Abstract
Lysozyme is a commercially valuable enzyme, and is applied in many fields, concerning products such as foods, drugs, and the like. In this work, lysozyme was isolated and purified from buffalo milk using sephadex G-50 and cation exchanger carboxymethyl cellulose. Lysozyme active fractions from buffalo milk were assayed against Gram positive substrate Micrococcus luteus at 450 nm and a decline in absorbance of 0.001 per min was observed. The optimum activity of lysozyme (158.3 ± 1.7 units/mL) was at 7.5 pH and 37°C temperature. Lysozyme activity at pasteurization temperatures 62.5°C, 30 min and 75°C, 15 s were (156.08 ± 1.03 and 156 ± 2 units/mL) not affected significantly; however, 47% activity of lysozyme was reduced at 100°C for 5 min. Antibacterial susceptibility testing of lysozyme (chicken egg white lysozyme and buffalo milk lysozyme) was performed on Micrococcus luteus (ATCC 4698) and Escherichia coli (ATCC 25235). Both lysozymes showed no inhibition effect against Escherichia coli.
INTRODUCTION
Agriculture, the most important sector of the Pakistan’s gross domestic product (GDP), provides employment to 45% population as well as input for the agro-based industry. Moreover, the single most important subsector of agriculture is livestock, which accounts for approximately 55.1% of the agriculture value and added 11.5% to GDP during 2010–2011. One of the major products of livestock is milk and this sector produced 46.44 billion liters during the same year.[Citation1] In Pakistan, buffaloes not only fulfill the protein requirements of the human population by milk and meat, but are also have a great share in providing the traction power for various agricultural purposes. Out of the total milk produced in the country, buffalo contributes about 68%, followed by cattle (27%), and sheep/goat/camel (5%). Due to their versatile qualities they are rightly called as Black Gold of Pakistan.[Citation2]
Milk is conventional and vigorous food source for energy, proteins, vitamins, and minerals. Besides its significance as a nutrient source, it had also the aptitude to kill bacteria and could be implemented to improve the quality life of milk. Numerous proteins, like immunoglobulins lactoferrin, antiviral lipids, vitamin-binding proteins and the enzymes, lysozyme, lactoperoxidase, and xanthine oxidase, found in milk under diverse conditions reveal antimicrobial activity. These immunoglobulins keep away the young ones from infection until their own immune system is developed.[Citation3]
Lysozyme (EC 3.2.1.17; muramidase) is a single polypeptide chain consisting of 129 amino acids, in which lysine is the N-end amino acid and leucine is the C-end one. It is a globular basic protein characterized by molecular weight of 14.3 kDa and cross-linked by four disulfide bonds.[Citation4,Citation5] It is an important antimicrobial agent in milk, which kills bacteria by cleaving the β-1,4-glycosidic bond between C-1 of N-acetyl muramic acid and C-4 of N-acetyl glucosamine residues of the peptidoglycan in the bacterial cell wall.[Citation6,Citation7] Lysozyme appears to inhibit not only bacteria where the peptidoglycan layer is a major component of their cell-wall, but also viruses and eukaryotic microorganisms devoid of a typical peptidoglycan layer, suggesting that it acts by other mechanisms of action than the hydrolytic activity.[Citation8] Lysozymes exist ubiquitously in diverse organisms, including animals, plants, fungi, and bacteria, and are classified into six major types: chicken-type (c-type), goose-type (g-type), invertebrate type (i-type), plant type, phage type, and bacterial type.[Citation6,Citation9] It is also present in various food commodities such as, hen egg-white (3.5%), buffalo milk (3.85 μg/mL), cow milk (1.67 μg/mL), and various vegetables (cauliflower 32.5; cabbage 2.7; red radish 2.2; white radish and turnip 0.5–1.8 μg/mL of juice).[Citation5,Citation10,Citation11] C-type and g-type lysozymes are found primarily in vertebrates. C-type and g-type lysozymes are similar in three-dimensional structures but share little homology at primary structure and differ in catalytic mechanism, enzymatic property, and genetic organization.[Citation6,Citation12−Citation14] In c-type lysozymes Glu35 facilitate the breakdown of C-O linkage in substrate while in g-type same function is performed by Glu73.[Citation15,Citation16] C-type is found in the hen egg-white and is known as chicken-type or chicken lysozyme (C-lysozyme) and g-type found in the goose egg-white and is known as goose type or G-lysozyme. Human lysozymes are considered to be the C-lysozyme type. However, cow milk may contain both C-lysozyme and G-lysozymes as both types are found in various other body fluids and in the stomach tissue of the cow.[Citation17] It is used as a preservative in foods and pharmaceuticals as well as in oral health care products to restore saliva’s own antimicrobial capacity. The food products consist of meat, fish, milk and dairy products, fruits, and vegetables; and health care products are mouth-rinses, moisturizing gels, and chewing gums.[Citation5,Citation18] The enzyme can limit the migration of neutrophils into damaged tissue and might function as an anti-inflammatory agent.[Citation19]
Moreover, there has been increasing demand for lysozyme due to its potent antimicrobial activity against a wide range of microorganisms and hence, in food preservation and safety. The objective of this study was to purify and characterize lysozyme from milk of Pakistani buffalo with specific properties such as high activity and stability over the broad range of processing conditions in the food industry.
MATERIALS AND METHODS
The facilities in Microbiology Lab, National Institute of Food Science and Technology, and Protein Molecular Biology Lab., Department of Chemistry and Biochemistry, University of Agriculture, Faisalabad, Pakistan were utilized for conducting the research. Chicken egg-white lysozyme (L-6876) and carboxy methylcellulose (CMC; C-0806) were purchased from Sigma-Aldrich, USA. Sephadex G-50 was purchased from MP Biosciences, USA. Potassium dihydrogen phosphate, dipotassium hydrogen phosphate, sodium hydro-oxide and potassium hydroxide were purchased from Merck Chemicals, Germany. Micrococcus luteus (ATCC 4698) and Escherichia coli (ATCC 25235) were purchased from Micro Biologics UK. Blood agar base, Nutrient agar, and Muller Hinton agar was purchased from Oxoid, Hampshire, UK.
Collection of Milk
Milk samples were procured from the livestock farm of University of Agriculture, Faisalabad, Pakistan and defatted with the laboratory scale milk cream separator and stored at –20°C until use. Frozen milk samples were thawed at ambient temperature and various analytical measurements were performed.
Determination of Lysozyme Activity
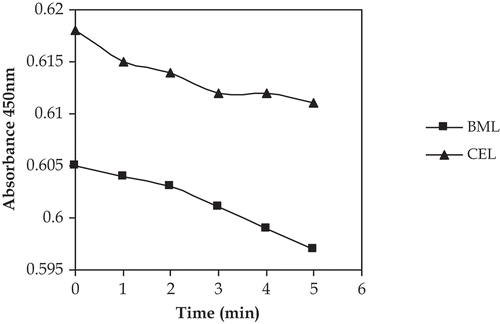
Lysozyme activity was as described by the Shugar[Citation20] with some modifications. Micrococcus luteus culture suspension (0.03 g/200 mL) was prepared in potassium phosphate buffer (0.066 M, pH 6.24). Then 1 mL of culture was taken and 2 mL of buffer was dissolved into it to make the absorbance between 0.6 and 0.7. The absorbance was measured with IRMECO UV-Vis spectrophotometer (Model U2020). The initial absorbance of Micrococcus luteus was 0.631 at 450 nm. Chicken egg-white lysozyme solution was prepared by dissolving 0.064 mg/mL of potassium phosphate buffer (pH 6.24, 0.066M). This reaction mixture contained 2.5 mL of culture solution and 0.1 mL of chicken egg-white lysozyme. Then decrease in absorbance at 450 nm per min was taken as enzyme activity with increase in time. The decrease in absorbance was 0.001 per min. ().
FIGURE 1 Effect of chicken egg white lysozyme (CEL) and buffalo milk lysozyme (BML) on the Micrococcus luteus.
Isolation and Purification of Lysozyme
Buffalo milk lysozyme (BML) isolation and purification was performed with method described by Priyadarshini and Kansal.[Citation21] Sephadex G-50 was soaked in to distilled autoclaved water (200 mL) and kept for 72 h. After 72 h, slurry was poured slowly with the walls of glass column by gravity flow and settle down. Washing of the sephadex was done with the sodium phosphate buffer (0.05M, pH 6.5 containing 0.2M NaCl). Then defatted milk sample was applied to the gel filtration using column packed with sephadex G-50. Lysozyme was eluted from sephadex G-50 column with 0.05M sodium phosphate buffer containing 0.2M NaCl (pH 6.5). Elutions absorbance was analyzed at 280 nm. Lysozyme active fractions were pooled up and concentrated.
Ion-Exchange Chromatography Using CMC
The sample was stirred gently for up to 4 h with CMC that had been already regenerated by suspending in 0.1M NaOH, 0.5M NaCl, and 0.1M HCl containing 0.5M NaCl for specific interval followed by filtration through Buchner funnel. Resin was packed in glass column and lysozyme was eluted with 0.05M sodium phosphate buffer containing 1M NaCl (pH 6.5). Lysozyme active fractions were pooled up and concentrated.
Lysozyme Characterization
Characterization of lysozyme was determined according to Priyadarshini and Kansal[Citation22] to study the effect of pH (5.5, 6.5, 7.5, 8.5, and 9.5) and temperature (4, 37, 62.5, 72, and 100°C) for optimum activity of lysozyme.
Antibacterial Susceptibility Testing
The disk diffusion method for the activity of lysozyme against M. luteus and E. coli was determined by the method as described by Bauer et al.[Citation23] Bacterial cell culture was prepared by adopting direct colony suspension method (0.5 McFarland turbidity standard). This inoculum suspension was streaked uniformly on dried Muller Hinton agar plates. Filter paper discs (10 mm diameter) containing 50 μL lysozyme (1, 2, 4, 10, and 20 μg/mL) were dispensed on the surface of agar plates. After 16–18 h of incubation (35°C), diameter of the zones of complete inhibition was measured including the diameter of the disc.
Statistical Analysis
Data obtained was subjected to statistical analysis by using the analysis of variance (ANOVA) techniques and means were compared for their significance according to method of Duncan’s Multiple Range tests.[Citation24]
RESULTS AND DISCUSSION
Isolation of BML by Gel Filtration Technique
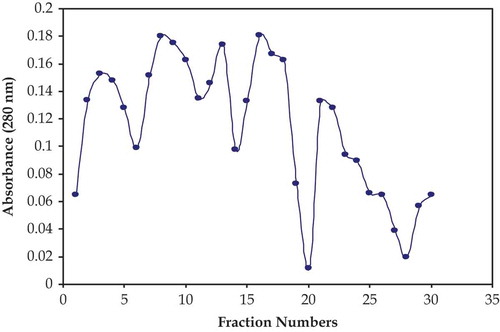
Isolation was performed by the column packed with sephadex G-50. When the entire milk sample was poured in to the resin remaining some of the sample on the resin surface then sodium phosphate buffer (0.05M, pH 6.5 containing 0.2M NaCl) was applied and lysozyme was eluted from the resin. Almost 30 different fractions of 3 mL were collected. Then absorbance was monitored for these samples at 280 nm. The samples having high value were assayed for the lysozyme activity. Lysozyme activity was recovered in the vials (no. 8, 13, 16, and 21). Results regarding the isolation of lysozyme from buffalo milk through sephadex G-50 presented in . Instead of these vials, others high values were also present. These high points were other type of proteins. The results of the present investigation are in accordance with the earlier findings. Fox and Kelly[Citation25] studied many indigenous enzymes and proteins in the milk. These were lactoperoxidase, lipase, catalase, and xanthine oxidase. Sugai et al.[Citation26] reported on equine milk and investigated three milk proteins that were lysozyme, α-lactalbumin, and β-lactoglobulin. Elagamy[Citation27] also studied the camel milk proteins concluded that camel milk was contained different types of proteins like α-lactalbumin, β-lactoglobulin, serum albumin, lactoferrin, immunoglobulin, along with lysozyme. Mohamed and Larsson-Raznikiewicz[Citation28] also studied the effects of heat on casein protein of camel milk. Priyadarshini and Kansal[Citation21] used the size exclusion technique using sephadex G-50 for the isolation of lysozyme from buffalo milk. Duhaiman[Citation29] also purified lysozyme from low molecular camel milk proteins using sephadex G-75 and reported higher yield of lysozyme. Saleh and Ibrahimi[Citation30] purified the lysozyme through Sephadex G-25 column and obtained better recovery of lysozyme activity.
Purification of BML Using Cation Exchanger (CMC)
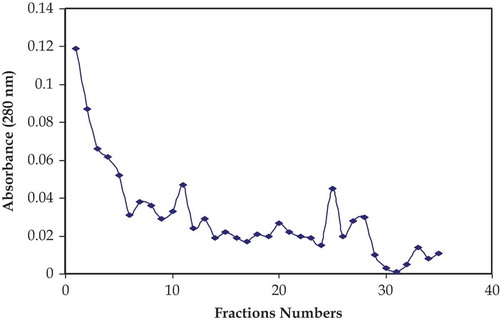
All the active fractions from the sephadex G-50 were pooled up, and from that solution 2.5 mL of buffalo milk sample was taken and applied to the column of cation exchanger and eluted with sodium phosphate buffer (0.05M, pH 6.5 containing 1M NaCl). Thirty-five different factions (3 mL) were taken in test tubes. Then absorbance was monitored at 280 nm. The samples having high absorbance were assayed for the lysozyme activity. Maximum activity of lysozyme was observed in vials 11 and 25. Fractions were pooled and further studied. Results regarding the purification of lysozyme from buffalo milk through CMC exchanger were shown in . Saleh and Ibrahimi[Citation30] studied on the rabbit tear lysozyme. After isolation from sephadex G-25, the most active fractions were pooled and lysozyme sample was applied to column of CMC for purification. They used the ammonium acetate buffer (pH 8) for the elutions of lysozyme and recovered 59% of lysozyme activity. Priyadarshini and Kansal[Citation21] applied the ion exchange chromatography using same exchanger CMC for the purification of lysozyme from buffalo milk and obtained almost 90% activity of lysozyme.
TABLE 1 Effect of pH levels on lysozyme activity (units/mL) at various temperatures
Characterization of Lysozyme
After purification through sephadex G-50 and CMC exchanger, effect of temperature and pH on the activity of lysozyme was studied and their optimum temperature and pH values were observed.
Effect of Temperature
Different temperatures (4, 37, 62.5, 72, and 100°C) were studied for the activity of lysozyme and found that with the increase in temperature the lysozyme activity was decreased and remained maximum at 4, 37, 62.5, and 72°C for 3 days, 24 h, 30 min, and 15 sec, respectively (). The activity was greatly reduced at 100°C for 5 min. At 4°C storage for 3 days, lysozyme maintained its optimum activity. The results obtained are in line with the earlier findings. Priyadarshini and Kansal[Citation22] reported thermal stability of BML. Lysozyme was subjected to temperatures of 63, 74, and 100°C for 1, 15, and 30 min. Lysozyme lost only 10% of its activity after 15 min at 74°C and 30% of its activity after 1 min at 100°C. After 30 min at 74°C about 34% of activity was lost. Inactivation of lysozyme occurred after 30 min at 100°C. Saleh and Ibrahimi[Citation30] found that enzyme was very stable at 4°C with more than 80% activity retained after ten days. Elagamy[Citation27] showed the effect of heat treatment of lysozyme. He found that heating at 65 or 75°C for 30 min had no significant effect on lysozyme activity in BML. Priyadarshini and Kansal[Citation31] found that BML was fully stable while the cow milk lysozyme was partly inactivated by pasteurization. Pahud[Citation32] also reported that lysozyme activity was unaffected if it was incubated at temperatures 20–70°C for 10 min.
Effect of pH
Buffer solutions of different pH (5.5, 6.5, 7.5, 8.5, and 9.5) were prepared and their effect was studied on lysozyme activity. Activity was decreased with the increase in pH with the highest activity at pH 7.5. The mean values for the effect of different levels of pH on lysozyme activity at various temperatures were shown in , declared that enzyme activity was maximum at pH 7.5 and 37°C. There was no significant effect observed on the activity of lysozyme at temperatures 4, 37, 62.5, and 72°C, but a drastic reduction in activity of lysozyme occurred at 100°C. Enzymatic activity of lysozyme was reduced at 100°C but having the same pattern, with increase in pH, activity of enzyme increased (optimal 7.5 pH). As pH was preceded toward the alkaline stage, its activity was decreased. Priyadarshini and Kansal[Citation21] declared that activity of lysozyme increased with the increase in pH and it was maximum at 7.4 pH and 37°C temperature. But it gradually decreased with the increase in pH. Priyadarshini and Kansal[Citation22] reported that optimum pH for lysozyme was 7.4. McKenzie and White[Citation33] worked on bovine milk lysozyme and reported the optimum pH and temperature for the activity of lysozyme that was 7.4 and 37°C. Ye et al.[Citation34] found that the g-type lysozymes exhibit peak activities at pH 6.0 to pH 6.8. Zhao et al.[Citation5] lysozyme has an optimum pH and temperature of 7.0 and 30°C, respectively. The affect of storage of lysozyme at 4°C was in line with the findings of Saleh and Ibrahimi.[Citation30] They found that lysozyme was very stable at 4°C with more than 80% activity retained after 10 days. They reported that lysozyme was more active in the pH range of 5.5–7 and activity dropped rapidly above pH 7.0.
Halliday et al.[Citation35] reported that feline and canine milk lysozyme showed optimum activity at a pH level of 7.4. Smolelis and Hartsell[Citation36] observed the increased rates of lysis of Micrococcus lysodeikticus by lysozyme with increase in temperature up to 60°C and pH was 6.0–7.0 for lysozyme activity. Sidhan and Gurnani[Citation37] affirmed that optimum pH for rat liver lysozyme was 5.4 in phosphate buffer and 37°C. With further increase in temperature, the activity of the enzyme decreased. Thompson[Citation38] reported 6.0–7.0 pH for lysozyme action on Micrococcus lysodeikticus. Smolelis and Hartsell[Citation36] noted that rates of lysis of Micrococcus lysodeikticus was increased with increase in temperature up to 60°C at 6.7 pH. Miyauchi et al.[Citation39] studied the effects of pH and temperature on purified fish lysozyme toward M. lysodeikticus. Optimum pH and temperature were measured in the range of 2.4–8.0 and 20–90°C, respectively.
Antimicrobial Susceptibility Testing of Lysozyme
Antimicrobial susceptibility testing was performed by the method of Bauer et al.[Citation23] The best suitable substrate for the lysozyme activity was peptidoglycan wall of the bacteria. These polysaccharides contents were higher in the Gram positive bacteria. So, Micrococcus luteus was best substrate for the lysozyme activity. Different concentrations of lysozymes were used against bacterial culture. Lysozyme performed suitable functioning on the Micrococcus luteus but there was no affect on E. coli which was Gram negative (). In Gram negative bacteria, the murein layer was limited, therefore, the attack of lysozyme was too limited. Results shown were coinciding with the findings of Priyadarshini and Kansal.[Citation21] They also found the results regarding the inhibition of M. luteus and E. coli on petri plates with different concentrations of lysozyme. Nikaido and Vaara[Citation40] found that human lysozyme lysed and killed several gram-positive microorganisms by damaging their surface exposed peptidoglycan. In contrast, most gram-negative organisms were resistant to killing by the protein. In these organisms an outer membrane shielded the peptidoglycan murein sacculus from the external environment. Martinez and Carroll[Citation41] reported that as lysozyme could not easily penetrate the outer membrane, the organisms were resistant to its effects and the protein had routinely been considered to have at most a secondary function in the host defense against these pathogens.
TABLE 2 Zone inhibition of Micrococcus luteus and Escherichia coli by standard chicken egg white lysozyme (CEL) and buffalo milk lysozyme (BML)
CONCLUSION
The purified BML showed optimum activity at 7.5 pH and 37°C. With increase in pH value from 5.5 to 7.5, enzyme activity of lysozyme was increased but it gradually decreased as pH moved toward an alkaline level. BML was more heat stable and it could hold the temperatures up to 72–75°C but drastic effects occurred as temperature moved toward higher levels. BML inhibited the M. luteus by creating zones but had no effect on the E. coli.
ACKNOWLEDGMENT
The authors are thankful to the National Institute of Food Science and Technology, University of Agriculture, Faisalabad for providing the laboratory facilities for conducting the research.
REFERENCES
- GoP. Economic Survey of Pakistan (2010–2011); Government of Pakistan, Economic Adviser’s Wing, Finance Division: Islamabad, 2011.
- Bilal, M.Q.; Suleman, M.; Raziq, A. Buffalo: Black gold of Pakistan. Livestock Res. for Rural Development. 2006; 18 p., http://www.lrrd.org/lrrd18/9/bila18128.htm.
- Al-Jabri, A.A. Honey, milk, and antibiotics. African Journal of Biotechnology 2005, 4, 1580–1587.
- Masschalck, B.; Deckers, D. Michiels. Lytic and nonlytic mechanism of inactivation of gram-positive bacteria by lysozyme under atmospheric and hydrostatic pressure. Journal of Food Protection 2002, 65, 916–923.
- Cegielska, R.R.; Leśnierowski, G.; Kijowski, J. Properties and application of egg white lysozyme and its modified preparations—A review. Polish Journal of Food and Nutrition Sciences 2008, 58 (1), 5–10.
- Zhao, L.; Sun, J.-S.; Sun, L. The g-type lysozyme of scophthalmus maximus has a broad substrate spectrum and is involved in immune response against bacterial infection. Fish and Shelfish Immunology 2011, 30, 630–637.
- Li, D.; Zhang, T.; Xu, C.; Ji, B. Effect of pH on the interaction of baicalein with lysozyme by spectroscopic approaches. Journal of Photochemistry and Photobiology. B: Biology 2011, 104, 414–424.
- Benkerroum, N. Antimicrobial activity of lysozyme with special relevance to milk. African Journal of Biotechnology 2008, 25, 4856–4867.
- Bachali, S.; Jager, M.; Hassanin, A.; Schoentgen, F.; Jollès, P.; Fiala-Medioni, A. Phylogenetic analysis of invertebrate lysozymes and the evolution of lysozyme function. Journal of Molecular Evolution 2002, 54, 652–664.
- Abd El-Aziz, M. Study on lysozyme level, distribution and effect of heat treatment in buffalo and cow milk. Journal Annals of Agricultural Science 2006, 51 (2), 439–446.
- Chandan, R.C.; Ereifej, K.I. Determination of lysozyme in raw fruits and vegetables. Journal of Food Science 1981, 46 (4), 1278–1279.
- Callewaert, L.; Michiels, C.W. Lysozymes in the animal kingdom. Journal of Biosciences 2010, 35, 127–160.
- Irwin, D.M.; Gong, Z. Molecular evolution of vertebrate goose-type lysozyme genes. Journal of Molecular Evolution 2003, 56, 234–242.
- Sahoo, N.R.; Kumar, P.; Bhusan, B.; Bhattacharya, T.K.; Dayal, S.; Sahoo, M. Lysozyme in livestock: A guide to selection for disease resistance: A review. Journal of Animal Sciences Advances 2012, 2 (4), 347–360.
- Hirakawa, H.; Ochi, A.; Kawahara, Y.; Kawamura, S.; Torikata, T.; Kuhara, S. Catalytic reaction mechanism of goose egg white lysozyme by molecular modeling of enzyme substrate complex. Journal of Biochemistry 2008, 144, 753–761.
- Helland, R.; Larsen, R.L.; Finstad, S.; Kyomuhendo, P.; Larsen, A.N. Crystal structures of g-type lysozyme from Atlantic cod shed new light on substrate binding and the catalytic mechanism. Cellular and Molecular Life Sciences 2009, 66, 2585–2598.
- Losnedahl, K.J.; Wang, H.; Aslam, M. Antimicrobial proteins in milk. Journal of Diary Sciences 1996, 84, 1123–1127.
- Tenovuo, J. Clinical applications of antimicrobial host proteins lactoperoxidase, lysozyme, and lactoferrin in xerostomia: Efficacy and safety. Oral Diseases 2002, 8, 23–29.
- Saraswathi, N.T.; Sankarayanan, R.; Vijayan, M. Effect of stabilizing additives on the structure and hydration of proteins. Acta Crystallographica Section D 2002, 58, 1162–1167.
- Shugar, D. The measurement of lysozyme activity and the ultra-violet inactivation of lysozyme. Biochimica et Biophysica Acta 1952, 8 (3), 302–309.
- Priyadarshini, S.; Kansal, V.K. Purification, characterization, antibacterial activity and N-terminal sequencing of buffalo-milk lysozyme. Journal of Dairy Research 2002a, 69, 419–431.
- Priyadarshini, S.; Kansal, V.K. Biochemical characterization of buffalo (Bubalus bubalis) milk lysozyme. Journal of Dairy Research 2003, 70, 467–472.
- Bauer, A.W.; Kirby, W.M.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. American Journal of Clinical Pathology 1966, 45 (4), 493–496.
- Steel, R.G.D.; Torrie, J.H.; Dickey, D. Principles and Procedures of Statistics, 3rd Ed; McGraw Hills Book Co. Inc.: New York, 1996.
- Fox, P.F.; Kelly, A.L. Indigenous enzymes in milk: Overview and historical aspects—Part 2. International Dairy Journal 2006, 16 (6), 517–532.
- Sugai, S.; Ikeguchi, M.; Shimizu, A. Characteristic properties of equine milk proteins. International Journal of Food Science and Technology 1999, 34, 437–443.
- Elagamy, E.I. Effect of heat treatment on camel milk proteins with respect to antimicrobial factors: A comparison with cow’s and buffalo milk proteins. Food Chemistry 2000, 68 (2), 227–232.
- Mohamed, M.A.; Larsson-Raznikiewicz, M. Heat treatment of camel milk—Effects up on casein fraction. Milchwissenschaft 1991, 46, 562–565.
- Duhaiman, A.S. Purification of camel milk lysozyme and its lytic effect on Escherichia coli and Micrococcus lysodeikticus. Comparative Biochemistry and Physiology—Part B 1988, 91 (4), 793–796.
- Saleh, A.M.; Ibrahimi, I.M. Electrophoretic polymorphisrn in rabbit tear lysozyme. Comparative Biochemistry and Physiology 1995, 112B (1), 21–30.
- Priyadarshini, S.; Kansal, V.K. Lysozyme activity in buffalo milk: Effect of lactation period, parity, mastitis, season in India, pH, and milk processing heat treatment. Asian-Australasian Journal of Animal Sciences 2002b, 15 (6), 895–899.
- Pahud, J.J.; Widmer, F. Calf rennet lysozyme. Biochemical Journal 1982, 201, 661–664.
- McKenzie, H.A.; White, F.H. Determination of lysozyme activity at low levels with emphasis on the milk enzyme. Analytical Biochemistry 1986, 157 (2), 367–374.
- Ye, X.; Zhang, L.; Tian, Y.; Tan, A.; Bai, J.; Li, S. Identification and expression analysis of the g-type and c-type lysozymes in grass carp Ctenopharyngodon idellus. Developmental and Comparative Immunology 2010, 34, 501–509.
- Halliday, J.A.; Bell, K.; Shaw, D.C. Feline and canine milk lysozymes. Comparative Biochemistry and Physiology—Part B. 1993, 106 (4), 859–865.
- Smolelis, A.N.; Hartsell, S.E. Factors affecting the lytic activity of lysozyme. Journal of Bacteriology 1952, 63, 665–674.
- Sidhan, V.; Gurnani, S. Kinetic characterization of rat liver nuclear lysozyme. Journal of Biosciences 1982, 4 (2), 191–195.
- Thompson, R. Lysozyme and its relation to antibacterial properties of various tissues and secretions. Archives of Pathology Journal 1940, 30, 1096–1134.
- Miyauchi, K.; Matsumiya, M.; Mochizuki, A. Purification and characterization of lysozyme from purple Washington clam Saxidomus purpurata. Fisheries Sciences 2006, 72, 1300–1305.
- Nikaido, H.; Vaara, M. Molecular basis of bacterial outer membrane permeability. Microbiology and Molecular Biology Reviews 1985, 49, 1–32.
- Martinez, R.J.; Carroll, S.F. Sequential metabolic expressions of the lethal process in human serum-treated Escherichia coli: Role of lysozyme. Infection and Immunity 1980, 28, 735–745.