Abstract
Gum arabic is the complex exudate of the Acacia senegal and Acacia seyal tree. This dried sap with immense commercial value is a global commodity, generally harvested in Africa and Western Asia. Non-digestibility, low solution viscosity, and generally recognized as safe status renders its popularity in the food industries. For its desirable emulsifying, stabilizing, binding, and shelf-life enhancing properties, it has found application in many foods. Current literature suggests its cardio-, reno-, gut-, and dental- protective, satiety-inducing, antimicrobial, anti-inflammatory, and anticoagulant implications. Its foray into drug delivery, sensor, tumor imaging, and nano-technology has met with appreciable success, fueling further investigation into its unexplored functionality. The objective of this review was to highlight the recently-unraveled pharmacological potential of this gum with an aim to provide holistic information on its wide spectrum of utility and prospects.
INTRODUCTION
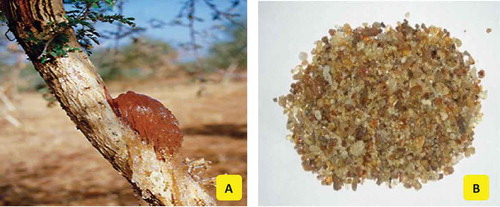
Gum arabic (GA) or acacia gum is the exudate from the Acacia senegal and Acacia seyal trees, belonging to Leguminosae family (). It’s a complex, branched heteropolysaccharide, either neutral or slightly acidic and composed of 1, 3-linked β-d-galactopyranosyl units. L-arabinose, L-rhamnose, and D-glucuronic acid have also been detected as constituents of this polymer.[Citation1] The side chains are composed of two to five 1, 3-linked β-d-galactopyranosyl units, which join the main chain by 1, 6-linkages. Some sources report that this gum is a mixture of polysaccharides and glycoproteins. The light-orange or pale-white pieces of GA are water soluble. Geographical distribution of these plants ranges from west of Africa to Indian peninsula. Most of the GA is harvested from the arid lands of Sudan, Chad, Nigeria, Senegal, and Ethiopia. Sudan is the largest exporter, accounting for up to 80% of the trade, followed by Nigeria. The dried saps are harvested as translucent masses, cleaned of extraneous materials, kibbled or powdered, and exported to Western countries. GA has acquired its name from Arabian traders who introduced it to Europe and played a key role in its popularity.
GA is one of the oldest and best-known among all natural gums. The usage of GA dates back to 5000 years ago. GA has been regarded as a therapeutic for chronic kidney diseases in Middle Eastern countries.[Citation2] For its edibility, vigorous water solubility, generally recognized as safe (GRAS) status, lack of aftertaste, and other desirable characteristics, GA has found broad utility in the food industry. For its emulsifying, stabilizing, thickening, and binding attributes, it is incorporated in food formulations ranging from ice creams, jellies, candies, soft drinks, beverages, syrups, and chewing gums. The film-forming properties make it ideal for confectionery coatings and glazes. The ability to enhance the shelf-life of flavors renders it attractive as a food additive. The European Union has given its approval to GA for food applications. Codex Alimentarius, the collection of internationally recognized standards, codes of practice and guidelines has also recommended it. In pharmaceuticals and herbal medicines, it coats pills and lozenges. Also, it is used in cosmeceuticals for formulation of creams and lotions. Due to its excellent binding property, it is a key ingredient in traditional lithography, printing, and water color paints. GA has found use in the textile industry as well for its ability to enhance tensile strength of the yarns. In this context, it seems worthwhile to keep a tab on the developments in GA technology in last decade and track the course of future advances.
CONSTITUENTS AND PROPERTIES
The quality of GA are evaluated based on the parameters like color, odor, moisture and ash content, viscosity, pH, specific rotation, tannins, and concentration of several metals. Mineral generally found are Ca, Na, K, P, and traces of Pb, Co, Cu, Zn, Ni, Cd, Cr, and Mn. If the quality of GA conforms well to international standards, only then are they exported. So, the proportions of the constituents are important parameter in quality regulation. Nasir et al.[Citation3] reported that GA solutions contain particularly high concentrations of Ca2+, Mg2+, and K+. Katayama et al.[Citation4] investigated the chemical and physical changes in GA induced by the treatment with ionizing radiations. The irradiation at high solute concentrations caused polymerization of GA even without any additives.
UTILITY SPECTRUM OF GA
This biopolymer has found an overwhelming array of uses. Below is a brief summarization of the breakthroughs in recent times. The applications validated until now have been presented in .
TABLE 1 Various applications of gum arabic
Dietary Fiber and Food Additive
GA conforms to the definitions of dietary fiber, i.e., a non-starch polysaccharide, resistant to intestinal enzymes and fermentable in the colon to liberate short-chain fatty acids. For its low calorific value, it is suitable for food-fortifications. Makri and Doxastakis[Citation5] analyzed the characteristics of oil-in-water emulsions after addition of GA, xanthan, or locust bean gum. The additives decreased the amount of protein adsorbed and enhanced the emulsions viscosity rendering stability. Pua et al.[Citation6] optimized the concentration of soy lecithin and GA in producing drum-dried jackfruit powder using response surface methodology (RSM). It was observed that jackfruit puree could be formulated by incorporating 2.65% of soy lecithin and 10.28% of GA into it. A matured GA, SUPER GUM was investigated for its ability to aid in the stabilization of water-in-oil-in-water emulsions and it was found to provide stability over a wide pH range, compared to other emulsifiers.[Citation7] Mirhosseini et al.[Citation8] optimized the orange beverage emulsion characteristics by RSM. The optimum composition was predicted to be 10.78% (w/w) GA, 0.24% (w/w) xanthan gum, and 12.43% (w/w) orange oil. Oil-in-water emulsions are crucial for the beverage industry to provide flavor, color, and turbidity to the soft drink. Food-grade emulsifiers for stabilizing the cloudy emulsion are always sought after. Klein et al.[Citation9] assessed several GA-based emulsifiers for optimum formula. The microfluidized emulsions were characterized by particle size distribution analysis (DLS) and optical microscopy. The emulsion composed of 3 wt% blends of whey protein isolate and GA in the ratio 3:1, along with 20 wt% canola oil proved suitable. Egwim and Oloyede[Citation10] reported the efficacy of GA as binder in immobilization of α-amylase in industrial bioreactors. The biopolymer performed better than guar gum in retaining enzyme activity and predicted system operations at a cost-effective manner. Pecivova et al.[Citation11] determined the effect of GA and apple-derived pectin on the sensory attributes of pizza flans. The addition enhanced the quality and flavor of the flans. However, a higher amount of GA tended to increase dryness, leading to adverse effect on the texture and taste.
Enhancement of Vegetable Shelf-Life
The perishability of fruit and vegetables is a major concern. An edible coating on them to retain freshness, quality, to enhance their shelf-life, and delay further ripening is a prevalent strategy to avert the above issues. Wax coatings of fruit and vegetables are common for they accentuate the appearance, retain moisture, and reduce weight loss. The coatings used on fruits and vegetables must meet the food additive regulations of the U.S. Food and Drug Administration. As a substitute of paraffin wax, performance of GA was assessed. Maqbool et al.[Citation12] suggested that a composite of 10% GA and 1% chitosan can be used to make an edible coating, commercially suitable for extending the storage life of bananas up to 33 days. At 13°C and 80% relative humidity (RH), the fruits could be stored significantly longer than the controls. The desirable attributes like fruit firmness, total carbohydrates, and reducing sugars were higher than the control. The shelf-life prolongation is supposed to be due to the reduced rate of respiration and ethylene evolution. El-Anany et al.[Citation13] evaluated the effect of a blend of soybean gum, jojoba wax, glycerol, and GA, on the shelf-life and quality of Anna apple during cold storage at 0°C and 90–95% RH. The results indicated that the coated apples attain delayed change in weight loss, firmness, titratable acidity, total soluble solids, decay, and color, compared to the uncoated ones. Ali et al.[Citation14] evaluated the potential of GA in coating tomatoes for their shelf-life extension after postharvest storage. It was observed that tomato coated with 10% GA shows a significant delay in changes of weight, firmness, titratable acidity, soluble solids concentration, ascorbic acid content, decay percentage, and color development compared to the uncoated fruit. The sensory evaluation proved that the above dose of GA maintains the overall quality of the vegetable during the storage period of 20 days at 20°C, without any spoilage and off-flavor. The management of anthracnose caused by Colletotrichum spp. is a major concern for the tropical fruit industry because of the resulting financial losses. Maqbool et al.[Citation15] studied the antifungal effect of GA combined with essential oils such as lemongrass and cinnamon oils in checking the postharvest anthracnose of banana and papaya. A synergistic blend of 10% GA, 0.05% lemongrass, and 0.4% cinnamon showed inhibition of the mycelia growth and spore germination. It was observed that GA, along with cinnamon oil, significantly delays the ripening process and maintains the quality more effectively during the entire storage period. Jiang et al.[Citation16] reported the efficacy of a blend of GA and natamycin in improving the quality and shelf-life of shiitake mushroom, for as long as 16 days. Idris et al.[Citation17] studied and compared the efficacy of GA as a color preservative and inhibitor of non-enzymatic browning of dehydrated tomato during storage with sodium metabisulfite. Tomato homogenates were treated with 1, 2.5, 5, 7.5, and 10.0% GA and 1.0% sodium metabisulfite, respectively, dehydrated, and color changes were monitored during four months of storage. Water activity decreased and Brix values increased significantly in GA-treated samples. Lightness value increased significantly with the addition of 5–10% GA compared to the dehydrated control. GA, at a dose of 5–10% (w/w) preserved color of dehydrated tomatoes during the studied storage period.
Microencapsulation for Stability
The effect of the addition of maltodextrin and GA in freeze-dried strawberry powder was studied. On incorporating the additive, the critical water activity increased significantly causing increase in its glass transition temperature. The hygroscopicity of freeze-dried strawberry powder could be minimized and stability improved.[Citation18] For its excellent emulsifying property, GA is used as a flavour encapsulator and stabilizer of citrus oil emulsion concentrates in soft drinks.[Citation19] To retain the flavor profile of cardamom oleoresin against sensitivity to light, heat, and oxygen, its microencapsulation was conducted. Oleoresin was spray-dried using GA, maltodextrin, and modified starch. The microcapsules were evaluated for the content and stability of volatiles, non-volatiles, entrapped 1, 8-cineole, and α-terpinyl acetate for six weeks. GA offered greater protection to the oleoresin than other polymeric constituents.[Citation20] In microencapsulation of cumin oleoresin too, GA offered superior protection compared to maltodextrin and modified starch (HiCap 100). However, the blend of the three polymers at a ratio 4:1:1 provided best protective effect.[Citation21] The ethanol extract of ginkgo leaf has the potency to enhance alkaline phosphatase (ALP) activities and increase collagen I in mouse osteoblast MC3T3-E1 cells, but oxidation-induced denaturation poses a challenge. Haidong et al.[Citation22] studied the oxidative stability of the polyphenol-rich extracts of ginkgo leaf microencapsulated with GA. The microcapsules increased the collagen I in mouse osteoblast MC3T3-E1 cells. Further, the pharmacological experiment showed that rabbits pre-treated with microcapsules of ginkgo leaf extracts had enhanced ALP activities and were at less risk of ischemia/reperfusion-induced oxidative injury.
Cardiovascular, Renal, and Intestinal Effects
Nasir et al.[Citation3] reported that the treatment with GA significantly increases both the intestinal and renal excretion of Mg2+ and Ca2+. The latter was accompanied by decreased urinary excretion of inorganic phosphate and decreased plasma concentrations of 1, 25-dihydroxy vitamin D. GA increased 24 h creatinine clearance and urinary antidiuretic hormone excretion, and decreased daily urine output as well as the urinary excretion of Na+. Collectively, the treatment with GA resulted in moderate but significant increases of creatinine clearance and altered electrolyte excretion, favoring in renal insufficiency. Glover et al.[Citation23] evaluated the cardiovascular and renal effects of dietary GA, Acacia (sen) SUPERGUM in normal individuals and a group of diabetic nephropaths. The normal diet supplemented with 25 g of SUPERGUM/day for a period of 8–12 weeks, showed a consistent fall in the mean systolic blood pressure of healthy volunteers. However, there were no effects of GA on renal function and haemodynamics of patients with diabetic nephropathy. Nasir et al.[Citation2] reported on the basis of their work that 10% GA when consumed with water increases urinary excretion of Ca2+ and decreases plasma phosphate and urea concentrations. GA decreases blood pressure and proteinuria in diabetic mice and might be of clinical importance in diabetic nephropathy.
Satiety and Anti-Obese Effects
Calame et al.[Citation24] studied the satiating effect induced by two blends of GA (EmulGold [EG] and PreVitae [PV]). The energy intake was determined 3 h after consumption of the GAs dissolved in water. At a dose of 40 g, both EG and PV yielded a significant reduction in energy intake of more than 100 and 200 kcal, respectively. At doses of 10 or 20 g, the reduction in energy intake amounted to more than 100 kcal for both. The results of this study show that both blends of GA are able to decrease the caloric intake significantly so may be used in a dietary strategy to control body weight gain. Ushida et al.[Citation25] studied the possible anti-obesity effect of GA as a dietary fiber. Three month old female mice were given a GA-drink (1% w/v) for 180 days. The GA in drinking water reduced age-dependent fat deposition. This inhibition of fat deposition effect was assumed to be due to the β3-adrenergic stimulation of adipocytes in which TNFα down-regulation. The modification of large intestinal microflora, as evidenced by the alteration in cecal short-chain fatty acid and 16S rDNA profile might have contributed to such reduction in TNF-α expression in the adipose tissues.
Antimicrobial Activity
Nishi et al.[Citation26] investigated the adjuvant role of oxidized GA in amphotericin B therapy against fungal pathogen Candida albicans and Cryptococcus neoformans, and leishmania causative agent Leishmania donovani. The drug conjugate was stable, non-haemolytic, and free of any toxicity to internal organs of the treated animal while exerting appreciable anti-fungal and anti-leishmanial activity. Ballal et al.[Citation27] conducted both in vitro and in vivo study to test the inhibitory effect of GA on malaria. The human erythrocytes were in vitro infected with Plasmodium falciparum. Mice were in vivo infected with Plasmodium berghei ANKA by intraperitoneal injection of parasitized murine erythrocytes. The oral administration of 10% GA, ten days prior to the infection, showed attenuated level of parasites in blood. The GA degradation product butyrate is attributed to the reduced vigor of malaria.
Protective Effect Against Dental Erosion
GA is considered to have an ability to enhance remineralization, because of its high concentration of Ca2+. Onishi et al.[Citation28] evaluated the cariostatic activities of GA using histopathological methods. After incubation in demineralization solution, human molar teeth were exposed to 10 mg/ml of GA, sodium fluoride at 1000 ppm (NaF, a salt with well-established cavity fighting ability), or double distilled water, then subjected to demineralization-remineralization cycles. Contact microradiographs of the molars exposed to GA were similar to that of those exposed to NaF, showing significant remineralization of caries. Beyer et al.[Citation29] investigated the protective effect of GA against acidic soft drink-caused enamel erosion. The enamel samples were exposed to polymer-modified citric acid solutions for 30, 60, and 120 s, respectively. Atomic force microscopy (AFM)-based nano-indentation was used to analyze the nano-mechanical properties of the treated enamel samples and scanning electron microscopy was used to scan their surface morphologies. Enamel samples treated with GA-enriched citric acid showed higher hardness and smoother surface compared to the enamel surfaces of samples treated only with citric acid. It is hypothesized that GA is adsorbed as a protective layer on the eroded enamel surface moderating the harshness of the acid.
Anti-Inflammatory and Anticoagulation Effect
Wapnir et al.[Citation30] suggested the inflammation modulation capacity of GA alone or in combination with cathartics (substance that accelerates in defecation). In male juvenile rats, the NF-κB p65 activity was highest after administration of cathartics followed by GA. Mucosal IL-1β was overexpressed in tissues from cathartic-treated rats and rats given high-GA solutions. Abd El-Mawla and Osman[Citation31] investigated the effects of water extract of GA on the tissues and enzymes of intestine and pancreas of rats intoxicated with the drug Meloxicam. When given 0.2 mg/kg bw meloxicam followed by 1 g gum in the same doses per day for a period of 21 days, pronounced protective effect of GA extract on the histology of rat intestine was observed. Also, a significant increase in the intestinal enzymes; lipase, amylase, ALP, and lactate dehydrogenase (LDH) were recorded. Ali et al.[Citation32] studied the role of inflammation and oxidative stress in adenine-induced chronic renal failure and the possible ameliorative effect of GA. Rats were given adenine combined with GA for four weeks, at the end of which urine, blood, and kidneys were subjected to tests. Adenine-induced increase in the concentrations of urea and creatinine in plasma, decrease in creatinine clearance, and increase in the inflammatory mediators were normalized by GA intake. Further, the oxidative stress and DNA damage were ameliorated. Hadi et al.[Citation33] conducted a randomized test to investigate the effect of GA on the coagulation system of Wistar rats. After administering GA along with drinking water for four weeks, blood samples were collected and coagulation parameters were determined. The bleeding time of rats treated with 6 gGA/100 ml was significantly prolonged; whereas, the prothrombin time of rats treated with 10 gGA/100 ml were significantly longer.
BIOMATERIAL/HYDROGEL
By means of a conventional cross-linking/co-polymerization reaction of modified GA, acrylamide and potassium acrylate in the presence of magnetite (Fe3O4) nano-particles, a magnetic field-sensitive smart hydrogel was synthesized and its potential as a magnetic biomaterial investigated. Characterizations through Fourier transform infrared spectrometer (FT-IR), nuclear magnetic resonance spectroscopy (NMR), X-ray diffraction spectroscopy (XRD), scanning electron microscopy (SEM), and energy-dispersive X-ray spectroscopy (EDX) revealed that the intelligent hydrogel was efficiently formed. The noble hydrogel might effectively be applied as biomaterial in tissue engineering.[Citation34]
Drug Delivery Agent
Drug expicients made of synthetic materials are generally toxic and expensive. So, non-toxic natural expicients to deliver the bioactive formula is sought-after. An injectable, biodegradable drug depot with controlled release of drugs for long-term delivery is required. Batra et al.[Citation35] recorded sustained release of FeSO4 for 7 h from GA pellets. An increase in the amount of GA in the pellets decreased the rate of release due to its inherent gelling property. The gel layer acts as a barrier and retards the rate of diffusion of FeSO4 through the pellet. Lu et al.[Citation36] reported that GA can be used in preparation of a monolithic osmotic tablet system for slow release of water-insoluble drugs. The model drug naproxen was released to about 81% of its initial content at pH 6.8 after 12 h. The diffusion was independent of environment media and stirring rate, suggesting the use of GA in oral drug-controlled delivery. Reis et al.[Citation37] prepared a pH-responsive hydrogel from GA chemically modified with glycidyl methacrylate. The cross-linking reaction led to the formation of a hydrogel. At high pH values, the water transport depended on polymer relaxation. The pH-responsive hydrogel appeared promising for further tests as drug carriers. Primaquine, a potent malaria drug, forms a cross-linked gel with periodate-oxidized GA by rapid mixing of the two constituents. It was observed that higher degree of GA oxidation leads to higher cross-linking density. In vitro release of primaquine into phosphate buffered saline (PBS) at 37°C demonstrated that the extent of release depended on the cross-linking density and drug payload. Repeated extraction using PBS soon after gel formation showed that not all of the primaquine was conjugated to the polysaccharide and the release seen in vitro was mostly from the unconjugated drug especially from matrices with higher cross-linking density. The gels were found to degrade in PBS and MTT assay against L929 mouse fibroblasts showed only very mild cytotoxicity at a concentration of 0.025 g/mL. This conjugate with desirable characteristics can be of considerable importance in battling against a host of diseases.[Citation38] Zhang et al.[Citation39] synthesized and characterized a magnetic iron oxide nano-particles (MNP) coated with 15.6% GA. The GA coating enhanced colloidal stability and provided reactive functional groups suitable for coupling of bioactive compounds. Significant cellular uptake of GA-MNP was evaluated in 9L glioma cells which suggested that the composite might be utilized as a MRI-visible drug carrier in achieving intracellular drug delivery. Avadi et al.[Citation40] reported that chitosan and GA-induced diffusion and relaxation could effectively control the release of nano-particles from polymer chains.
Sensor and Tumor Imaging
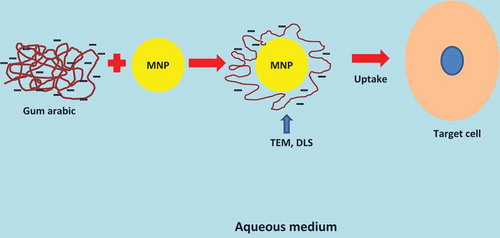
Tiwari[Citation41] fabricated an electrically active, water‐soluble radical copolymer of GA with redox property. With a battery of high-end technical characterization, the shelf-life and electrical conductivity of the copolymer was monitored. Results held possibilities of their application in the fabrication of semiconductor sensor devices. Zhang et al.[Citation39] studied that GA coating on MNP enhanced the colloidal stability and provided reactive functional groups suitable for coupling of bioactive compounds. MRI confirmed the accumulation of GA-MNP at the 9L glioma tumors site after intravenous administration to the rats while electron spin resonance (ESR) spectroscopy reported the fold-increase in GA-MNP accumulation to be 12 ().
FIGURE 2 Graphical illustration of the stabilizing effect of negatively charged gum arabic on iron oxide magnetic nano-particles. Gum arabic provides functionalization and cell selectivity. It provides increased electrostatic repulsion for better dispersal and stabilization of the particles, facilitating uptake by the cells.
Applications in Nanotechnology
Several studies have been conducted on the potential utility of GA in nano-constructs and molecular imaging. GA acts as reducing and oxidizing agent, so vital for molecular functionalization of nano-materials. Functionalization by this biopolymer has been evidenced to cause toxicity elimination, pathogen detection, stability improvement, and better dispersion of nano-particles in water.
Williams et al.[Citation42] functionalized magnetite nano-particle surfaces by GA coating, to enhance stability. Particle characterization was performed using transmission electron microscopy (TEM) and dynamic light scattering (DLS) to analyze the morphology and quantify the size distribution of the nano-particles, respectively. The results from DLS indicated that the GA-treated nano-particles formed smaller agglomerates as compared to the untreated samples over a 30 h time frame. Thermogravimetric analyses indicated an average weight loss of 23%, showing that GA has a strong affinity toward the iron oxide surface. GA shows promise for dispersing nanoparticles in aqueous solutions. Gold nanoparticles (AuNPs) are very stable against oxidation, thus significant in the advancement of clinically useful diagnostic and therapeutic nano-medicines. However, the huge potential of AuNP-based nano-medicinal products is blighted by toxicity, rendering it unsuitable for intravenous mode. Kattumuri et al.[Citation43] demonstrated that GA can be used as a non-toxic material in the production of readily administrable biocompatible AuNPs for diagnostic and therapeutic applications in nano-medicine. Gamma-Al2O3 nano-particles were synthesized and modified using GA to avoid agglomeration (clustering) in aqueous solutions. Results showed that the nano-particles dispersed well in aqueous solutions after GA modification. Also it increased adsorptive desulfurization and biodesulfurization capacity, assumed to be due to improvement in the dispersion and biocompatibility of γ-Al2O3 nano-particles post GA modification.[Citation44] The adsorption of GA onto hydroxyapatite and magnetic nano-particles were studied.[Citation45] Roque et al.[Citation46] studied the surface modification of MNPs with GA via adsorption and covalent coupling. The covalent immobilization of GA at the surface of aldehyde-activated or aminated MNPs yielded negatively charged surfaces which showed promise for better biomolecular attachment. Chockalingam et al.[Citation47] synthesized GA-modified MNPs capable of isolating S. aureus bacteria at 8–10 colony forming units (cfu)/ml within ˜3 min. This alternative fast detection technique can be employed for other microbial detection. Wu and Chen[Citation48] synthesized AuNPs using GA as reducing as well as stabilizing agent. GA proved helpful in reducing the Au (III) ions completely and stabilizing the nano-particles.
Onyari et al.[Citation49] studied various polylactic acid and GA blends and showed that it is possible to prepare films of varying hydrophobic-hydrophilic properties for various applications. Uniform distribution of the nano-cellulose fillers in starch film matrix posed a hurdle in exploiting the full potential of nanomaterials. GA as additive distributed the nano-cellulose uniformly by reducing surface energy and improved the tensile strength. Further, the vapor permeability of the starch film was reduced two-fold.[Citation50]
Earning Opportunity
GA oozes naturally from the barks or nicks are made or stripping done to induce flow. The tappers collect the gum in a sequence of plucking, sorting, and filling in bags. Acacia senegal is part of large-scale sustainable-agriculture, forest-management, and rural economic-development strategies. Hundreds of thousands of poor Sudanese are dependent on acacia gum for their livelihoods. If tapped properly, GA can be a sustainable source of income in the poverty-ridden Africa.
Issues Encountered
Ingestion of GA as food additive still faces regulatory issues from the European Commission (EC).[Citation51] An obstacle to regulatory approval is the wide natural variability and inconsistent quality of commercial GA which changes its molecular parameters and functional properties. Irregularity in supply and wide fluctuating prices combined with availability of cheap and accessible substitutes are threats looming over the popularity of GA. Guar gum hydrolysate emulsification properties are comparable to those of GA.[Citation52] Locust gum, xanthan, and mesquite gum also pose as rivals.[Citation53]
Boosting Acacia Agro-Forestry
GA production in Sudan has developed over the years in a well-established traditional bush-fallow system in which the gum tree (Acacia senegal) is rotated with annual crops. Following the Sahel drought, the gum area has suffered deforestation and GA production has conspicuously declined. Many original adopters have abandoned gum production and the bush-fallow system. Several programs have been developed to revive and boost gum production. Rahim et al.[Citation54] studied the decision-making behavior of farmers in west Sudan. The financial condition and off-farm works were found to have significant influence in the disadoption decision of the farmers. It was inferred that emphasizing on annual crops in the region is likely to reduce the seasonal labor migration and improve GA production. Further, it is opined by economists that the expansion of gum agro-forestry can be boosted by enhancing the cost of GA in international market so that farmers can get reasonable profit.
Future Prospects
GA is an ideal model for unraveling the properties and interaction of food formulation and nano-conjugates. Salazar-Montoya and Jimenez-Avalos[Citation55] analyzed the flow and viscoelasticity behavior of dispersions of 15 to 45% (w/v) GA, 1 to 8% (w/v) soy proteins, and their mixtures. The interaction between the polysaccharide and protein led to a better understanding of the rheological behavior of complex mixtures at high concentrations, allowing these model systems to be useful in the future food applications. Extrapolating from the recent evidence bases it is clear that GA is a key polysaccharide in formulation of stable nano-dispersions. These findings can be harnessed for optimal retention of bioactive components (astaxanthin). Further, the GA-based encapsulation of food additives are expected to be benefitted from extreme conditions and released to food gradually, i.e., aspartame sweetener. Future prospects of GA seems bright and pivotal for the food and medical industries.
CONCLUSION
GA has vital role in industrial manufacturings, spanning from food, pharmaceutics, paint, textile, and printing industries. It has proved itself indispensable as stabilizer, emulsifier, bulking agent, shelf-life enhancer, encapsulating agent for bioactive components, and satiating agent. Its anti-inflammatory effects on multiple organs have elevated its status as a safe food additive. Now, GA is increasingly being implicated in nano-medicines and biosensors. Among other effective uses, controlled drug delivery and better biodispersion of nano-paticles are most pronounced. On the other hand, its agro-forestry is facing steep competition from cash crops. Incentive or subsidies from authorities may motivate farmers to stick to Acacia plantations. The dwindling GA production must be bolstered for sustainable economic growth of cash-strapped and unemployment-ridden African countries.
REFERENCES
- Shirwaikar, A.; Shirwaikar, A.; Prabu, S.L.; Kumar, G.A. Herbal excipients in novel drug delivery systems. Indian Journal of Pharmaceutical Sciences 2008, 70, 415–422.
- Nasir, O.; Umbach, A.T.; Rexhepaj, R.; Ackermann, F.; Bhandaru, M.; Ebrahim, A.; … Lang, F. Effects of gum arabic (Acacia senegal) on renal function in diabetic mice. Kidney and Blood Pressure Research 2012, 35, 365–372.
- Nasir, O.; Artunc, F.; Saeed, A.; Kambal, M.A.; Kalbacher, H.; Sandulache, D.; … Lang, F. Effects of gum arabic (Acacia senegal) on water and electrolyte balance in healthy mice. Journal of Renal Nutrition 2008, 18, 230–238.
- Katayama, T.; Nakauma, M.; Todoriki, S.; Phillips, G.O.; Tada, M. Radiation-induced polymerization of gum arabic (Acacia senegal) in aqueous solution. Food Hydrocolloids 2006, 20, 983–989.
- Makri, E.A.; Doxastakis, G.I. Study of emulsions stabilized with Phaseolus vulgaris or Phaseolus coccineus with the addition of Arabic gum, locust bean gum, and xanthan gum. Food Hydrocolloids 2006, 20, 1141–1152.
- Pua, C.K.; Hamid, N.S.A.; Rusul, G.; Rahman, R.A. Production of drum-dried jackfruit (Artocarpus heterophyllus) powder with different concentration of soy lecithin and gum arabic. Journal of Food Engineering 2007, 78, 630–636.
- Su, J.; Flanagan, J.; Singh, H. Improving encapsulation efficiency and stability of water-in-oil-in-water emulsions using a modified gum arabic (Acacia [sen] SUPER GUM™). Food Hydrocolloids 2008, 22, 112–120.
- Mirhosseini, H.; Tan, C.P.; Hamid, N.S.A.; Yusof, S. Effect of Arabic gum, xanthan gum, and orange oil contents on ζ-potential, conductivity, stability, size index, and pH of orange beverage emulsion. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2008, 315, 47–56.
- Klein, M.; Aserin, A.; Svitov, I.; Garti, N. Enhanced stabilization of cloudy emulsions with gum arabic and whey protein isolate. Colloids and Surfaces B: Biointerfaces 2010, 77, 75–81.
- Egwim, E.C.; Oloyede, O.B. Assessment of gum arabic and agar gum as binders for the immobilization of α-amylase. Journal of Biochemical Technology 2011, 3, 222–224.
- Pecivova, P.; Dula, T.; Hrabe, J. The influence of pectin from apple and gum arabic from acacia tree on the quality of pizza. International Journal of Food Properties 2013, DOI:10.1080/10942912.2011.587931
- Maqbool, M.; Ali, A.; Alderson, P.G.; Zahid, N.; Siddiqui, Y. Effect of a novel edible composite coating based on gum arabic and chitosan on biochemical and physiological responses of banana fruits during cold storage. Journal of Agricultural Food Chemistry 2011a, 59, 5474–5482.
- El-Anany, A.M.; Hassan, G.F.A.; Ali, F.M.R. Effects of edible coatings on the shelf-life and quality of Anna apple (Malus domestica Borkh) during cold storage. Journal of Food Technology 2009, 7, 5–11.
- Ali, A.; Maqbool, M.; Ramachandran, S.; Alderson, P.G. Gum arabic as a novel edible coating for enhancing shelf-life and improving post-harvest quality of tomato (Solanum lycopersicum L.) fruit. Postharvest Biology and Technology 2010, 58, 42–47.
- Maqbool, M.; Ali, A.; Alderson, P.G.; Mohamed, M.T.M.; Siddiqui, Y.; Zahid, N. Post-harvest application of gum arabic and essential oils for controlling anthracnose and quality of banana and papaya during cold storage. Postharvest Biology and Technology 2011b, 62, 71–76.
- Jiang, T.; Feng, L.; Zheng, X.; Li, J. Physicochemical responses and microbial characteristics of shiitake mushroom (Lentinus edodes) to gum arabiccoating enriched with natamycin during storage. Food Chemistry 2013, 138, 1992–1997.
- Idris Y.M.A.; Ibrahim, Y.A.; Mariod, A.A. Color of dehydrated tomato: Effects of gum arabic. International Journal of Food Properties 2013, 16, DOI:10.1080/10942912.2011.565535
- Mosquera, L.H.; Moraga, G.; Martínez-Navarrete, N. Critical water activity and critical water content of freeze-dried strawberry powder as affected by maltodextrin and arabic gum. Food Research International 2011, DOI:org/10.1016/j.foodres.2011.05.019
- Yadav, M.P.; Igartuburu, J.M.; Yan, Y.; Nothnagel, E.A. Chemical investigation of the structural basis of the emulsifying activity of gum arabic. Food Hydrocolloids 2007, 21, 297–308.
- Krishnan, S.; Kshirsagar, A.C.; Singhal, R.S. The use of gum arabic and modified starch in the microencapsulation of a food flavoring agent. Carbohydrate Polymers 2005, 62, 309–315.
- Kanakdande, D.; Bhosale, R.; Singhal, R.S. Stability of cumin oleoresin microencapsulated in different combination of gum arabic, maltodextrin, and modified starch. Carbohydrate Polymers 2007, 67, 536–541.
- Haidong, L.; Fang, Y.; Zhihong, T.; ZaiGuo, H. Use of combinations of gum arabic, maltodextrin, and soybean protein to microencapsulate Ginkgo leaf extracts and its inhibitory effect on skeletal muscle injury. Carbohydrate Polymers 2012, 88, 435–440.
- Glover, D.A.; Ushida, K.; Phillips, A.O.; Riley, S.G. Acacia(sen) SUPERGUM™ (Gum arabic): An evaluation of potential health benefits in human subjects. Food Hydrocolloids 2009, 23, 2410–2415.
- Calame, W.; Thomassen, F.; Hull, S.; Viebke, C.; Siemensma, A.D. Evaluation of satiety enhancement, including compensation, by blends of gum arabic. A methodological approach. Appetite 2011, 57, 358–364.
- Ushida, K.; Hatanaka, H.; Inoue, R.; Tsukahara, T.; Phillips, G.O. Effect of long term ingestion of gum arabic on the adipose tissues of female mice. Food Hydrocolloids 2011, 25, 1344–1349.
- Nishi, K.K.; Antony, M.; Mohanan, P.V.; Anilkumar, T.V.; Loiseau, P.M.; Jayakrishnan, A. Amphotericin B-gum arabic conjugates: Synthesis, toxicity, bioavailability, and activities against Leishmania and fungi. Pharmaceutical Research 2007a, 24, 971–980.
- Ballal, A.; Bobbala, D.; Qadri, S.M.; Foller, M.; Kempe, D.; Nasir, O.; … Lang, F. Anti-malarial effect of gum arabic. Malaria Journal 2011, 10, 139.
- Onishi, T.; Umemura, S.; Yanagawa, M.; Matsumura, M.; Sasaki, Y.; Ogasawara, T.; Ooshima, T. Remineralization effects of gum arabic on caries-like enamel lesions. Archives of Oral Biology 2008, 53, 257–260.
- Beyer, M.; Reichert, J.; Heurich, E.; Jandt, K.D.; Sigusch, B.W. Pectin, alginate, and gum arabic polymers reduce citric acid erosion effects on human enamel. Dental Materials 2010, 26, 831–839.
- Wapnir, R.A.; Sherry, B.; Codipilly, C.N.; Goodwin, L.O.; Vancurova, I. Modulation of rat intestinal nuclear factor NF-κB by gum arabic. Digestive Diseases and Sciences 2008, 53, 80–87.
- Abd El-Mawla, A.M.; Osman, H.E. Effects of gum acacia aqueous extract on the histology of the intestine and enzymes of both the intestine and the pancreas of albino rats treated with Meloxicam. Pharmacognosy Research 2011, 3, 114–121.
- Ali, B.H.; Al-Husseni, I.; Beegam, S.; Al-Shukaili, A.; Nemmar, A.; Schierling, S.; … Schupp, N. Effect of gum arabic on oxidative stress and inflammation in adenine-induced chronic renal failure in rats. PLoS ONE 2013, 8, e55242.
- Hadi A-H.A.; Elderbi, M.A.; Mohamed, A-W.H. Effect of gum arabic on coagulation system of albino rats. International Journal of PharmTech Research 2010, 2, 1762–1766.
- Paulino, A.T.; Guilherme, M.R.; Mattoso, L.H.C.; Tambourgi, E.B. Smart hydrogels based on modified gum arabic as a potential device for magnetic biomaterial. Macromolecular Chemistry and Physics 2010, 21, 1196–1205.
- Batra, V.; Bhowmick, A.; Behera, B.K.; Ray, A.R. Sustained release of ferrous sulfate from polymer-coated gum arabic pellets. Journal of Pharmaceutical Sciences 1994, 83, 632–635.
- Lu, E.X.; Jiang, Z.Q.; Zhang, Q.Z.; Jiang, X.G. A water-insoluble drug monolithic osmotic tablet system utilizing gum arabic as an osmotic, suspending, and expanding agent. Journal of Controlled Release 2003, 92, 375–382.
- Reis, A.V.; Guilherme, M.R.; Cavalcanti, O.A.; Rubira, A.F.; Muniz, E.C. Synthesis and characterization of pH-responsive hydrogels based on chemically modified Arabic gum polysaccharide. Polymers 2006, 47, 2023–2029.
- Nishi, K.K.; Jayakrishnan, A. Self-gelling primaquine-gum arabic conjugate: An injectable controlled delivery system for primaquine. Biomacromolecules 2007b, 8, 84–90.
- Zhang, L.; Yu, F.; Cole, A.J.; Chertok, B.; David, A.E.; Wang, J.; Yang, V.C. Gum arabic-coated magnetic nano-particles for potential application in simultaneous magnetic targeting and tumour imaging. The AAPS Journal 2009, 11, 693–699.
- Avadi, M.R.; Sadeghi, A.M.M.; Mohammadpour, N.; Abedin, S.; Atyabi, F.; Dinarvand, R.; Rafiee-Tehrani, M. Preparation and characterization of insulin nano-particles using chitosan and arabic gum with ionic gelation method. Nanomedicine: Nanotechnology, Biology, and Medicine 2010, 6, 58–63.
- Tiwari, A. Gum arabic‐graft‐polyaniline: An electrically active redox biomaterial for sensor applications. Journal of Macromolecular Science, Part A 2007, 44, 735–745.
- Williams, D.N.; Gold, K.A.; Holoman, T.R.P.; Ehrman, S.H.; Wilson, O.C. Surface modification of magnetic nano-particles using gum arabic. Journal of Nano Research 2006, 8, 749–753.
- Kattumuri, V.; Katti, K.; Bhaskaran, S.; Boote, E.J.; Casteel, S.W.; Fent, G.M.; … Katti, K.V. Gum arabic as a phytochemical construct for the stabilization of gold nano-particles: In vivo pharmacokinetics and X-ray-contrast-imaging studies. Small 2007, 3, 333–341.
- Zhang, H.Y.; Shan, G.B.; Liu, H.Z.; Xing, J.M. Surface modification of γ-Al2O3 nano-particles with gum arabic and its applications in adsorption and biodesulfurization. Surface and Coatings Technology 2007, 201, 6917–6921.
- Roque, A.C.A.; Wilson Jr, O.C. Adsorption of gum arabic on bioceramic nano-particles. Material Science and Engineering: C 2008, 28, 443–447.
- Roque, A.C.A.; Bicho, A.; Batalha, I.L.; Cardoso, A.S.; Hussain, A. Biocompatible and bioactive gum arabic coated iron oxide magnetic nano-particles. Journal of Biotechnology 2009, 144, 313–320.
- Chockalingam, A.M.; Babu, H.K.R.R.; Chittor, R.; Tiwari, J.P. Gum arabic modified Fe3O4 nano-particles cross linked with collagen for isolation of bacteria. Journal of Nanobiotechnology 2010, 8, 30.
- Wu, C-C.; Chen, D-H. Facile green synthesis of gold nano-particles with gum arabic as a stabilizing agent and reducing agent. Gold Bulletin 2010, 43, 234–240.
- Onyari, J.M.; Mulaa, F.; Muia, J.; Shiundu, P. Biodegradability of Poly (lactic acid), preparation and characterization of PLA/gum arabic blends. Journal of Polymers and the Environment 2008, 16, 205–212.
- Vigneshwaran, N.; Ammayappan, L.; Huang, Q. Effect of gum arabic on distribution behaviour of nanocellulose fillers in starch film. Applied Nanoscience 2011, 1, 137–142.
- Phillips, G.O.; Ogasawara, T.; Ushida, K. The regulatory and scientific approach to defining gum arabic (Acacia senegal and Acacia seyal) as a dietary fibre. Food Hydrocolloids 2008, 22, 24–35.
- Sarkar, S.; Singhal, R.S. Esterification of guar gum hydrolysate and gum arabic with n-octenyl succinic anhydride and oleic acid and its evaluation as wall material in microencapsulation. Carbohydrate Polymers 2011, 86, 1723–1731.
- López-Franco, Y.L.; Córdova-Moreno, R.E.; Goycoolea, F.M.; Valdez, M.A.; Juárez-Onofre, J.; Lizardi-Mendoza, J. Classification and physicochemical characterization of mesquite gum (Prosopis spp.) Food Hydrocolloids 2012, 26, 159–166.
- Rahim, A.H.; Ruben, R.; van Ierland, E.C. Examining disadoption of gum arabic production in Sudan. Agroforestry Systems 2008, 73, 115–126.
- Salazar-Montoya, J.A.; Jimenez-Avalos, H.A.; Ramos Ramirez, E.G. Effects of gum arabic concentration and soy proteins on the flow and viscoelasticity of their dispersion. International Journal of Food Properties 2012, 15, 891–902.