M. Multi residue determination of acidic pesticides in water by HPLC-DAD with confirmation by GC-MS using conversion to the Methyl ester with Trimethylsilydi-azomethane. Journal of Chromatographic Science 2003, 41, 343–349.
- Nguyen, T.H.D.; Wang, X.C. Volatile, taste components, and sensory characteristics of commercial brand oyster sauces: Comparisons and relationships. International Journal of Food Properties 2012, 15, 518–535.
- Vagenas, G.; Roussis, I.G. Fat-derived volatiles of various products of cows’, ewes’, and goats’ milk. International Journal of Food Properties 2012, 15, 665–682.
- Utsumi, Y.; Miyazawa, M. Oxygen radical absorbance capacity of volatile oils from Japanese edible wild plants (diplazium squamigerum, laportea macrostachya, and vitis coignetiae). International Journal of Food Properties 2011, 14, 1090–1101.
- Kaftan, A.; Elmaci, Y. Aroma characterization of virgin olive oil from two Turkish olive varieties by SPME/GC/MS. International Journal of Food Properties 2011, 14, 1160–1169.
- Kaban, G. Volatile compounds of traditional Turkish dry fermented sausage (sucuk). International Journal of Food Properties 2010, 13, 525–534.
- Wang, L.; Li, J.; Pan, Z.F.; Zheng, X.S.; Lv, Z.X. Determination of ginkgolic acids in Ginkgo biloba leaves by gas chromatography with on-line pyrolyticmethylation. Chinese Journal of Chromatography 2008, 26, 731.
- Wang, L.; Qian, J.; Hu, Z. Determination of dihydroxyacetone and glycerol in fermentation broth by pyrolytic methylation/gas chromatography. AnalyticaChimicaActa 2006, 557, 262–266.
- Wang, H.D.; Chi, H.L.; Shi, O.P.; Ye, H. Analysis of chlorophenoxy acid herbicides in water by large-volume on-line derivatization and gas chromatography-mass spectrometry. Journal of Chromatography A 2000, 896, 111–116.
- Pan, Z.F.; Wang, L.L.; Mo, W.M.; Wang, C.; Hu, W.; Zhang, J.J. Determination of benzoic acid in soft drinks by gas chromatography with on-line pyrolytic methylation technique. Analytica Chimica Acta 2005, 557, 218–223.
- Xie, W.C.; Gu, X.H.; Luo, C.G.; Wang, G.; Tang, J. Study on the pyrolysis behavior of geranyl-beta-D-glucopyranoside by gas chromatography-mass spectrometry. Chinese journal of chromatography 2006, 24, 39–42.
- Osete-Cortina, L.; Domenech-Carbo, M.T. Analytical characterization of diterpenoid resins present in pictorial varnishes using pyrolysis-gas chromatography-mass spectrometry with on line trimethylsilylation. Journal of chromatography 2004, 1065, 265–278.
- Wang, L.L.; Zhou, Y.; Jia, Y.L.; Tang, C.L.; Zhou, H.J.; Pan, Z.F. Determination of multi-chemical constitutes for Ginkgo biloba leaves by gas chromatography with pyrolytic methylation. Journal of Zhejiang University of Technology 2010, 4, 15.