Abstract
Young’s modulus and Poisson’s ratio changes in Japanese radish and carrot during boiling were examined. Young’s modulus decreased rapidly within 2 min, then the decrease moderated. Carrot (3.0 to 0.5 MPa) exhibited increased modulus values relative to radish (1.8 to 0.3 MPa) during the same boiling period. Poisson’s ratio decreased slightly during boiling; carrot Poisson’s ratio (0.40 to 0.33) was smaller than radish (0.35 to 0.23) during the same boiling period. Boiling in saline solutions increased moduli reductions under the same treatment conditions. These results indicated that carrot maintained hardness, but reduced volume relative to radish during boiling.
INTRODUCTION
Dietary fiber has traditionally been defined as the indigestible portions of plant-derived foods, including fruits and vegetables.[Citation1] These plant sources can be consumed in raw and cooked forms. The cooked fruit and vegetable texture is typically softened, and quite different from the raw material. The elastic properties of fruits and vegetables are a primary trait used to evaluate the product’s textural attributes.[Citation2,Citation3] Young’s modulus is a measure of the elastic property of a material, and is evaluated based on its stress–strain curve.[Citation2,Citation3] Poisson’s ratio is also a measure of a material’s elastic properties, and is defined as the strain ratio in the direction perpendicular to the applied force, to the strain in the direction of the applied force.[Citation2,Citation3] These elastic properties are often used as a parameter to assess food quality.[Citation4−Citation6]
Young’s modulus can be measured using precious testing devices.[Citation2] Poisson’s ratio can be measured using mechanical equipment.[Citation7,Citation8] However, mechanical methods are difficult to apply to Poisson’s ratio measurements for boiled food products due to the uncertain state of the contact point between the pinching instrument and the sample object. Therefore, a non-contact ultrasonic technique method was developed and used,[Citation9,Citation10] although the method remains complex, and is still difficult to apply to softened materials.
Anazodo and Chikwendu[Citation11] conducted an evaluation of corn cob samples under a compressive loading between two parallel steel plates using photographic measurements, which is a non-contact method to evaluate Poisson’s ratio. Pallottino et al.[Citation12] measured compressive deformation of Tarocco orange fruit to assess its mechanical properties using digital imaging devices. Recently, high quality digital cameras for commercial use have become available, which can capture high-resolution digital images and analyze material deformations using a commercial personal computer with graphic software.
In the present study, changes in Young’s modulus and Poisson’s ratio for cylindrical root tissue samples of Japanese radish and carrot at different boiling times in distilled water, and 1, 2, and 4% saline solution concentrations were examined using a precious testing device, and captured digital images of individual samples were compared before and after deformation.
MATERIALS AND METHODS
Materials
Fresh Japanese radish (Raphanus sativus L. cv. Daikon) and carrot (Daucus carota L. cv. US-Harumakigosun), harvested in 2008 in Hokkaido, Japan, were purchased at a local supermarket in Matsudo, Japan. Samples were stored in a refrigerator at 8°C, and laboratory tests were conducted the day following purchase. Refined salt (over 99% NaCl, manufactured by the salt industry center of Japan) was purchased, and used to prepare the saline solution.
Sample Preparation
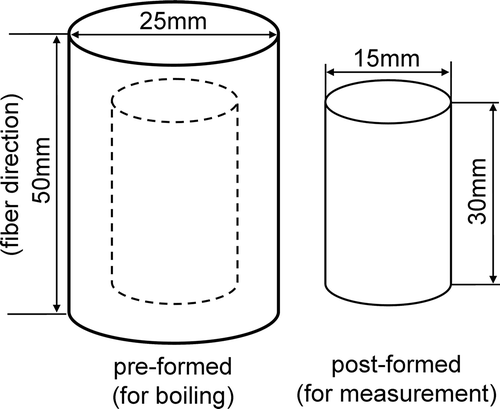
The surface of boiled vegetable tissues collapsed or distorted, rendering Poisson’s ratio measurements as challenging. Therefore, we developed a method to maintain sample shape pre- (pre-formed), and post- (post-formed) boiling (). A 25-mm diameter cylindrical piece of tissue was excised from the root vegetable center along the growth axis as a pre-formed sample in preparation for boiling (). The cylinder height was 50 mm, and the base faced horizontally. Samples were excised using a precious cutting apparatus with a sharp cylindrical knife (NS-3M, Hirano, Chiba, Japan). Following removal, pre-formed individual cylinders were placed in a pot containing 1 L of boiling distilled water or saline solution heated by a 600 W electric cooking stove (SK-65, Ishizaki Electric, Tokyo, Japan). The boiling time was up to 30 min in distilled water, and 10 min in saline solution, adding boiling water as necessary to maintain approximately 1 L of water. Saline solution concentrations were 1, 2, and 4% (w/v). Tissue cylinder temperature was measured in the root center by a fiber optic thermometer (FL-2000, Anritsu, Tokyo, Japan). After boiling, the pre-formed cylindrical samples were placed in a refrigerator at 8°C, and cooled approximately 30 min to equalize the thermal conditions similar to raw samples. A second formed cylinder was carefully excised from the center of cooled samples to measure Young’s modulus and Poisson’s ratio (). A precious slice cutter (SK-4N, Hirano) was used to remove a formed cylinder sample of 15 mm in diameter and 30 mm in height, and a circular level (ED-CI, Ebisu, Niigata, Japan) verified the cylindrical form. Verified cylinders were rapidly measured at room temperature and averaged for the reduplications.
Measurement Procedures
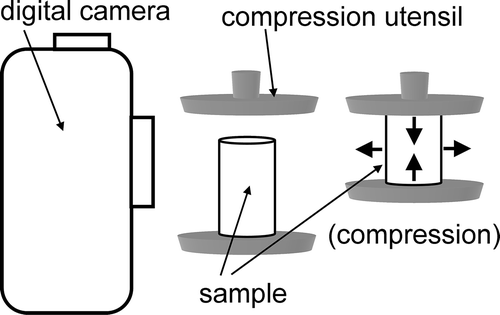
A creep meter (RE2-3305S, Yamaden, Tokyo, Japan) with a planar plunger (Φ30 mm) as the compression utensil was applied to compress the formed cylindrical sample from the base. The creep meter also measured normal stress against compression, calculated from the load divided by the base area, and compressive strain calculated as a percentage of compressed length divided by initial sample length (height). Compression rate was set to 1 mm/s, and the apparatus trigger force was set at 0.2 N. The initial contact point between sample and plunger, which is regarded as the initial sample length, was set at 0% compression level (zero compressive strain). Young’s modulus (E) was calculated from normal stress (σ) values and compressive strain (ϵ) as follows:
Poisson’s ratio (μ) is defined as the ratio of width change (ΔW) amount per initial width (W), and length change (ΔL) amount per initial length (L) as follows:
The widths of cylindrical samples were expanded and lengths were shortened because a compression test was applied in this study. A digital camera (E-510, Olympus, Tokyo, Japan) with a macro lens (35 MM, Zuiko, Tokyo, Japan) was used to capture a digital image of each cylindrical sample. The camera position was fixed during examination (). The image size was 3648 × 2736 pixels, and the image resolution was approximately 120 pixels/mm. Changes in cylinder width and length were calculated from differences in pixel number between images before and after compression using graphic software (Photoshop CS2, Adobe, San Jose, CA, USA).
Statistical Analyses
Young’s modulus and Poisson’s ratio were expressed as means ± standard error (SE). One-way analysis of variance (ANOVA) was applied to ascertain significant differences among means at P < 0.05 level of significance using JMP 9 software (SAS Institute Inc., Cary, NC, USA).
RESULTS AND DISCUSSION
Attributes of Cylindrical Samples
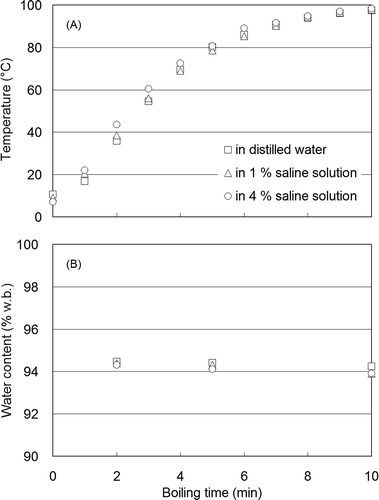
Japanese radish sample temperature changes measured from pre-formed cylinder centers and water content during boiling in distilled water, 1 and 4% saline solution concentrations are shown, respectively, in and . Radish internal temperature reached 100°C in approximately 10 min (). Thus, cylindrical samples at each 1 min stage within the total 10 min boiling duration possessed different thermal conditions, which would influence the elastic properties of vegetable tissues. Consequently, boiled samples at each boiling interval were rapidly removed from the distilled or saline boiling water, and cooled in a refrigerator. Results showed that the sample water content remained constant at approximately 94% for up to 10 min of boiling (). All Japanese radish samples exhibited these attributes under these conditions.
FIGURE 3 Changes in temperature (a), and water content (b), of cylindrical samples of Japanese radish during boiling in distilled water, and 1 and 4% saline concentration solutions.
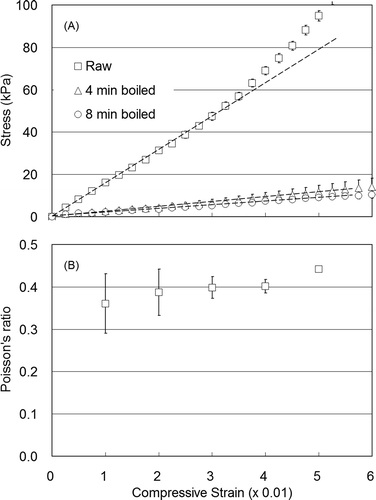
Stress–strain curves derived from formed cylinders of fresh and boiled Japanese radish samples (cut from pre-formed cylinders), and preliminary Poisson’s ratio at each compressive strain are provided in and , respectively. Young’s modulus was strictly defined for an elastic object, i.e., Hookean solid or the elastic region of compressive strain. The modulus is the elastic range slope value, and was depicted as a linear region in the stress–strain curve, as described above. These results were determined as the apparent Young’s modulus.[Citation2,Citation3] Consequently, the sample elastic range needs to be examined. Results showed that the linear region of the stress–strain curve ranged from 0 to approximately 0.03 of the compressive strain (), and the apparent Young’s modulus can be calculated from the slope (shown by dashed lines in ). Young’s modulus for individual samples for each treatment condition was calculated as a slope value from 0 to 0.03 of the compressive strain, and subsequently averaged for the re-duplication under the same treatment conditions. Poisson’s ratio was also calculated from the amount of change in the cylindrical sample shape, measured as a change in pixel number captured in digital images from before and after sample deformation. Poisson’s ratio standard errors at 0.01 or 0.02 compressive strains exceeded 0.03 or 0.04 (). Due to the linear region definition for Young’s modulus calculation and Poisson’s ratio errors, 0.03 of the compressive strain was applied to Poisson’s ratio measurement in this study.
Changes in Young’s Modulus and Poisson’s Ratio during Boiling
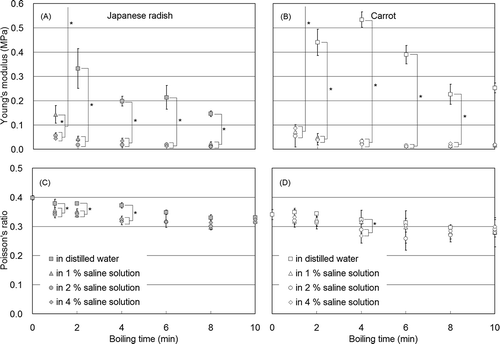
Changes in Young’s modulus and Poisson’s ratio from formed cylindrical Japanese radish and carrot samples during boiling in distilled water are depicted in and , respectively. Young’s modulus in Japanese radish decreased rapidly (within 2 min) from approximately 1.8 to 0.3 MPa. Following 10 min of boiling, no significant difference (P > 0.05) in Young’s modulus was detected at approximately 0.1 MPa, which indicated the samples had reached a stable state. Poisson’s ratio for Japanese radish decreased from approximately 0.40 to 0.33 within 8 min, and reached stability after boiling for 8 min. Changes in Young’s modulus for carrot showed a sudden decrease from 3.0 to 0.5 MPa within 2 min, and the modulus stabilized at approximately 0.1 MPa after boiling for 15 min. Carrot modulus values exhibited slight increases compared to Japanese radish, although a consistent trend was not observed. Poisson’s ratio in carrot, which was less than the Japanese radish ratio, exhibited a decreased tendency from approximately 0.35 to 0.23 during a 15-min boiling period. This result indicated that the volume reduction in carrot tissue against compression was greater than in Japanese radish.
FIGURE 5 Changes in Young’s modulus (a), and Poisson’s ratio (b) in Japanese radish and carrot during the boiling period (n = 6). n.s.: Indicates no significant differences; P > 0.05.
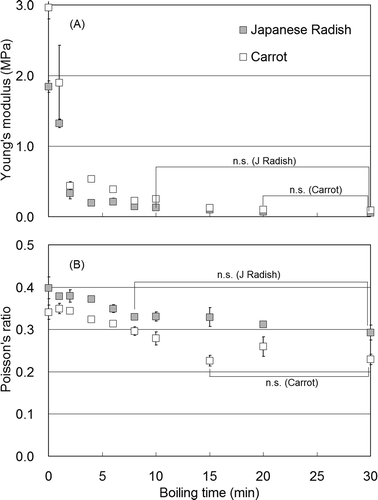
The pre-formed cylinder center temperature increased to approximately 40°C within 2 min of boiling (); however, Young’s modulus exhibited a rapid decrease, which indicated the tissues softened during treatment. Typically, vegetable tissue softening during boiling is associated with elution and/or pectin degradation in the cell wall.[Citation13] In contrast, Poisson’s ratio changes showed degree level decreases up to an approximately 10-min boiling duration. Tamura et al.[Citation14] concluded that physical tissue structure, including the xylem parenchyma primary cell wall matrix was changed to a sponge-like structure by boiling, and a pectin elution from the cell wall was increased during boiling. These observations suggested Poisson’s ratio was reduced during boiling with decreased Young’s modulus, although the effect would be species-specific, i.e., varies among different taxa.
Changes in Elastic Properties during Boiling in Saline Solutions
Changes in Young’s modulus and Poisson’s ratio for cylindrical samples during boiling in distilled water, and 1, 2, and 4% saline solution concentrations are depicted in –. Young’s modulus for Japanese radish () and carrot () boiled in saline solutions rapidly decreased, and saline results were significantly less (P < 0.05) than distilled water values from each boiling interval. In particular, 1 min of boiling was approximately 0.1 MPa, which remained at a stable level for over 10 min of boiling in distilled water. Tamura[Citation15] and Fuchigami et al.[Citation16] conducted sensory tests, and reported that boiling in less than 1% saline solution resulted in softer vegetables. In the present study, the samples boiled in 2 and 4% saline solutions should therefore show a rapid decrease in Young’s modulus values. Congruent with Young’s modulus, saline effects on Poisson’s ratio ( and ) were significant (P < 0.05) following less than 4 min of boiling. The ratios calculated based on boiling in saline solution were less than the ratios derived from boiling in distilled water. In addition, consistent with Young’s modulus results, differences among concentrations were not detected. These results showed increased tissue reduction following boiling in a saline solution; however, the samples treated in 1, 2, and 4% salt concentrations exhibited no significant change in tissue volume.
CONCLUSIONS
Sample deformation from all directions, including plastic range against compression, must typically be considered. However, the present method exhibits utility in evaluating the elastic properties of boiled and softened foods. The results of this study indicated Japanese radish was softer than carrot under the same boiling conditions; however, Japanese radish volume showed decreased reduction against compression compared to carrot samples. In terms of the eating attributes of these two root vegetables after boiling, carrots should be more easily swallowed than Japanese radish under the same cooking conditions, if the root vegetables become fragments following mastication. Future research will involve ascertaining temperature dependency by mathematical modeling for a complete assessment of the elastic properties of softened food materials. Differences among varieties of the same vegetables will also be reported.
REFERENCES
- Kiriyama, S.; Ebihara, K.; Ikegami, S.; Innami, S.; Katayama Y.; Takehisa, F. Searching for the definition, terminology and classification of dietary fiber and the new proposal from Japan. Journal of Japanese Association of Dietary Fiber Research 2006, 10, 11–23.
- Bourne, M.C. Food Texture and Viscosity: Concept and Measurement, 2nd Ed.; Academic Press: New York, 2002; 59 pp.
- Sahin, S.; Sumnu, S.G. Physical Properties of Foods; Springer: New York, 2006; 39 pp.
- Manjunatha, S.S.; Das Gupta, D.K. Instrumental textural characteristics of restructured carrot cubes. International Journal of Food Properties 2006, 9, 453–462.
- Polat, R.; Aktas, T.; Ikinci, A. Selected mechanical properties and bruise susceptibility of nectarine fruit. International Journal of Food Properties 2012, 15, 1369–1380.
- Tunick, M.H.; Onwulata, C.I.; Thomas, A.E.; Phillips, J.G.; Mukhopadhyay, S.; Sheen, S.; Liu, C.K.; Latona, N.; Pimentel, M.R.; Cooke, P.H. Critical evaluation of crispy and crunchy textures: A review. International Journal of Food Properties 2013, 16, 949–963.
- Chappell, T.W.; Hamann, D.D. Poisson’s ratio and Young’s modulus for apple flesh under compressive loading. Transactions of the ASAE 1968, 10, 608–612.
- Kiani Deh Kiani, M.; Maghsoudi, H.; Minaei, S. Determination of Poisson’s ratio and Young’s modulus of red bean grains. Journal of Food Process Engineering 2011, 34, 1573–1583.
- Grotte, M.; Duprat, F.; Pietri, E.; Loonis, D. Young’s modulus, Poisson’s ratio, and Lame’s coefficients of golden delicious apple. International Journal of Food Properties 2002, 5, 333–349.
- Elfawakhry, H.; Hussein, M.A.; Becker, T. Investigations on the evaluation of rheological properties of cereal based viscoelastic fluids using ultrasound. Journal of Food Engineering 2013, 116, 404–412.
- Anazodo, U.G.N.; Chikwendu, S.C. Poisson’s ratio and elastic modulus of radially compressed biomaterials—I: Small deformation approximation. Transactions of the ASAE 1983, 26, 923–929.
- Pallottino, F.; Costa, C.; Menesatti, P.; Moresi, M. Assessment of the mechanical properties of Tarocco orange fruit under parallel plate compression. Journal of Food Engineering 2011, 103, 308–316.
- Fuchigami, M. Relationship between pectic compositions and the softening of the texture of Japanese radish roots during cooking. Journal of Food Science 1987, 52, 1317–1320.
- Tamura, S.; Kawamura, C.; Senda, T.; Fuchigami, M. Effect of various salts on the softening of cooked Japanese radish roots and on the fine structure of the parenchyma cell wall after cooking. Journal of Home Economics Japan 1993, 44, 633–641.
- Tamura, S. Softening mechanism of cooked vegetables (part 1): Effects of added NaCl on softening cooked Japanese radish root. Journal of Home Economics Japan 1987, 38, 375–381 (in Japanese).
- Fuchigami, M.; Sasaki, A.; Sanmoto, A.; Tamura, S.; Okuda, H. Effects of various chlorides on the softening of cooked Japanese radish roots and on the pectic composition after cooking. Journal of Home Economics Japan 1993, 44, 643–648.