Abstract
In the current study, the volatile nitrosamines (N-nitrosodimethylamine, N-nitrosomethylethylamine, N-nitrosodiethylamine, N-nitrosopyrrolidine, N-nitrosodi-n-propylamine, N-nitrosopiperidine, N-nitrosodi-n-butylamine, N-nitrosodiphenylamine) were detected in 28 selected Chinese meat products. An ultrasonic solvent extraction and gas chromatography mass spectometry analysis using a HP-5MS capillary column probed to be the best method. The concentration of eight volatile nitrosamines showed a good linear relationship and the linear correlation coefficient was above 0.999. The limit of detection of the instrument (IDL) and limit of quantification of the instrument (IQL) was 1.134–9.894 μg/L and 3.779–32.980 μg/L, respectively. The limit of detection of the method (MDL) and limit of quantification of the method (MQL) was 0.057–0.495 μg/kg and 0.189–1.649 μg/kg, respectively. The recovery of the method was 82.575–108.364%. The volatile nitrosamines contents were detected by the current method in 28 selected Chinese meat products. The results showed that the N-nitrosamine levels of Chinese sausages and emulsion sausages were generally higher than that of cooked sausages, and the contents in sausage with garlic flavor were obviously low. The major volatile nitrosamines found in our samples were N-nitrosomethylethylamine, N-nitrosopyrrolidine, N-nitrosodi-n-butylamine, and N-nitrosodiphenylamine.
INTRODUCTION
N-nitrosamines are amines derivatives including R1R2N-N=O functional group. In 1978, the International Agency for Research on Cancer (IARC) classified a number of N-nitrosamines with respect to the cancer risk for humans.[Citation1] The researchers in IARC considered that N-nitrosodimethylamine (NDMA) and N-nitrosodiethylamine (NDEA) belonged to the group that was probably carcinogenic to humans, and N-nitrosodi-n-butylamine (NDBA), N-nitrosopiperidine (NPIP), and N-nitrosopyrrolidine (NPYR) belonged to the group that was possibly carcinogenic to humans. N-nitrosamines could induce tumors in a variety of organs, such as liver, lungs, kidneys, bladder, pancreas, esophagus, and tongue, depending on the species. Many food and beverages categories could find N-nitrosamines, such as cured meat, vegetable oil, cheese, drinking water, and beer.[Citation2] The human body can be poisoned by N-nitrosamines through various ways, especially from food intake. It is necessary to learn about N-nitrosamines contents in foods in order to make the risk assessment for the government and to help the consumers choose healthy food in their daily life.
N-nitrosamines are formed in the reaction between a substance having an amino group and a nitrosating agent, which are commonly used in the manufacture of meat products.[Citation3] Sodium nitrite and sodium nitrate are used as curing agents in meat, not only as preservatives (as they decrease the risk of botulism poisoning), but also for color and flavor. The formation of N-nitrosamines is a complex process and the presence of these compounds in meat products depends on various parameters associated with conditions of preparation, storage, and/or thermal processing of meat.[Citation4]
The analysis methods of N-nitrosamines in the meat products have been carried out by different analytical methods, including gas chromatography (GC) and gas-liquid chromatography with thermal energy analyzer or with a Coulson electrolytic conductivity detector, and GC high resolution mass spectrometry (MS). Positive-ion chemical ionization MS with methane or ammonia as reagent gases has been used to differentiate N-nitrosamines compounds.[Citation2] Volatile nitrosamines can be analyzed by GC with the advantages of relative non-polarity, low molecular weight, and sufficient vapor pressure. GC coupled to MS can achieve good sensitivity of volatile nitrosamines.[Citation5] Due to the complex matrix of samples and the low concentrations of N-nitrosamines in meat products, it is necessary to isolate and pre-concentrate volatile nitrosamines in the process of pretreatment. Distillation and solvent extraction in relatively low polarity organic solvents coupled to gas chromatography mass spectometry (GC-MS) is the official method of analysis.[Citation6] However, distillation procedure is a complex, time-consuming, labor intensive procedure which is not suitable for a wide range of study. Solid-phase microextraction (SPME) has replaced distillation procedure in many common applications. This method saves preparation time, solvent purchase, and disposal costs, and can improve the detection limits compared with distillation procedure. It has been used routinely in combination with GC and GC-MS, and successfully applied to a wide variety of compounds, especially for the extraction of volatile and semi-volatile organic compounds from environmental, biological, and food samples.[Citation7] SPME was also introduced for direct coupling with HPLC and LC-MS in order to analyze the weakly volatile compounds.[Citation8] Ultrasonic solvent extraction are widely used in the pharmacy, food, biological engineering, and other fields in present day which can increase the leaching efficiency with ultrasonic cavitation effect. In addition, mechanical vibration, diffusion, and pulverization can also accelerate the extraction of component. Traditional extraction methods are gradually replaced by ultrasonic solvent extraction with the advantages of the high efficiency, energy saving, simple operation, short time, and low cost.[Citation9,Citation10]
The aim of the present study was to compare the effect of the ultrasonic solvent extraction and SPME as an extraction method in the analysis of volatile nitrosamines in popular Chinese meat products by GC-MS, typifying the contents of eight volatile nitrosamines in popular Chinese meat products.
MATERIALS AND METHODS
Reagents
A N-nitrosamine standard mixture in dichloromethane (2000 μg/mL) containing NDMA, N-nitrosomethylethylamine (NMEA), N-nitrosodiethylamine (NDEA), NPYR, N-nitrosodi-n-propylamine (NDPA), NPIP, NDBA, and N-nitrosodiphenylamine (NDpheA) was provided by Dr. Ehrenstorfer Co. (Augsburg, Germany). Working standard solutions with concentrations of 0.01, 0.05, 0.1, 0.2, 0.5, and 1 μg/mL were prepared daily in methanol and stored at 4°C.
Methanol HPLC grade was provided by Merck Ltd (Beijing, China). Sodium chloride, dichloromethane, sodium sulfate were all of analytical grade and purchased from Beijing Chemicals Co. (Beijing, China). Syringe filters (0.45 μm) were purchased from Hercules (Beijing, China). The water was purified by means of an Elix-Milli-Q system (Millipore, Bedford, MA, USA). The carrier gas used for GC was helium obtained from Juyang Gas Co., Ltd (Changchun, China).
Sample Preparation
All samples selected in the study were purchased from local supermarkets and stores in Changchun, China in 2013. A total of 28 samples covered three categories of popular meat foods in China, including Chinese sausages, emulsion sausages, and cooked sausages. All samples were stored at –18°C before analysis.
An ultrasonic solvent extraction method and a SPME method were used as extraction methods in the current article. As for the ultrasonic solvent extraction, sample preparation was performed following the procedure proposed by Wu et al. (2012).[Citation10] Twenty grams of the minced sample was homogenized in a blender (DS-1, Shanghai Precision Instruments Co., Ltd, Shanghai, China) and put into a 100 mL beaker and 40 mL of dichloromethane was added. The sample was ultrasonic extracted for 15 min with an ultrasonic cell crushing apparatus (JY92-2D, Ningbo New Cheese Biological Technology Co., Ltd., Zhejiang, China) with a ultrasonic power of 500 W. The extraction procedure was repeated with 40 mL of dichloromethane in order to collect volatile nitrosamines completely. Then the extract liquid was mixed together and filtered through sodium sulfate. Subsequent concentration was performed at 35°C with a rotary evaporator (RE-52A, Shanghai Yarong Biochemical Instruments Co., Ltd, Shanghai, China) and when the extract liquid was concentrated to about 1 mL, a nitrogen evaporator (MD200, Shanghai Moyi Trade Co., Ltd, Shanghai, China) was used for blowing the liquid to dry. One milliliter of methanol was added to a definite volume and stirred for 1 min in a vortex shaker (QL-866, Haimen Qilinbeier Instruments Co., Ltd, Haimen, China). Finally, the methanol aqueous sample solution was filtered through a nylon 0.45 μm syringe filter for GC-MS analysis.
As for a SPME method, fused silica fibers coated with polydimethylsiloxane-divinylbenzene (PDMS-DVB) were used with a manual SPME holder (Supelco, LA, USA). The fiber was conditioned prior to use in a GC split-splitless inlet, at the temperature and length of time recommended by the manufacturer. Two grams of ground sample was poured into open-top vials with PTFE/silicone septum. Five microliters of saturated NaCl solution and a magnetic stir bar were added to the sample vial, followed by the sample. The sample was placed on a magnetic stirrer (Bante, China) at 65°C to establish the equilibrium between the headspace and sample prior to the exposure of the fiber to the headspace. After a 30 min of extraction time, the fiber was retracted and removed and then introduced into the injection port of the GC for thermal desorption at 200°C for 5 min.[Citation11CitationCitationCitationCitation−Citation16]
GC-MS Analysis
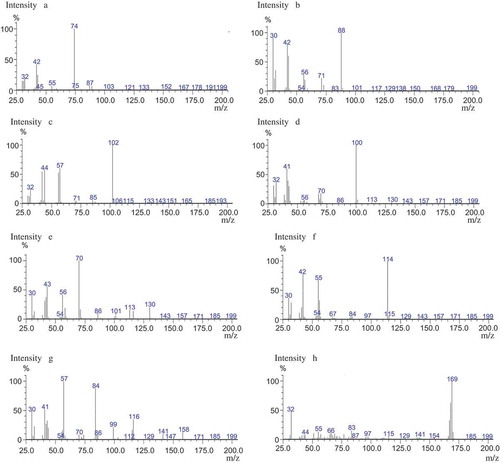
A gas chromatograph coupled with a mass spectrometer (GCMS-QP2010, Shimadzu, Japan) equipped with an inert ion source and provided with a split-splitless injection port was used to determine the N-nitrosamine levels.[Citation17,Citation18] A HP-5MS capillary column (30 m long, 0.25 mm I.D., and 0.25 μm film thickness, Agilent, USA) and a Rxi-5MS capillary column (30 m long, 0.25 mm I.D., and 0.25 μm film thickness, Restek, USA) were used for optimizing the method. The helium carrier gas was maintained at a constant flow of 1 mL/min. The injection port was held at 200°C and used in the splitless mode, applying a pressure pulse of 49.5 kPa. The GC oven temperature was programmed as follows: Start temperature of 40°C (held 1 min) and increase to 200°C at 9°C/min, held for 1 min. Ionization was carried out in the electron-impact (EI) mode (70 eV). The temperatures of the ion source and the transfer line were 230 and 250°C, respectively. The solvent cut time was 3.5 min. In order to establish the retention time for each analyte, stock solutions were previously injected in scan mode over the m/z range of 30–200. Then the analytes were quantified in the selected ion monitoring (SIM) mode using the target ion and qualifier ions. Selected ions chosen for each compound were NDMA (74), NMEA (88), NDEA (102), NPYR (100), NDPA (70), NPIP (114), NDBA (84), and NDpheA (169), respectively.
Validation Method
In order to check the performance of the analysis method, some quality parameters such as IDL (limit of detection of the instrument), IQL (limit of quantification of the instrument), MDL (limit of detection of the method), MQL (limit of quantification of the method), linearity, recovery, and precisions were established.
The limit of detection (LOD) and limit of quantification (LOQ) were determined at a signal-to-noise ratio of three and ten, respectively, measured at the approximate retention time of the corresponding analyte peak. The MDL and MQL were determined on base of 20 g of the minced sample which were concentrated into one milliliter of analyte. The linearity was obtained from a graph using the external standard method with seven concentration levels (0.01, 0.05, 0.1, 0.2, 0.5, and 1 μg/mL). The approach employed to evaluate the accuracy of the method was based on the recovery of known amounts of each nitrosamine spiked into sausage samples in three levels. The amount of the standard analyte added was between two and four times the estimated amount of the analyte in the sample. Reproducibility was expressed as the relative standard deviation (RSD) determined by the area of the chromatographic peak of six analyses of the same sample.
Statistical Analysis
Statistical analysis was performed using SPSS 11.5 software (Chicago, USA). The significance of difference was calculated by one-way ANOVA test, and the results with p < 0.05 were considered to be statistically significant. Multiple range tests were used to observe the statistical differences in ANOVA. Graphs were drawn with OriginPro 8.0 software (OriginLab Corporation, Northampton, MA, USA).
RESULTS AND DISCUSSION
Optimization of Experimental Conditions
The capillary column (HP-5MS and Rxi-5MS) and extraction method (ultrasonic solvent extraction and SPME method) was compared in the present article. As for the extraction method, considering that SPME equipment was used with a manual holder, the extracted sample was injected manually by using of SPME method. Meanwhile, the extracted sample was injected automatically by using of ultrasonic solvent extraction method. The automatic one referred to a direct injection, and the manual one referred to the fiber injection after carrying out the SPME procedure. Since the matrix can influence the extraction efficiency, 500 μL of the eight different nitrosamines at 10 μg/mL were added to the food matrix before injection. showed the total ion chromatograms (TICs) of analytes with direct injection and fiber injection in the scan mode. Using SPME method with PDMS-DVB coated fibers only allowed the extraction of five out of eight N-nitrosamines, which were NDEA, NDPA, NPIP, NDBA, and NDpheA. The results showed a good agreement with S. Ventanas and J. Ruiz.[Citation13] While by using the method of ultrasonic solvent extraction, eight N-nitrosamines could be detected with TICs mode. Since ultrasonic solvent extraction has lots of advantages in simple operation, short time, and low cost, ultrasonic solvent extraction with automatic injection was used in the current study.
FIGURE 1 (a) TICs of volatile N-nitrosamine standard with automatic injection in scan mode corresponding to extraction by using ultrasounds. (b) TICs of volatile N-nitrosamine standard with manual injection in scan mode corresponding to extraction by using SPME.
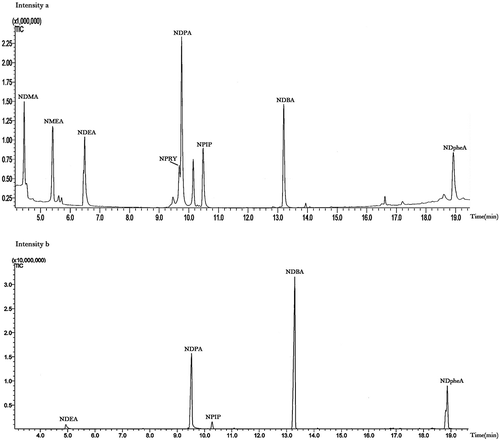
As for the optimization of capillary columns, a HP-5MS capillary column and a Rxi-5MS capillary column was used for the determination of eight volatile nitrosamines. showed the TICs of eight N-nitrosamine standard solutions injected for six times with HP-5MS capillary column. showed the mass spectrum of eight volatile N-nitrosamines. The results showed that the HP-5MS capillary column had better repeatability compared with that of Rxi-5MS capillary column. The RSD run-to-run was shown in , which showed a good RSD ranking from 1.005 to 2.055%.
FIGURE 2 Total ion chromatograms (TICs) of volatile N-nitrosamine standard injected for six times with HP-5MS capillary column.
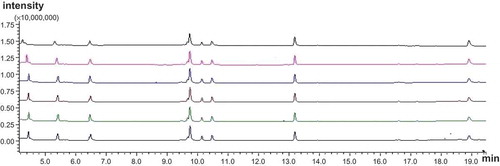
FIGURE 3 Mass spectrum of volatile N-nitrosamines: (a) N-nitrosodimethylamine, (b) N-nitrosomethylethylamine, (c) N-nitrosodiethylamine, (d) N-nitrosopyrrolidine, (e) N-nitrosodi-n-propylamine, (f) N-nitroso piperidine, (g) N-nitrosodi-n-butylamine, and (h) N-nitrosodiphenylamine.
also presented the quality parameters of the proposed ultrasonic extraction/GC-MS analysis method for the eight nitrosamines including the recovery, IDL, IQL, MDL, and MQL. The recovery of the method was 82.575–108.364%. The IDL and IQL of the method were 1.134–9.894 μg/L and 3.779–32.980 μg/L, respectively. The MDL and MQL of the method were 0.057–0.495 μg/kg and 0.189–1.649 μg/kg, respectively. Calibration curves of eight volatile nitrosamines at the working ranges indicated in and good linearity were achieved with the correlation coefficient (R2) higher than 0.999.
TABLE 1 Quality parameters of the proposed ultrasonic extraction/GC-MS analysis method for the eight volatile nitrosamines
TABLE 2 Calibration curve and linear range of eight volatile nitrosamines
TABLE 3 Contents of volatile N-nitrosamine (NAs) in different products assessed using the proposed ultrasonic extraction/GC-MS analysis method
Volatile Nitrosamine Contents in Chinese Meat Products
The volatile nitrosamine contents in total of 28 selected Chinese meat products covered three categories were determined by the current GC-MS method and the results were shown in . Chinese sausages are popular traditional food in China. The average values of each volatile N-nitrosamine in different meat products were also listed in . The total values of volatile N-nitrosamine contents in selected meat products ranged from 0.424 to 51.018 μg/kg. As a whole, volatile nitrosamines were present in all selected food categories of Chinese meat products. Meanwhile, the volatile N-nitrosamine levels varied with the food categories, the reason was that the raw material and processing methods had significant influence on the formation of volatile N-nitrosamines.[Citation19] The highest level of volatile N-nitrosamines was observed in grilled sausage with corn flavor. After being calculated, the average volatile N-nitrosamine levels followed the order: emulsion sausages (19.993 μg/kg), Chinese sausages (12.590 μg/kg), cooked sausages (6.264 μg/kg), which showed significant difference between each other (p < 0.05). Therefore, the volatile N-nitrosamine levels of Chinese sausages and emulsion sausages were generally higher than that of cooked sausages. Different addition of nitrites may cause different volatile N-nitrosamine contents between them and also lead to different shelf lives. Emulsion sausage is the most popular meat products in China. The result indicated that the levels of volatile N-nitrosamines in grilled sausages and smoked sausage were found higher, probably due to the heat treatment, which benefit to the formation of the N-nitrosamines in some meat products.[Citation20] Cooked sausages have a short shelf life because they are produced without preservatives or chemical additives. Even though they were also produced with heat treatment, cooked sausages still had lower nitrosamines. Volatile N-nitrosamine contents in emulsion sausages with garlic flavor were apparently lower than that in other emulsion sausages. The vegetables are rich of antioxidant compounds such as ascorbic acid and phenolic compounds. Research showed that allyl sulfur compounds in garlic might act as nitrite scavengers. Therefore, addition of garlic flavor might have effect on the contents of nitrosamines. The results were consistent to Choi et al.[Citation21] which demonstrated that garlic could reduce the N-nitrosamine contents. In addition, the major volatile nitrosamines found in our samples were NMEA, NPYR, NDBA, and NDpheA, whereas samples with NPIP occurred least. Indeed, a maximum level of 3 μg/kg for NDMA in retail meat products (except for canned meat) has been set in China according to national standards GB 2762-2012.[Citation22] In all the selected meat products, only sliced ham and fish steak are above the limitation except for canned meat. NDMA in others varied from 0.217 to 2.858 μg/kg. There are barely any limitation standards for other volatile nitrosamines.
CONCLUSION
The ultrasonic solvent extraction followed by GC-MS detection and quantification using a HP-5MS capillary column proved to be the best method for analysis of volatile nitrosamines in meat products. Satisfactory results, such as precision, linearity, and accuracy were obtained, which assure that the determining method was suitable for this purpose. The method is simple, permits fast analysis, little manipulation, and presents adequate sensitivity. Results show that volatile nitrosamines were existed in most kinds of meat products. Volatile N-nitrosamine levels of Chinese sausages and emulsion sausages were generally higher than that of cooked sausages, and the contents in sausage with garlic flavor were obviously low. The major volatile nitrosamines found in our samples were NMEA, NPYR, NDBA, and NDpheA. Overall, the results demonstrated that GC-MS method can be applied as an appropriate technique for volatile nitrosamines analysis in meat products.
FUNDING
This work was supported by the Fund of National Basic Research Program of China (“973” Program, 2012CB720805).
Additional information
Funding
REFERENCES
- IARC. Overall evaluation of carcinogenicity: anupdating of IARC monographs. Vols. 1–42, Suppl. 7, Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. International Agency for Research on Cancer, Lyon, 1987.
- Yurchenko, S.; Mölder, U. Volatile N-nitrosamines in various fish products. Food Chemistry 2006, 96 (2), 325–333.
- Drabik-Markiewicz, G.; Dejaegher, B.; Mey, E.D.; Kowalska, T.; Paelinck, H.; Heyden, Y.V. Influence of putrescine, cadaverine, spermidine, or spermine on the formation of N-nitrosamine in heated cured pork meat. Food Chemistry 2011, 126 (4), 1539–1545.
- Drabik-Markiewicz, G.; Dejaegher, B.; Mey, E.D.; Impens, S.; Kowalska, T.; Paelinck, H.; Heyden, Y.V. Evaluation of the influence of proline, hydroxyproline, or pyrrolidine in the presence of sodium nitrite on N-nitrosamines formation when heating cured meat. Analytica Chimica Acta 2010, 657 (2), 123–130.
- Campillo, N.; Vinas, P.; Martínez-Castillo, N.; Hernández-Córdoba, M. Determination of volatile nitrosamines in meat products by microwave-assisted extraction and dispersive liquid-liquid microextraction coupled to gas chromatography-mass spectrometry. Journal of Chromatography A 2011, 1218 (14), 1815–1821.
- National Standards of China. Determination of N-nitrosamines in foods. 2003, GB/T 5009.26–2003. Beijing (China): National Standards.
- Vas, G.; Vékey, K. Solidphase microextraction: A powerful sample preparationtoolprior to mass spectrometric analysis. Journal of Mass Spectrometry 2004, 39 (3), 233–254.
- Kataoka, H.; Lord, H.L.; Pawliszyn, J. Applications of solid-phase microextraction in food analysis. Journal of Chromatography A 2000, 880 (1–2), 35–62.
- Jurado-Sánchez, B.; Ballesteros, E.; Gallego, M. Comparison of microwave assisted, ultrasonic assisted, and soxhlet extractions of N-nitrosamines and aromatic amines in sewage sludge, soils, and sediments. Science of Total Environment 2013, 463–464, 293–301.
- Wu, Y.Y.; Liu, F.J.; Li, L.H.; Yang, X.Q.; Deng, J.C.; Chen S.J.; Ma, H.X. Determination and optimization of N-nitrosamines in salted fish by gas chromatography-mass spectrometry. South China Fisheries Science, 2012, 8 (4), 16–22.
- Grebel, J.E.; Young, C.C.; Suffet, I.H. Solid-phase microextraction of N-nitrosamines. Journal of Chromatography A 2006, 1117 (1), 11–18.
- Andrade, R.; Reyes, F.G.; Rath, S. A method for the determination of volatile N-nitrosamines in food by HS-SPME-GC-TEA. Food Chemistry 2005, 91 (1), 173–179.
- Ventanas, S.; Ruiz, J. On-site analysis of volatile nitrosamines in food model systems by solid-phase microextraction coupled to a direct extraction device. Talanta 2006, 70 (5), 1017–1023.
- Hou, H.; Zhao, X.; Li, B.F. Solid-phase microextraction method for the determination of volatile compounds in hydrolysates of Alaska Pollock frame. International Journal of Food Properties 2013, 16 (4), 790–802.
- Hayaloglu, A.A.; Karabulut, I. SPME/GC-MS characterization and comparision of volatiles of eleven varieties of Turkish cheese. International Journal of Food Properties 2013, 16 (7), 1630–1653.
- Pérez, D.M.; Alatorre, G.G.; Alvarez, E.B.; Silva, E.E.; Alvarado, J.J. Solid-phase microextraction of N-nitrosodimethylamine in beer. Food Chemistry 2008, 107 (3), 1348–1352.
- Jurado-Sánchez, B.; Ballesteros, E.; Gallego, M. Comparison of the sensitivities of seven N-nitrosamines in pre-screened waters using an automated preconcentration system and gas chromatography with different detectors. Journal of Chromatography A 2007, 1154 (1–2), 66–73.
- Yurchenko, S.; Mölder, U. The occurrence of volatile N-nitrosamines in Estonian meat products. Food Chemistry 2007, 100 (4), 1713–1721.
- Glória, M.A.; Barbour J.F.; Scanlan, R.A. Volatile nitrosamines in fried bacon. Journal of Agricultural and Food Chemistry 1997, 45 (5), 1816–1818.
- Rywotycki, R. The effect of selected functional additives and heat treatment on nitrosamine content in pasteurized pork ham. Meat Science 2002, 60 (4), 335–339.
- Choi, S.Y.; Chung, M.J.; Lee, S.J.; Shin, J.H.; Sung, N.J. N-nitrosamine inhibition by strawberry, garlic, kale, and the effects of nitrite-scavenging and N-nitrosamine formation by functional compounds in strawberry and garlic. Food Control 2007, 18 (5), 485–491.
- National Standards of China. Maximum levels of contaminants in foods. 2012. GB 2762-2012. Beijing (China): National Standards.
