Abstract
Grapes (Vitis vinifera L. origin var: Carignane, Cabernet Sauvignon, Merlot, Grenache, Columbard, and Semillon) were cultivated and processed according to accepted organic agriculture and organic wine techniques. Aged wines (5 years) were evaluated for changes of their phenolic acids. The highest reduction of gallic acid concentrations were determined in Cabernet Sauvignon (24.36 mg/L) and Carignane (16.00 mg/L) wines. The quantities of p-hydroxybenzoic acid decreased mostly in Carignane (22.47 mg/L) and Columbard (20.84 mg/L) wines. The decreases of syringic acid were dominant in Cabernet Sauvignon (2.34 mg/L) and Carignane (1.69 mg/L) wines. In the case of ferulic acid, the highest reduction was determined in Cabernet Sauvignon (3.97 mg/L) wines. The decreases of p-coumaric acid contents were the same and mostly in Merlot (1.06 mg/L) and Grenache (1.035 mg/L) wines. The principal component analyses results demonstrated the relations among aged wines produced from different grape varieties and their phenolic acids. The distribution of data was accumulated into two groups. The first group included total phenols, gallic acid, p-hydroxybenzoic acid, syringic acid, p-coumaric acid, and Merlot/Carignane/Grenache wines. The second one included ferulic acid and Cabernet Sauvignon wine.
Keywords:
INTRODUCTION
Grapes and wines contain large amounts of phenolic compounds, mostly flavonoids at high concentrations of 1000–1800 mg/L. Most phenolics found in wines may act as antioxidants.[Citation1−Citation4] Anthocyanins, non-anthocyanic phenols (including flavan-3-ols, flavonols, and hydroxycinnamic acids) and polymeric pigments resulting from the reactions between anthocyanins and other phenols are the main compounds influencing the sensory properties of wines. Anthocyanins and polymeric pigments are responsible for the red color of wines, while non-anthocyanic phenols, such as flavan-3-ols and flavonols, are responsible for astringency and bitterness of wines.[Citation5] The antioxidant properties of wines are mainly due to flavonols and stilbenes.[Citation6,Citation7]
The constituents of red wine, especially flavonoids, have been of interest due to their health effects over the human body. Regarding the implicit quality of wine, consumers are particularly concerned with the health benefits of the product requiring an unadulterated wine that does not contain residues of chemical treatments (organic wine). Non-anthocyanin phenolic compounds present in wine mainly include hydroxybenzoic and hydroxycinnamic acids and their derivatives, stilbenes, phenolic alcohols, and flavonoid compounds, such as flavonols and flavanols. Together with anthocyanins, these compounds constitute important quality parameters of wines.
Organic wine is defined as “wine made from organically grown grapes.” In the meantime, organic wine (regarding both the crop and production methods) varies among certifiers. However, in all cases in order to be classified as organic, the farmer must be able to prove which vineyard the grapes came from, which officially recognized body certified the vineyard as organic, and from what date certified organic practices began. The main differences between organic and nonorganic wine production is the fact that “organic” suggests minimal processing and no use of chemical additives.
The study done with Vitis vinifera L. cv. Merlot wines elaborated from grapes of the same vineyard in five consecutive vintages (1997–2001) and presenting different aging time (1–5 years) in bottle (non-oxidative conditions) demonstrated that aging time in bottle seemed to have a larger influence over the concentration of main non-flavonoid phenolics (hydroxybenzoic and hydroxycinnamic acids and their derivatives), stilbenes, alcohols, and other phenols. The relative contents of the main groups of anthocyanin and non-anthocyanin phenolic compounds were slightly modified under the non-oxidative conditions present during bottle aging.[Citation8] To our knowledge, no research has reported the changes of phenolic acids during aging of organic wines. The aims of this study were to produce organic wines from different grape varieties, age them for 5 years, and evaluate their phenolic acid contents. The changes in gallic, p-hydroxybenzoic, syringic, p-coumaric, and ferulic acids at the end of the fifth year were reported.
MATERIALS AND METHODS
Materials
Organic grapes supplied from the Yazgan Company were cultivated under the control of the International Nutritional and Agriculture Certification (INAC) in Kemal Pasa, Ulucak Company. The experimental vineyard was 18 years old and trained as wired Goblet. It consists of six varieties (Vitis vinifera var: Merlot, Grenache, Carignane, Cabernet Sauvignon, Semillon, and Columbard). A mixture of vetch and barley (80 kg + 20 kg) was used for the nutrition of organic vine stocks. In addition, 30 kg of farm manure was applied per hectare every 2 years. The soil was cultivated twice in winter and spring by plow. During the vegetation period, the soil was worked twice more by cultivator. A sulphur treatment was applied five times for the control of powdery mildew (Uncinula necator), which is the main disease in the Aegean Region. Copper compounds were applied once or twice against downy mildew (Plasmopara viticola) according to the forecasting system. European grapevine moth (Lobesia botrana) was controlled by three or four sprayings of Bacillus thuringiensis. The timing of these applications was made by using the forecasting system. All grapes were transported to the Food Engineering Department of Ege University, Izmir, Turkey and crushed within 24 h of harvest.
Wine Processing
Grapes were hand harvested from a vineyard at Kemal Pasa in Ulucak. Maturity was evaluated according to the sugar (25–27 Brix) and acidity (4–5 g/L tartaric acid) contents of each grape variety. Non-mechanically crushed grapes were allowed to ferment until dryness (density <1000). Utilization of SO2 was restricted up to 25 mg/L SO2. For the production of white wines, must was inoculated with ‘Fermivin’ (2%, Sacchromyces cerevisiae 7013 INRA; Gist-Brocades Co., Delft, the Netherlands) for the red wines production with ‘Fermirouge’ (2%, S. cerevisiae 7000 INRA; Gist-Brocades Co.).
The pomace (residue from pressing) obtained during red wine production was stirred and pushed down twice daily. Alcohol fermentation was allowed to dryness in glass vessels. Fining (the process by which cloudiness is precipitated by dragging, using clays, such as bentonite or kaolin, or organic products, such as is in glass or egg white) and filtering (the removal of undesirable matter suspended in wine) were done without using any stabilizing and fining agents. Bottled wines were stored at 15°C. By using different grape varieties (Vitis vinifera var: Merlot, Grenache, Carignane, Cabernet Sauvignon, Semillon, and Columbard), three parallel production runs were made. The aging period was considered as 5 years. The rutine wine analyses, such as pH, alcohol content, total acidity and volatile acidity were determined according to procedures described in International Organization of Vine and Wine (OIV) methods.[Citation9] The wines were stored in glass bottles at 15°C in an air-conditioned room.
Total Phenols Analysis
The total phenolic content was determined by the Folin-Ciocalteu method[Citation10] by carrying out the following modification to reduce the assay volume. To 3.90 ml H2O was added 0.1 ml sample followed by 0.5 ml (25 ml Folin Ciocalteu reagent in 75 ml H2O) Folin-Ciocalteu reagent (Merck, Darmstadt, Germany) mix. After 3–6 min, 0.5 ml of saturated sodium carbonate (20 g Na2CO3 in 100 ml H2O) (Merck) was added. After vigorous mixing with a vortex and waiting for 30 min, the reading was performed at 725 nm (Pharmacia LKB Novaspec† II spectrophotometer, Uppsala, Sweden). The results were expressed as gallic acid equivalents (GAE) using a calibration curve against a gallic acid (Merck) standard (100 mg/L).
High-Performance Liquid Chromatography Analysis
Gallic acid, p-hydroxybenzoic acid, syringic acid, ferulic acid and p-coumaric acid were obtained as standards from Sigma Chemical Company (Frankfurt, Germany). Solvents used for chromatography were methanol and phosphoric acid (of high-performance liquid chromatography ultra gradient grade) supplied by J.T. Baker (New Jersey, USA) and Riedel-de Haen AG (Darmstadt, Germany), respectively. Membranes (0.45-μm pore size) used for filtration of the samples were obtained from Sartorius AG (Darmstadt, Germany) (16555 Minisart).
The liquid chromatographic system (HP 1100 series) supplied by SEM Company (Izmir, Turkey) was equipped with an electrochemical detector (HP1049A Programmable Electrochemical Detector), a pump (HP 1100 series G1310A isocratic pump), a manual injector (HP 1100 series G1328A Rheodyne 7725I) with 20-μl loop and a chromatographic data processing software (HP Chem Station LC Rev. A.06. 03.509). Separation was performed by a (C18) column (Hichrom 5 C18, 7.75 × 300 mm, 5 μm particle size).
The operating conditions were carried out at 21 ± 4°C. The separation of phenolic compounds were performed with a flow rate of 1 ml/min for 80 min. Amperometric detection was carried at ±0.65 V (versus Ag/AgCl, 0.5 mA full scale) in the electrochemical cell. The used solvents proportions were methanol/0.01 N phosphoric acid (30/70 v/v). Both solvents were degassed (ELMA LC 30/H ultrasonic bath, Darmstadt, Germany) for 15 min before use. Each compound was tentatively identified by retention time under the same conditions. Quantitative determinations were carried out by the external standard method based on peak areas.
Statistical Evaluation
Significant differences between averages were obtained at a 95% significance level. Using multivariate exploratory techniques, principal component analysis was performed. Principal component analysis permits visualization of the original arrangement of wines in an n-dimensional space, by identifying the directions in which most of the information is retained. The cluster analysis was performed as a joining type (tree cluster) by using raw data with complete linkage and the Euclidean distance. The scale plot was demonstrated as link/dmax/100. In order to be comparable, all values were standardized.
RESULTS AND DISCUSSION
The identification of phenolic compounds having antioxidant properties and found in wines has raised much interest. Wine phenolics belong to two main groups: the flavonoids (flavan-3-ols, flavonols, and anthocyanins) and the non-flavonoids (derivatives of benzoic acid, hydroxycinnamates, and stilbenes). The majority of phenolic compounds arise from the condensation of flavan-3-ol units (such as (--)-epicatechin and (+)-catechin) to yield oligomers (proanthocyanidins) and polymers (condensed tannins). In typical red wines, the proanthocyanidins and condensed tannins are the most sizable fraction, being in the range of 25–50% in a very young wine (immediately after maceration) and even higher in older wines.[Citation11] The results of general analysis of wines (pH, alcohol content, total acidity and volatile acidity) demonstrated that all samples are in the range of the standard values for the red wines of this type.[Citation9]
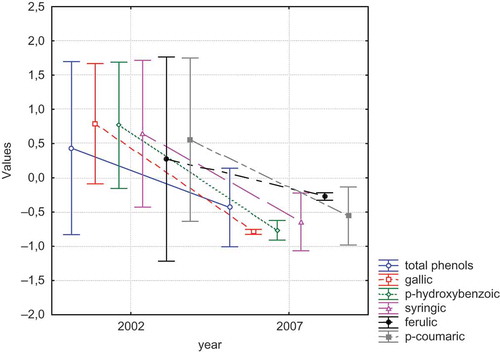
The phenolic acid contents of wines during 5 years (2002 and 2007) are presented by . As could be seen from the figure, the phenolic acid contents of all wines decreases significantly after 5 years. According to other studies,[Citation12] the concentrations of total and flavonoid phenols as well as red pigments tended to decrease with age. The highest decease was determined for p-coumaric acid (0.047 mg/L) followed by syringic acid, p-hydroxybenzoic acid, gallic acid and ferulic acid. Monagas et al.[Citation13] demonstrated that hydroxybenzoic acid and its derivatives (gallic, protocatechuic, vanillic, and syringic acids, methyl and ethyl gallates), hydroxycinnamic acids and its derivatives (trans-caftaric, cutaric, caffeic and p-coumaric acids, hexose esters of trans-p-coumaric acid), stilbenes (trans- and cis-resveratrol-3-O glucosides, and trans- and cis-resveratrol), phenolic alcohols, and other related compounds (tyrosol and tryptophol), flavanols, and flavonols presented different evolution patterns during aging.
During aging of wines, the fining agents also affect the phenolic compounds concentrations in wines. In a study done by Stankovic et al.,[Citation14] it was demonstrated that the use of bentonite causes color decreases while gelatine leads to increases of wine color intensity. During aging the main changes of phenolic compounds involve following chemical interactions: thermal and oxidative degradation of anthocyanins, decomposition of anthocyanins by ketons; reactions involving interactions of tannins with protein and polyssacharides, formation of carbocations from cyanidins, polymerization reactions of procyanidins, copigments formations of anthocyanidins, and their condensation reactions with tannins.[Citation15] During these processes, some specific changes are recorded, such as transformation of gallic acid to ellagic acid under oxidative conditions—hydrolysis of flavonol glycosides and tartaric esters of hydroxycinnamic acids to their corresponding free forms. Schwarz et al.[Citation16] demonstrated that hydroxycinnamic acids can also react with anthocyanins, resulting in pyranoanthocyanin-type pigments. Changes that occurred during aging in the bottle were the decrease of the concentration of trans-caftaric and cutaric acids accompanied by an increase of trans-caffeic acid and especially of trans-p-coumaric acid.[Citation13] Comparison of the data showed that the concentrations of anthocyanins, flavan-3-ols, and hydroxycinnamic acids decreased in all wines during aging. The concentrations of hydroxycinnamic esters decreased regularly in all wines (by about 6% after 5 months, 12% after10 months) while that of free hydroxycinnamic acids increased, probably due to hydrolysis of tartaric esters and acylated anthocyanins.
Although fermentation occurs under anaerobic conditions, oxidative processes, both enzymatic and non-enzymatic, are thought to play a significant role in changing the phenolic composition of wine This is accompanied by the oligomerization of the original phenolics. As wine has been bottled, access to additional oxygen is practically terminated and the subsequent transformations of wine phenolics occur under anaerobic conditions. However, the storage of wine in corked bottles still results in slow polymerization and condensation reactions. This reversible transformation is likely limited by the partial precipitation of condensed wine phenolics because of their low solubility in wine.
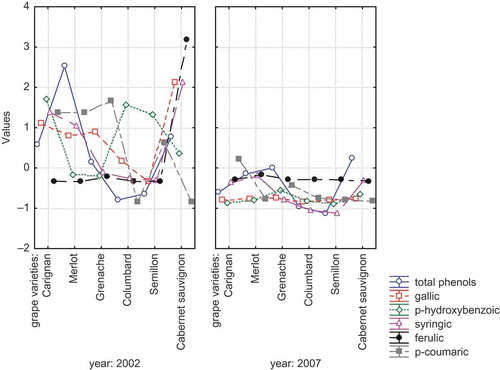
The mean values of phenolic acids in aged wines produced from different grape varieties (Vitis vinifera L. cv.: Carignane, Cabernet Sauvignon, Merlot, Grenache, Columbard, and Semillon) are presented in . Evaluating the aging years and grape varieties of wines the highest decreases of gallic acid concentrations were determined in Cabernet Sauvignon (24.37 mg/L) followed by Carignane (16.00 mg/L) wines. The order of wines concerning the decreases for p-hydroxybenzoic acid content during aging was determined as: Carignane (22.47 mg/L) > Columbard (20.84 mg/L) > Semillon (19.24 mg/L). The decreasing order regarding the syringic acid content was as: Cabernet Sauvignon (2.34 mg/L) > Carignane (1.69 mg/L) > Merlot (1.19 mg/L). The highest decreases of ferulic acid content were determined in Cabernet Sauvignon (3.96 mg/L), Merlot (0.17 mg/L), and Carignane (0.05 mg/L) wines. The maximum reduction of p-coumaric acid was determined for Grenache (1.03 mg/L) and Merlot (1.05 mg/L) wines. The statistical significance of years was determined only for gallic acid (p < 0.01). Positive correlations were determined of total phenol with gallic acid (r = 0.60) and with syringic acid (r = 0.66).
The gallic acid, as the strongest radical scavenger[Citation17] present in wines, has an indirect effect over the other phenolic acids present in wines. In our previous studies,[Citation9] we determined the positive correlation of gallic acid with syringic acid and with total phenols. According to Revilla and Gonzalez-San Jose,[Citation18] no significant changes were registered for these esters under the aging conditions employed, even in the presence of high levels of gallic acid. The same situation can also be considered for the other hydroxybenzoic acids (protocatechuic, vanillic, and syringic acids) studied in the different wines. Revilla and Gonzalez-San Jose[Citation18] also found no significant changes in the total concentration of gallic, protocatechuic, and syringic acids in Tempranillo wines elaborated from vinifications treated with pectolitic enzymes during 24 months of bottle-aging.
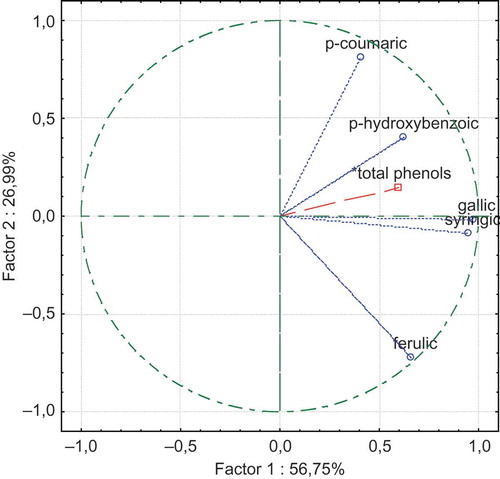
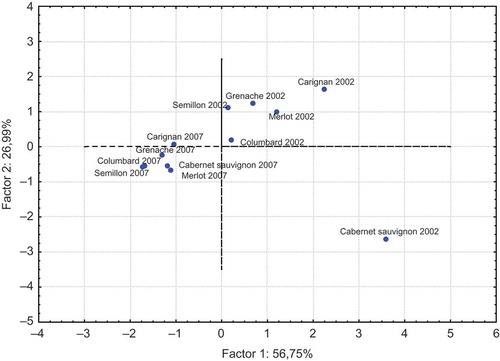
Principal component analysis was run on the average set of data to examine attribute relationship and to demonstrate the differences in different grape varieties at the end of production (2002) and after aging (2007). The eigenvalues of the correlation matrix were determined to be 56.75, 26.99, 12.72, 2.44, and 1.10%. The distribution of phenolic acids and total phenolic content values of wines by PCA are found in . The results demonstrated that the localization of examined phenolic acids except ferulic acid are in the same coordinate (upper-left). The distribution of wines according to their aging year and grape variety origin by PCA could be seen in . The localization of wines with different grape origins varied with aging years. The data demonstrated also the different patterns of phenolic acids found in Cabernet Sauvignon wine.
As the figure presenting the distribution of phenolic acids and total phenols found in aged wines () and graphic giving the localization of wines according to the their aging year and grape variety origin () have the same x- and y-values (57.75 × 26.99), they could be evaluated together in order to determine the relationship among analyzed parameters and wine samples. By plotting and over each other, interesting results were obtained. There was determined a strong relation among syrincic acid, p-hydroxybenzoic acid, gallic acid, p-coumaric acid, and Carignane, Merlot, and Grenache wines of the year 2002. The close relation was recognized also between Cabernet Sauvignon wine and its ferulic acid during the year 2002.
CONCLUSIONS
In conclusion, this article reported valuable data about the phenolic acids changes during aging of organic wines. The statistical significance of aging year was determined for gallic acid (p < 0.01) content of wines. Positive correlations were determined for total phenol with gallic acid (r = 0.60) and with syringic acid (r = 0.65). The intercepts of analyzed parameters and wines produced from different grape varieties in n-dimensional space demonstrated the close relation among syringic acid, p-hydroxybenzoic acid, gallic acid, p-coumaric acid, and Carignane, Merlot, and Grenache wines of 2002. The close relation was recognized also between Cabernet Sauvignon wine and its ferulic acid during the year 2002. These results emphasized the changes in phenolic acids during aging of organic wines produced from Vitis vinifera L. origin var: Carignane, Cabernet Sauvignon, Merlot, Grenache, Columbard, and Semillon grapes.
REFERENCES
- Frankel, E.N.; Waterhouse, A.L.; Teissedre, P.L. Principal phenolic phytochemicals in selected California wines and their antioxidant activity in inhibiting oxidation of human low-density lipoprotein. Journal of Agricultural and Food Chemistry 1995, 43, 890 –894.
- Jayaprakasha, G.H.; Singh, R.P.; Sakariah, K.K. Antioxidant activity of grape seed (Vitis vinifera) extracts on peroxidation models in vitro. Food Chemistry 2001, 73, 285 –290.
- Natella, F.; Ghiselli, A.; Guidi, A.; Ursini, F.; Scaccini, C. Red wine mitigates the postprandial increase of LDL susceptibility to oxidation. Free Radical Biology and Medicine 2001, 5, 1036 –1044.
- Landrault, N.; Poucheret, P.; Ravel, P.; Grasc, F.; Cros, G.; Teissedre, P.L. Antioxidant capacities and phenolics level of French wines from different varieties and vintages. Journal of Agricultural and Food Chemistry 2001, 49 (7), 3341 –3348.
- Yang, T.; Juan, F.G.; Sun, D. Advances in wine ageing technologies for enhancing wine quality and accelerating wine ageing process. Critical Reviews in Food Science and Nutrition 2014, 54 (6), 817–835.
- Augustin, S.; Claudine, M.; Christine, M.; Christian, R.; Liliana, J. Dietary polyphenols and prevention of diseases. Critical Reviews in Food Science and Nutrition, 2005, 45, 287 –306.
- Eiro, M.J.; Heinonen, M. Anthocyanin color behavior and stability during storage: Effect of intermolecular copigmentation. Journal of Agricultural and Food Chemistry 2002, 50, 7461 –7466.
- Suarez, R.; Monagas, M.; Bartolome, B.; Gomez-Cordoves, C. Phenolic composition and colour of Vitis vinifera L. cv merlot wines from different vintages and aging time in bottle. Ciência e Técnica Vitivinícola 2007, 22, 35 –44.
- Yıldırım, H.K.; Akçay, Y.D.; Güvenç, U.; Altındışlı, A.; Sözmen, E.Y. Antioxidant activities of organic grape, pomace, juice, must, wine and their correlation with phenolic content. International Journal of Food Science and Technology 2005, 40 (2), 133 –142.
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolic with phosphomolibdic-phosphotungustic acid reagent.American Journal of Enology and Viticulture 1965, 16, 144 –158.
- Roginsky, V.; De Beer, D.; Harbertson, J.F.; Kilmartin, P.A.; Barsukova, T.; Adams, D.O. The antioxidant activity of California red wines does not correlate with wine age. Journal of the Science of Food and Agriculture 2006, 86, 834 –840.
- Okuda, T.; Takayanagi, T.; Sato, M.; Yokotsuka, K. Changes in radical scavenging activity of Japanese Cabernet sauvignon red wines during ageing. Journal of Wine Research 2002, 13 (2), 93 –100.
- Monagas, M.; Gómez-Cordovés, C.; Bartolomé, B. Evolution of polyphenols in red wines from Vitis vinifera L. during aging in the bottle. II. Non-anthocyanin phenolic compounds. European Food Research and Technology 2005, 220, 331 –340.
- Stankovic, S.; Jovic, S.; Zivkovic, J.; Pavlovic, P. Influence of age on red wine colour during fining with bentonite and gelatin. International Journal of Food Properties 2012, 15, 326 –335.
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Durboridieu, D. Handbook of Enology Volume 2: The Chemistry of Wine, Stabilization and Treatments; John Wiley and Sons: Chichester, 2006; 91 –108.
- Schwarz, M.; Jerz, G.; Winterhalter, P. Isolation and structure of Pinotin A, a new anthocyanin derivative from Pinotage wine. Institut für Lebensmittelchemie, Technische Universität Braunschweig, Deutschland Vitis 2003, 42 (2), 105 –106.
- Pandey, K.B.; Rizvi, S.I. Ferric reducing and radical scavenging activities of selected important polyphenols present in foods. International Journal of Food Properties 2012, 15, 702 –708.
- Revilla, I.; Gonzalez-San Jose, M.L. Addition of pectolytic enzymes: An enological practice which improves the chromaticity and stability of red wines. International Journal of Food Science and Technology 2003, 38, 29 –36.