Abstract
The effect of lycopene, β-carotene, and γ-tocopherol on the oxidative stability of soybean triacylglycerol was evaluated under Schaal oven conditions at 60°C. Peroxide value, conjugated diene, hydroperoxides, and thiobarbituric acid reactive substances were used as indicators for primary and secondary lipid oxidation products, whereas Rancimat method was used to study the oil stability. Results indicated that lycopene and β-carotene acted as pro-oxidants, whereas γ-tocopherol acted as antioxidant in triacylglycerol. Pro-oxidative activity of lycopene was higher than β-carotene at 100 and 200 ppm levels under Schaal oven thermal conditions. The combination of lycopene and γ-tocopherol (1:2) acted as antioxidant with better efficiency than γ-tocopherol alone in inhibiting the hydroperoxide formation in triacylglycerols.
INTRODUCTION
Oxidative stability of oil is the resistance to oxidation during processing and storage.[Citation1] It is an important parameter to determine quality and shelf life of edible oils. Low-molecular weight off-flavor compounds, produced during oxidation, reduce consumer’s acceptability. Oxidation of edible oils is influenced by light, heat, fatty acids composition, metals, pigments, phospholipids, and antioxidants.[Citation2] Oxidation causes rancid odors, discoloration, nutritional loss, and harmful compound synthesis.[Citation2] Synthetic antioxidants, such as butylated hydroxyl anisole (BHA), butylated hydroxyl toluene (BHT), and tert-butyl hydroquinone (TBHQ) have been used from a long time to retard oxidative deterioration in fats and oils. However, synthetic antioxidants have been reported to have a toxic effect on experimental animals.[Citation3] The use of natural antioxidants is a better choice to preserve oil and oil-based products. Several compounds, including γ-tocopherol,[Citation4] rosemary extract,[Citation5] and phospholipid,[Citation6] have been found to be more effective than BHA and BHT in preventing oil oxidation.
The effect of carotenoids on lipid oxidation is important for the food processing industry. Carotenoids might act as antioxidants by quenching singlet oxygen or by reacting with free radicals or pro-oxidant. The antioxidant/pro-oxidant properties of carotenoids are affected by their concentration, oxygen partial pressure, and the nature of the environment. The mechanisms of reactions between carotenoids and radical species may involve radical addition, hydrogen abstraction, and electron transfer, but the precise antioxidant/pro-oxidant mechanisms remain unclear.[Citation7] Carotenoids impart color to the food and consumer preference for natural colors is increasing. The nutraceutical status of lycopene has accelerated research activities to improve functional, as well as sensory quality of food products. The objective of the present study was to evaluate the effects of carotenoids and γ-tocopherol on the oxidative stability of soybean triacylglycerol.
MATERIALS AND METHODS
Material
Refined soybean oil was procured from the local source. Lycopene, β-carotene, and γ-tocopherol standards were obtained from Sigma Chemical Co., St. Louis, MO, USA.
Preparation of Triacylglycerol
A chromatographic column (34 mm internal dia; 400 mm length) was connected to a vacuum pump and packed subsequently with four adsorbents.[Citation8] The bottom layer consisted of 40 g of activated silicic acid and the next layers were 20 g mixture of Celite: 514-activated charcoal (1:2 w/w) and 80 g of mixture of Celite: 545-sucrose (1:2 w/w) and the top layer was of 40 g of activated silicic acid. The column was pre-run with n-hexane until the solvent was recovered at the bottom. Refined soybean oil containing an equal volume of n-hexane was made to pass through the chromatographic column. The eluent was evaporated under vacuum at 30°C to obtain triacylglycerols (TAG).
Physico-Chemical Characteristics of Refined Oil and Triacylglycerol of Soybean
The specific gravity (SG), melting point, and refractive index (RI) using Abee´s Refractometer at 20°C, and color using lovibond (Model F the Tintometer Limited, Amesbury, England) were determined. Free fatty acid content (FFA; as % oleic acid), iodine value (IV; Wijs), peroxide value (PV; meq O2/kg oil), saponification number (SN) and the percentage of unsaponifiable matter of refined oil and TAG were determined according to the methods of AOAC.[Citation9]
Fatty Acid Profile of Refined Oil and Triacylglycerol of Soybean
Refined oil and triacylglycerol of soybean (200–500 mg) along with pentadecanoic acid (C15:0) as an internal standard were refluxed with 0.5N KOH for 3–5 min, added ammonium chloride-methanol-sulphuric acid mixture (ammonium chloride 2 g; methanol 60 mL; conc. sulphuric acid 3 mL) and refluxed for 18 min. Contents were cooled, petroleum ether was added (BP 40-60ºC), shaken, the ether layer was separated, and the ether under the vacuum evaporated.[Citation9] The residue was dissolved in 3–10 mL of petroleum ether and used for determining the fatty acids profile by a gas chromatograph (GC 14A Shimadzu, Japan) fitted with 30-meter (0.32 mmdia) glass capillary column coated with OMEGA Wax 320 and an flame ionisation detector. Identification of fatty acid methyl esters was based on comparison of retention times with authentic fatty acid methyl esters. The fatty acid composition was expressed as weight percent of the total fatty acid methyl ester.
Oxidative Stability Tests
The Schaal oven test was conducted by placing soybean TAG (20 gm) containing lycopene, β-carotene, γ-tocopherol, and lycopene/γ-tocopherol (2:1) into screw capped culture tubes (19 mm internal dia; 93 mm ht) in duplicate and then placed in a dark forced-air oven at 60°C. Samples were removed from the oven at 2 day intervals up to 11 days for analysis.[Citation10] The oxidative stability was evaluated by determining PV, thiobarbituric acid reactive substances (TBARS) and conjugated diene hydroperoxide values (CD).[Citation11] TBARS were calculated by multiplying the absorbance readings at 532 nm by a factor of 0.145, determined from a standard line prepared using 1,1,3,3-tetramethoxypropane as a precursor of malonaldehyde.
Metrohm Rancimat (Model 743, Herisau, Switzerland) was used to determine the oil stability index (OSI). A stream of filtered, cleaned, dried air was allowed to bubble through the hot lipid samples (3.0 g) at a rate of 15 L/h contained in a reaction vessel placed in an electric-heating block. The effluent air containing volatile organic acids from the lipid sample was collected in a measuring vessel containing distilled water (60 mL). The conductivity of the water as oxidation proceeded was measured automatically. The OSIs of the oil samples were automatically recorded at 120°C. The time needed for the appearance of a sudden rise in water conductivity was registered as the induction time in hours.[Citation12]
Statistical Analysis
All analyses were carried out in triplicate and were reported as mean ± SD. An ANOVA test (SPSS 12.0, SPSS Inc., Chicago, IL, USA) was used to compare the mean values of each treatment. Significant differences between the means of parameters were determined by using the Duncan test (p < 0.05).
RESULTS AND DISCUSSION
Physico-Chemical Characteristics of Refined Oil and Triacylglycerol of Soybean
The physico-chemical properties of refined oil and TAG of soybean are given in . The moisture content (0.10%) and SG (0.92) were similar for refined oil and TAG of soybean. Results indicated negligible differences in the refractive indices between the samples of refined oil and TAG. The color value of refined soybean oils decreased with separation and purification of TAG. The lower color value of TAG might be due to the binding of carotenoids with the stationary phase of the eluting column. Saponification values of TAG 192 mg KOH/g of oil were significantly (p < 0.05) higher than refined soybean oil 183 mg KOH/g. Acid value of oil (0.59 mg KOH/g) showed a significant (p < 0.05) differences with that of TAG (0.27 mg KOH/g of oil). Wij’s iodine number of refined oil (123 g I/100 g oil) was significantly (p < 0.05) lower than the purified TAG (129 g I/100 g oil). PV of refined oil (2.3 meq O2/kg of oil) was higher than TAG (2.6 meq O2/kg of oil). Unsaponifiable matter was 1.19% for refined oil but absent in TAG. FFA was 0.28 and 0.13% for refined oil and TAG of soybean, respectively. Hui[Citation13] reported RI (1.47), saponification value (188–195 mg KOH/g of oil), IV (125–138 gI/100 g), acid value (0.48–0.62 mg KOH/g of oil), and PV (1.98–2.41 meq O2/kg of oil) of soybean oil. The present results on the physico-chemical properties of soybean oil were close to previously reported values. Statistical analysis showed non-significant differences in moisture, SG, RI, and PV between refined oil and TAG, while color value, saponification value, acid value, iodine number, and FFA varied significantly (p < 0.05).
TABLE 1 Physico-chemical parameters of refined oil and triacylglycerol of soybean (n = 3)
The fatty acid composition of the refined oil and TAG of soybean is presented in . Polyunsaturated fatty acids constituted 59.77 and 64.46%, respectively for refined oil and TAG. Linoleic acid (C18:2) was the major fatty acid representing 52.2 and 56.2%, respectively for refined oil and TAG of soybean. Linolenic acid (C18:3) represented 7.57 and 8.26% for refined oil and TAG. Second major fraction was of monounsaturated fatty acid with 23.44 and 19.41%, respectively for refined oil and TAG. Oleic acid (C18:1) was the major fatty acid in this class which constituted 23.2% for refined oil and 19.2% for TAG. Docosanoicacid (C20:1) was in the range of 0.21–0.24%. Saturated fatty acids contents were 16.18% and 15.30%, respectively for refined oil and TAG of soybean. Palmitic acid (C16:0) was the highest in this group followed by stearic (C18:0), arachidic (C20:0), lignoceric acid (C24:0), behenic (C22:0), and myristic (C14:0), respectively, for refined oil and TAG. It was found that the fatty acid compositions of soybean oil consisted of linoleic (53.96%), oleic (24.02%), palmitic (10.66%), linolenic (6.05%), stearic (4.28%), arachidic (0.52%), behenic (0.24%), margaric (0.06%), palmitoleic (0.06%), myristic (0.06%), and margaroleic (0.04%) acids. These results indicate that the oxidative stability of soybean oil was low.[Citation14] Fatty acid composition of TAG of soybean oil was 11.21% palmitic, 0.09% palmitoleic, 3.70% stearic, 23.62% oleic, 54.74% linoleic, 5.54% linolenic, and 0.26% decosanoic.[Citation15] Thus, the results are in agreement with previous studies on fatty acid profile of refined and TAG of soybean oil. Statistical analysis revealed that palmitic, oleic, linoleic, and linolenic acid differed significantly (p < 0.05) in refined oil and TAG of soybean while other did not vary significantly.
TABLE 2. Fatty acid profile of refined oil and triacylglycerol of soybean (n = 3)
Effect of Carotenoids and γ-Tocopherol on Oxidative Stability of TAG
The effects of γ-tocopherol, β-carotene, lycopene, and a combination of lycopene:γ-tocopherol (1:2) on the oxidative stability of soybean TAG were evaluated. The effectiveness of natural antioxidants on the PV of TAG of soybean is shown in . It is evident that both lycopene and β-carotene acted as pro-oxidants thereby accelerating the peroxide formation in soybean TAG. However, γ-tocopherol and combination of lycopene-γ-tocopherol decreased the PV as compared to control sample. Increase in lycopene and β-carotene concentrations from 100–200 ppm increased the formation of peroxides. However, increase in γ-tocopherol concentration (200 ppm) did not result in changes in PV.
FIGURE 1 Effect of carotenoids and γ-tocopherol on (a) peroxide value (PV); (b) thiobarbituric acid reactive substances (TBARS); (c) conjugated diene (CD) in soybean triacylglycerol stored at 60ºC (n = 3).
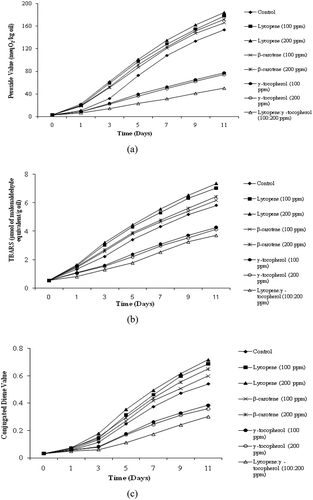
Statistical analysis showed a significant (p < 0.05) increase in PV of soybean TAG with time for control and test samples. The increase was the highest in lycopene and β-carotene followed by control which indicated pro-oxidation action of the two. However, γ-tocopherol and combination of lycopene-γ-tocopherol showed less increase in the PV value compared to control indicating antioxidant effect in TAG of soybean. Results indicated that combination of lycopene with γ-tocopherol was the most effective antioxidant. γ-tocopherol at 100 ppm and a combination of lycopene and γ-tocopherol (100:200 ppm), respectively, inhibited hydroperoxide formation of triglycerides under light at 25ºC.[Citation16]
The effectiveness of different antioxidant in vegetable oil has been frequently reported using TBARS.[Citation8,Citation17] Synthesis of the TBARS in soybean TAG treated with antioxidants at 60ºC is presented in . The TBARS values increased with time in control and treated samples of soybean TAG at 60ºC during 11 days of incubation. The increase in TBARS for lycopene and β-carotene treated samples was higher than control that indicated pro-oxidant effect. Lycopene and β-carotene at 200 ppm level exhibited higher TBARS values than control. However, the samples containing γ-tocopherol and combination of lycopene-γ-tocopherol indicated lower value for TBARS than the control. This establishes the antioxidative activity of lycopene-γ-tocopherol combination in the soybean TAG. Statistical analysis showed a significant (p < 0.05) increase in TBARS in control as well as treated samples of soybean TAG, but a combination of lycopene-γ-tocopherol indicated the least increase, thereby showing its antioxidative properties.
Effect of natural antioxidants on the formation of CD in soybean TAG is shown in . Lycopene and β-carotene at 200 ppm level exhibited higher CD values than control. Combination of lycopene-γ-tocopherol was more effective than γ-tocopherol in preventing the formation of CD. The trends in antioxidant effectiveness of CD values were similar to those obtained for primary oxidation products as reflected in PV. The comparatively higher values for PV, TBARS, and CD values for TAG were found at all the time intervals. Henery et al.[Citation18] determined the stability and antioxidant effects of carotenoids and γ-tocopherols in safflower seed oil reported that lycopene was more pro-oxidative than β-carotene whereas α- and γ-tocopherol delayed the onset of oxidation. Soybean oil samples containing 50 ppm of unheated all-trans β-carotene or lycopene stored under light showed significantly lower PV than control samples.[Citation19] Haila et al.[Citation16] studied the effects of lycopene, lutein, annatto, and γ-tocopherol on autoxidized triglycerides. Lutein and lycopene were pro-oxidants, whereas annatto and γ-tocopherol effectively inhibited hydroperoxide formation. A combination of lutein and γ-tocopherolwas more efficient than γ-tocopherol in inhibiting the hydroperoxide formation of triglycerides.
Effect of lycopene, β-carotenoid and lycopene:γ-tocopherol (1:2) on the OSI at 120°C has been given in . The control sample had an OSI value of 5 h that decreased or increased with the treatments. The highest oxidative stability is for the combination of lycopene: γ-tocopherol (1:2) followed by γ-tocopherol, control, β-carotene, and lycopene. The high oxidative stability of combination of lycopene:γ-tocopherol (1:2) is due to the antioxidative property of γ-tocopherol. The OSI significantly decreased with the increased in carotenoids concentration. Addition of carotenoids has a strong negative influence on the oxidative of the oils resulted in decrease in resistance to oxidation.
FIGURE 2 Oxidative stability index (OSI) of soybean triacylglycerol treated with carotenoids and γ-tocopherol.
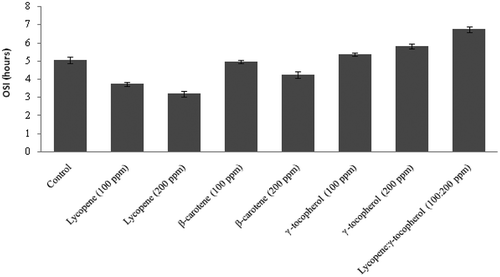
The pro-oxidant effects of lycopene and β-carotene support the earlier findings since they promote the oxidation of vegetable oils under light[Citation18,Citation20,Citation21] and under dark.[Citation22,Citation23] However, some researchers have reported antioxidant properties of lycopene and β-carotene.[Citation24–Citation27] Lycopene and β-carotene, like unsaturated fatty acids, are susceptible to oxidation due to the presence of the polyene system. An increase in lycopene and β-carotene concentrations from 100–200 ppm increased the formation of peroxides, because both β-carotene and lycopene may react with oxygen to form peroxyl radicals,[Citation28] these peroxyl radicals may enhance the propagation stage of the oxidation reaction because they supply the system with more oxidizable substrates. The auto oxidation products of β-carotene have been identified to be complex mixture of products with epoxy, hydroxyl, and carbonyl groups.[Citation29,Citation30] The commercial lycopene, as well as the laboratory prepared lycopene extract from tomato paste, exhibited a pro-oxidant effect in the beginning of the storage of sunflower and rapeseed oils and later a moderate stabilizing effect. The stability of sunflower and rapeseed oils was the maximum with a combination of commercial rosemary extract and lycopene extract.[Citation31] Decrease in PV, TBARS, and CD values, and increased OSI of soybean TAG with lycopene-γ-tocopherol combination might be due to protection of γ-tocopherol to free radical auto oxidation of lycopene as a result of its free radical chain termination mechanism.
CONCLUSION
The pro-oxidant effect of carotenoids was inhibited with the addition of γ-tocopherol. The advantage of a combination of a lycopene and γ-tocopherol as an antioxidant may be due to the effect of γ-tocopherol to retard the formation of degradation products of the carotenoid. The present study demonstrated pro-oxidative activity of lycopene and β-carotene but strong antioxidative activity of lycopene in combination with γ-tocopherol in lipid system. The study suggests that whenever carotenoids are used as a coloring agent in lipid-containing foods, the potential pro-oxidant effects of carotenoids should be considered.
REFERENCES
- Guillen, M.D.; Cabo, N. Fourier Transform Infrared Spectra Data Versus Peroxide and Anisidine Values to Determine Oxidative Stability of Edible Oils. Food Chemistry 2002, 77, 503–510.
- Lercker, G.; Rodriguez-Estrada, M.T. Cholesterol Oxidation Mechanism. In Cholesterol and Phytosterol Oxidation Products: Analysis, Occurrence and Biological Effects; Guardiola, F.; Dutta, P.C.; Codony, R.; Savage, G.P; Eds.; AOCS Press: Champaign, IL, 2002; 1–26.
- Whysner, L.; Wang, C.X.; Zang, E.; Iatropoulos, M.J.; Williams, G.M. Dose Response of Promotion by Butylatedhydroxyanisole in Chemically Initiated Tumours of the Rat Fore Stomach. Food and Chemical Toxicology 1994, 32(3), 215–222.
- Hildebrand, D.H. Phospholipids Plus Tocopherol Increase Soybean Oil Stability. Journal of the American Oil Chemists Society 1984, 61(3), 552–555.
- Wada, S.; Fang, X. Synergistic Antioxidant Effects of Rosemary and Alpha-Tocopherol at Different Storage Temperature and Its Application for Inhibiting Dried Sardine Meat Oxidant. Journal of the Japan Oil Chemists Society 1994, 43(2), 109–115.
- Gordon, M.H.; Kourimska, L. The Effects of Antioxidants on Changes in Oils During Heating and Deep Frying. Journal of the Science of Food and Agriculture 1995, 68, 347–353.
- Edge, R.; Mc Garvey, D.J.; Truscott, T.G. The Carotenoids as Antioxidants—A Review. Journal of Photochemistry and Photobiology B-Biology 1997, 41, 189–200.
- Khan, M.A.; Shahidi, F. Effects of Natural and Synthetic Antioxidants on the Oxidative Stability of Borage and Evening Primrose Triacylglycerols. Food Chemistry 2001, 75, 431–437.
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists International (18th ed.). In Oils and Fats; AOAC International: Gaithersburg, MA, 2005.
- Kıralan, M.; Bayrak, A. Oxidative and Antiradical Stabilities of Two Important Virgin Olive Oils from Ayvalik and Memecik Olive Cultivars in Turkey. International Journal of Food Properties 2013, 16, 649–657.
- American Oil Chemists’ Society. Oven Storage Test for Accelerated Aging of Oils. In Official Methods and Recommended Practices of the American Oil Chemists Society; The American Oil Chemists’ Society Press: Champaign, IL, Official Method, 1997; 5–97.
- Koprivnjak, O.; Skevin, D.; Valic, S.; Majetic, V.; Petricevic, S.; Ljubenkov, I. The Antioxidant Capacity and Oxidative Stability of Virgin Olive Oil Enriched with Phospholipids. Food Chemistry 2008, 111, 121–126.
- Hui, Y.H. Bailey’s Industrial Oil and Fat Products, 5th Ed; Wiley: New York, NY, 1996.
- Aysel, M.B.; Bayrak, A.; Kiralan, M.; Ozbucak, T. Individual and Combined Use of Rosemary and Origanum in Soybean Oil as Natural Antioxidants. International Journal of Food Properties 2013, 16, 995–1001.
- Nakaya, K.; Kohata, T.; Doisaki, N.; Ushio, H.; Ohshima, T. Effect of Oil Droplet Sizes of Oil-in-Water Emulsion on the Taste Impressions of Added Tastants. Fisheries Science 2006, 72, 877–883.
- Haila, K.; Lievonen, S.M.; Heinonen, M. Effects of Lutein Lycopene, Annatto, and γ-tocopherol on Autoxidation of Triglycerides. Journal of Agricultural and Food Chemistry 1996, 44, 2096–2100.
- Ganthavorn, C.; Hughes, J.S. Inhibition of Soybean Oil Oxidation by Extracts of Dry Beans (Phaseolus vulgaris). Journal of the American Oil Chemists Society 1997, 74, 1025–1030.
- Henry, L.K.; Catignani, G.L.; Schwartz, S.J. Oxidative Degradation Kinetics of Lycopene, Lutein, 9-cis and All-Trans β-Carotene. Journal of the American Oil Chemists Society 1998, 75, 823–829.
- Steenson, D.L.; Min, D.B. Effects of β-Carotene and Lycopene Thermal Degradation Products on the Oxidative Stability of Soybean Oil. Journal of American Oil Chemists Society 2000, 77(11), 1153–1160.
- Terao, J.; Yamauchi, R.; Murakami, H.; Matsushita, S. Inhibitory Effects of Tocopherols and β-carotene on Singlet Oxygen-Initiated Photo-Oxidation of Methyl Linoleate and Soybean Oil. Journal of Food Processing and Preservation 1980, 4, 79–93.
- Haila, H.; Heinonen, M. Action of α-Carotene on Purified Rapeseed Oil During Light Storage. Lebensmittel-Wissenschaft und Technologie 1994, 27, 573–577.
- Warner, K.; Frankel, E.N. Effects of β-Carotene on Light Stability of Soybean Oil. Journal of the American Oil Chemists Society 1987, 64, 213–218.
- Suzuki, T.; Usuki, R.; Kaneda, T. The Role of Carotenoids in the Oxidative Deterioration of Edible Oils. Journal of Japan Oil Chemists Society 1989, 38, 486–491.
- Fakourelis, N.; Lee, E.C.; Min, D.B. Effects of Chlorophyll and β-Carotene on the Oxidation Stability of Olive Oil. Journal of Food Science 1987, 52, 234–235.
- Lee, E.C.; Min, D.B. Quenching Mechanism of β-Carotene on the Chlorophyll Sensitized Photo-Oxidation of Soybean Oil. Journal of Food Science 1988, 53, 1894–1895.
- Lee, S.W.; Min, D.B. Effects, Quenching Mechanisms, and Kinetics of Carotenoids in Chlorophyll-Sensitized Photo-Oxidation of Soybean Oil. Journal of Agricultural and Food Chemistry 1990, 38, 1630–1634.
- Jung, M.Y.; Min, D.B. Effects of Quenching Mechanisms of Carotenoids on the Photosensitized Oxidation of Soybean Oil. Journal of the American Oil Chemists Society 1991, 68, 653–658.
- Tsuchihashi, H.; Kigoshi, M.; Iwatsuku, M; Niki, E. Action of β-Carotene as an Antioxidant Against Lipid Peroxidation. Archives of Biochemistry and Biophysics 1995, 323, 137–147.
- Kennedy, T.A.; Liebler D.C. Peroxyl Radical Scavenging by β-Carotene in Lipid Bilayers. Journal of Biological Chemistry 1992, 267, 4658–4663.
- Jorgensen, K.; Skibsted, L.H. Carotenoid Scavenging of Radicals. Zeitschrift Fur Lebensmittel-Untersuchung Und-Forschung A-Food Research and Technology 1993, 196, 423–429.
- Vajdak, M.; Schmidt, S.; Lizicarova, I.; Zahradnikova, L.; Sekretar, S. Effect of Lycopene and Antioxidants on the Oxidative Stability of Sunflower and Rape Seed Oils. Bulletin Potravinarskeho Vyskumu 2004, 43, 219–228.
