Abstract
The ethanol extracts of apple fruits harvested from the cultivars Aldas, Auksis, Ligol, and Lodel grown in Lithuania were analyzed by the high-performance liquid chromatography method. Chlorogenic acid was found to be a predominant component in the apple fruits of all the cultivars, except the cultivar Ligol. (–)-Epicatechin was the major compound in the ethanol extracts of apple fruits obtained from all the cultivars, while the amount of (+)–catechin was lower. The following quercetin glycosides were identified and quantified: hyperoside, isoquercitrin, avicularin, rutin, and quercitrin. Hyperoside was the major quercetin glycoside in apple fruits.
INTRODUCTION
The domestic apple (Malus domestica Borkh.) is one of the most widely cultivated fruit trees. More than 7000 cultivars have been reported worldwide.[Citation1] According to the data of 2010, about 67.57 million tons of apples are produced every year. China, the United States, and Turkey are the leading apple producing countries.[Citation2] This fruit plays an important role in a human diet, and it is one of the world’s most popular fruits.[Citation3,Citation4] Moreover, apples are a source of various biologically active substances, especially phenolic compounds.[Citation4,Citation5]
It is very important to analyze the phenolic content of apples as they are extensively used in the prevention of various diseases, preparation of safe and healthy foods, and development and production of dietary supplements. It has been demonstrated that fresh apples used in diet every day, their products, and apple-derived foods are rich in biologically active substances. During the recent years, especially great attention is paid to studies on the chemical composition of apples and the effects of biologically active substances present in these fruits. Literature sources report that apples accumulating numerous biologically active substances can be utilized for the prevention of some diseases.[Citation6−Citation9] Scientists are interested in studies investigating an antioxidant effect of phenolic compounds, which is considered one of the main biological activities possessed by these compounds.[Citation10,Citation11] Phenolic compounds, acting as natural antioxidants, scavenge free radicals and inhibit their production, stimulate the synthesis of antioxidant enzymes, and thus prevent oxidative stress resulting in damage to the structural molecules of the body.[Citation12−Citation15] The antioxidant activity of polyphenolic compounds is associated with other biological properties of these compounds, such as anti-inflammatory, antimicrobial, anticancer, cardiovascular system-improving, and other activities.[Citation16−Citation19]
Therefore, the aim of this study was to determine the qualitative and quantitative content of phenolic compounds in the ethanol extracts of apple fruits harvested from the cultivars Aldas, Auksis, Ligol, and Lodel grown under Lithuanian climatic conditions and to assess the variation of phenolic compounds in the apple fruit samples obtained from different cultivars.
MATERIALS AND METHODS
Apples
The following apple cultivars were included in the study: Auksis, Aldas, Ligol, and Lodel. The apple trees were grown in the garden of the Institute of Horticulture, Lithuanian Research Centre for Agriculture and Forestry, Babtai, Lithuania. Apple fruits were harvested from ten-year-old apple trees in 2012. The study sample comprised 20 ripe apple fruits of each cultivar. The apple slices were immediately frozen in a freezer (–35°C) with air circulation; then apple fruits were lyophilized with a ZIRBUS sublimator 3×4×5/20 (ZIRBUS technology, Bad Grund, Germany) at a pressure of 0.01 mbar (condenser temperature, –85°C). The lyophilized apple and fruits were ground to a fine powder by using a Retsch 200 mill (Haan, Germany). Loss on drying before analysis was determined by the method of the European Pharmacopoeia.[Citation20] Data were recalculated for absolute dry lyophilizate weight.
Chemicals
All the solvents, reagents, and standards used were of analytical grade. Acetonitrile and acetic acid were obtained from Sigma-Aldrich GmbH (Buchs, Switzerland) and ethanol from Stumbras AB (Kaunas, Lithuania). Hyperoside, rutin, quercitrin, phloridzin, procyanidin B1 and procyanidin B2, and chlorogenic acid standards were purchased from Extrasynthese (Genay, France), (+)-catechin and (–)-epicatechin from Fluka (Buchs, Switzerland), and avicularin and isoquercitrin from Chromadex (Santa Ana, USA). Deionized water, produced by the Crystal E high-performance liquid chromatography (HPLC, Adrona SIA, Riga, Latvia) water purification system, was used.
Extraction
An amount of 2.5 g of lyophilized apple powder (exact weight) was weighed, added to 30 mL of ethanol (70%, v/v), and extracted in a Sonorex Digital 10 P ultrasonic bath (Bandelin Electronic GmbH & Co. KG, Berlin, Germany) for 20 min at 40°C. Ultrasonic power was 480 W; frequency was 35 kHz. The conditions of extraction (type of extraction, duration, temperature, ultrasonic power, solvent, and its concentration) were chosen considering the results of extraction optimization. The extract obtained was filtered through a paper filter; the apple lyophilizate on the filter was washed twice with 10 mL of ethanol (70%, v/v) in a 50-mL flask. The extract was filtered through a membrane filter with a pore size of 0.22 μm (Carl Roth GmbH, Karlsruhe, Germany).
Instrumentation and Chromatographic Conditions
A Waters 2695 chromatograph equipped with a Waters 2998 photodiode array (PDA) detector (Waters, Milford, USA) was used for HPLC analysis. Chromatographic separation was managed, chromatograms were recorded, and data were processed with the Empower® v. 2.0. program software (Waters, Milford, USA). Chromatographic separations were carried out by using a YMC-Pack ODS-A (5 μm, C18, 250 × 4.6 mm i.d.) column equipped with a YMC-Triart (5 μm, C18, 10 × 3.0 mm i.d.) precolumn (YMC Europe GmbH, Dinslaken, Germany). The column was operated at a constant temperature of 25°C. The volume of the extract being investigated was 10 μL. The flow rate was 1 mL/min, and gradient elution was used. The mobile phase consisted of 2% (v/v) acetic acid in water (solvent A) and 100% (v/v) acetonitrile (solvent B). The following conditions of elution were applied: 0–30 min, 3–15% B; 30–45 min, 15–25% B; 45–50 min, 25–50% B; and 50–55 min, 50–95% B. Detection was simultaneously performed at three wavelengths: 280 nm (dihydrochalcones, catechins, procyanidins), 320 nm (phenolic acids), and 360 nm (flavonols). The spectrum of UV-visible light was recorded from 200 to 600 nm. All the phenolic compounds were identified by comparing their retention times and UV-Vis spectra with those of the standard compounds. The compounds identified were confirmed by adding the standard compound into the analyzed extract and monitoring the changes in the peak shape and UV-Vis spectral characteristics. The concentration of phenolics was calculated based on the peak areas by using standard compounds.
Statistical Data Analysis
All the experiments were carried out in triplicate. Means and standards deviations were calculated with the SPSS 20.0 software (Chicago, USA). A single factor analysis of variance (ANOVA) along with the post-hoc Tukey test was employed for statistical analysis. The Kolmogorov-Smirnov test was applied to compare two independent samples without requiring the data be normally distributed. To verify the hypothesis about the equality of variances, the Levene’s test was employed. Differences were considered to be significant at p < 0.05.
RESULTS AND DISCUSSION
As the chemical composition of the apples of different cultivars can vary considerably,[Citation3] it is very important to compare and assess the chemical composition of the apples of different cultivars and to ensure their quality. The application of the HPLC method and the use of methodology developed by us in the analysis of ethanol extracts obtained from apple fruits allow the identification of the qualitative and quantitative content of individual phenolic compounds and its variation in the fruits of different apple cultivars.
The following phenolic compounds of different groups were identified in the investigated ethanol extracts of apple fruit samples (cultivars Aldas, Auksis, Ligol, and Lodel): procyanidin B1, (+)-catechin, chlorogenic acid, procyanidin B2, (–)-epicatechin, rutin, hyperoside, isoquercitrin, avicularin, quercitrin, and phloridzin. The chromatograms of ethanol extracts of apple fruit samples obtained from the cultivars investigated are identical regarding the number of analytes and retention time. The example of chromatogram of the ethanol extract of the apple fruit sample is shown in .
TABLE 1 Quantitative content of quercetin glycosides in ethanol extracts obtained from the apple fruits of cultivars grown in Lithuania.
The identified phenolic compounds seen in the chromatogram of apple fruit extract (cultivar Aldas) were quantified (). A quantitative analysis provides the data on the quantitative content of biologically active compounds and its variation among different cultivars and species. The phytochemical analysis of plant raw materials enables the identification and evaluation of the chemotaxonomic markers of plant species and cultivars, and this aspect is very important in the taxonomic classification and well as the description, and differentiation of cultivated plant cultivars in order to ensure the quality of healthy, safe food rich in biologically active substances and its provision for a consumer.
In the investigated ethanol extracts of apple samples, quercetin glycosides were the most abundant group of phenolic compounds. These compounds are important for human health because of their strong antioxidant and anticancer activities.[Citation10,Citation14,Citation21] The consumption of products rich in quercetin glycosides reduces the risk of cardiovascular and neurodegenerative diseases.[Citation22−Citation24]
The following quercetin glycosides were identified and quantified in the ethanol extracts of apple fruit samples (cultivars Aldas, Auksis, Ligol, and Lodel): hyperoside, isoquercitrin, rutin, avicularin, and quercitrin. summarizes the characteristics of quantitative content. The highest total amount of quercetin glycosides was two times greater than the lowest amount of these compounds identified in the apple fruits of the cultivar Auksis (). Hyperoside was a predominant component among quercetin glycosides in the ethanol extracts of the apple samples of cultivars selected for this study. Rutin was the minor component among all quercetin derivatives identified (). These results are consistent with those of previously published studies,[Citation25,Citation26] which reported hyperoside and quercitrin to be predominant compounds; avicularin or avicularin along with hyperoside was reported less frequently.[Citation3,Citation27,Citation28]
The triplet of quercetin glycosides, namely rutin, hyperoside, and isoquercitrin, where always hyperoside was a predominant component and the concentration of rutin was lowest, was found to be characteristic of all apple cultivars analyzed in the present study. This is in line with the results of other studies[Citation26,Citation27] and therefore, suggests that the rutin-hyperoside-isoquercitrin triplet, where hyperoside predominates and the concentration of rutin is relatively low, could be considered one of the characteristic features of phytochemical composition in apples. This characteristic feature along with the compounds of dihydrochalcone class could be used for the chemotaxonomy of apple trees, which allows the identification of apple cultivars and products.
Quercetin glycosides identified and quantified in the ethanol extracts of apple fruit samples (Aldas, Auksis, and Lodel) can be ranked in the following ascending order by their quantitative content: rutin < isoquercitrin < quercitrin < avicularin < hyperoside. In the ethanol extracts of cultivar Ligol apples, the amount of quercitrin was found to be greater than that of avicularin (), distinguishing this cultivar from the remaining cultivars. This characteristic feature was mentioned by the Polish study as well.[Citation29]
The compounds of procyanidin class have been identified in the plants of the family Rosaceae[Citation30,Citation31] and various apple cultivars.[Citation26,Citation32,Citation33] Procyanidins are important for a human body as they exhibit antioxidant, anticancer, anti-inflammatory, and platelet aggregation- and cholesterol-reducing effects.[Citation34−Citation36] In the present study, procyanidins B1 and B2 were identified and quantified in the ethanol extracts of apple fruit samples. shows their quantitative content.
TABLE 2 Quantitative content of procyanidins, phenolic acids, and dihydrochalcones in ethanol extracts obtained from the apple fruits of cultivars grown in Lithuania.
The highest total amount of procyanidins was determined in the apple fruits of the cultivar Lodel; and the lowest, in the apple fruits of the cultivar Ligol. In the ethanol extracts of lyophilized apple samples of all the cultivars analyzed, the concentration of procyanidin B2 was higher than that of procyanidin B1 (). The highest concentration of procyanidin B1 was determined in the apple fruits of the cultivar Auksis. It was three-fold greater than its lowest concentration in the apple fruits of the cultivar Ligol (). Other literature sources report lower amounts of procyanidin B1 and B2 in the extracts of the lyophilized apple samples of the cultivar Ligol.[Citation29]
The compounds of the phenolic acid class have been identified while studying the chemical content of apple samples obtained from various apple cultivar.[Citation27,Citation28] In this study, it was important to analyze the variation in the amount of chlorogenic acid and to compare the obtained results with those of other studies. The analysis of the study results revealed that chlorogenic acid was the major phenolic compound in the ethanol extracts of apple fruit samples of all the cultivars, except the cultivar Ligol. Its quantitative content is shown in .
The amount of chlorogenic acid accounted for 44.9 to 74.9% of the total content of all phenolic compounds identified by the HPLC method. The results of this study have confirmed previous studies reporting that chlorogenic acid is one of the most predominant components in apples.[Citation27−Citation29] The lower amount of chlorogenic acid in the apple fruits of the cultivar Ligol was reported by the Polish study.[Citation29] Different apple cultivation and fertilization approaches could contribute to the differences in the quantitative composition; the impact of meteorological and geographical factors cannot be excluded as well.[Citation27,Citation37]
Phloridzin, a compound of the dihydrochalcone class detected in the apple fruit samples, can be regarded as a chemotaxonomic marker for the identification of apple cultivars as well as products made from apples.[Citation26,Citation38,Citation39] Phloridzin exhibit antidiabetic activity;[Citation40] therefore, plant raw materials accumulating this compound can be potentially useful for the prevention of diabetes mellitus. The apple fruits of the cultivar Aldas contained the greatest amount of phloridzin (151.7 ± 6.5 μg/g), and the lowest amount of this compound was determined in the apple fruits of the cultivar Ligol (75.4 ± 2.7 μg/g; ).
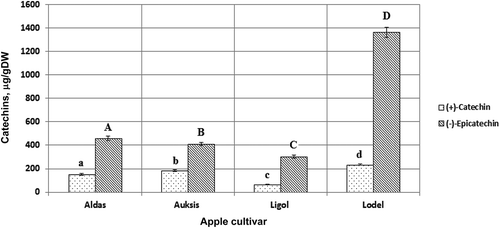
In this study, the compounds of catechin class—(+)-catechin and (–)-epicatechin—were identified and quantified in the ethanol extracts of apple fruits (). The highest concentration of (–)-epicatechin was identified in the apple fruits of the cultivar Lodel. It was 4.5-fold greater than its lowest concentration in the apple fruits of the cultivar Ligol. The apple fruits of the latter cultivar had the greatest amount of (+)-catechin among all the cultivars studied (). These data confirm the importance of the cultivar as a factor for the quantitative composition of apples, described in other literature sources.[Citation3,Citation28] Other studies reported similar quantitative characteristics of these compounds in the extracts obtained from apple fruits.[Citation4,Citation28,Citation41] The results reported in this study prompt further research on the chemical composition and biological effect of apple fruits by evaluating the antioxidant activity of individual phenolic compounds in vitro and in vivo, and confirm a potential of these plant raw materials in the development and production of healthy food rich in biologically active compounds and dietary supplements.
CONCLUSIONS
This study that investigated the phenolic content of ethanol extracts obtained from apple fruits of the cultivars Aldas, Auksis, Ligol, and Lodel grown under Lithuanian climatic conditions by the HPLC method showed that chlorogenic acid was predominant components in apple fruits. The following quercetin glycosides were identified and quantified in the apple fruits of the cultivars investigated: hyperoside, isoquercitrin, rutin, avicularin, and quercitrin. Hyperoside was the major compound of quercetin glycosides in apple fruits. The apple fruits of the cultivar Aldas had the greatest total amount of quercetin glycosides. The greatest total amount of catechins was determined in the apple fruits of the cultivar Lodel. The concentration of procyanidin B2 was higher than that of procyanidin B1; the apple fruits of the cultivar Lodel contained the greatest total amount of procyanidins. The results of this study provide new knowledge about the qualitative and quantitative content of phenolic compounds in the apple fruits of cultivars grown under Lithuanian climatic conditions and variation in this content depending on the cultivar. The results of studies will be used to identify promising apple cultivars grown under Lithuanian climatic conditions, the fruits of which accumulate large amounts of phenolic compounds and are valuable for the consumption and production of juice and other foods.
FUNDING
This study was supported by a grant from the Research Council of Lithuania (No. SVE-02/2011).
REFERENCES
- Noiton, D.A.M.; Alspach, P.A. Founding clones, inbreeding, coancestry, and status number of modern apple cultivars. Journal of the American Society for Horticultural Science. American Society for Horticultural Science 1996, 121, 773–782.
- FAO Stat database. 2012. http:// faostat.fao.org. (accessed November 20, 2012).
- Ceymann, M.; Arrigoni, E.; Schärer, H.; Nising, A.B.; Hurrell, R.F. Identification of apples rich in health-promoting flavan-3-ols and phenolic acids by measuring the polyphenol profile. Journal of Food Composition and Analysis 2012, 26, 128–135.
- Wu, J.; Gao, H.; Zhao, L.; Liao, X.; Chen, F.; Wang, Z.; Hu, X. Chemical compositional characterization of some apple cultivars. Food Chemisty 2007, 103, 88–93.
- Belviso, S.; Scursatone, B.; Re, G.; Zeppa, G. Novel data on the polyphenol composition of Italian ancient apple cultivars. International Journal of Food Properties 2013, 16, 1507–1515.
- Gerhauser, C. Cancer chemopreventive potential of apples, apple juice, and apple components. Planta Medica 2008, 74, 1608–1624.
- Pearson, D.; Tan, C.; German, B.; Davis, P.; Gershwin, M. Apple juice inhibits low density lipoprotein oxidation. Life Sciences 1999, 64, 1913–1920.
- Soler, C.; Soriano, J.M.; Mañes, J. Apple-products phytochemicals and processing: A review. Natural Product Communications 2009, 4, 659–670.
- Nowacka, M.; Śledź, M.; Wiktor, A.; Witrowa-Rajchert, D. Changes of radical scavenging activity and polyphenols content during storage of dried apples. International Journal of Food Properties 2014, 17, 1317–1331.
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism, and structure-activity relationships. The Journal of Nutritional Biochemistry 2002, 13, 572–584.
- Scalbert, A.; Johnson, I.T.; Saltmarsh, M. Polyphenols: Antioxidants and beyond. The American Journal of Clinical Nutrition 2005, 81, 215S–217S.
- Halliwell, B.; Murcia, M.A; Chirico, S.; Aruoma, O.I. Free radicals and antioxidants in food and in vivo: What they do and how they work. Critical Reviews in Food Science and Nutrition 1995, 35, 7–20.
- Hider, R.C.; Liu, Z.D.; Khodr, H.H. Metal chelation of polyphenols. Methods in Enzymology 2001, 335, 190–203.
- Nijveldt, R.J.; van Nood, E.; van Hoorn, D.E.C.; Boelens, P.G.; van Norren, K.; van Leeuwen, P.A.M. Flavonoids: A review of probable mechanisms of action and potential applications. The American Journal of Clinical Nutrition 2001, 74, 418–425.
- Li, L.; Li, X.; Ban, Z.; Jiang, Y. Variation in antioxidant metabolites and enzymes of “Red Fuji” apple pulp and peel during cold storage. International Journal of Food Properties 2014, 17, 1067–1080.
- Curin, Y.; Andriantsitohaina, R. Polyphenols as potential therapeutical agents against cardiovascular diseases. Pharmacological Reports 2005, 57, 97–107.
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Medicine and Cellular Longevity 2009, 2, 270–278.
- Petti, S.; Scully, C. Polyphenols, oral health, and disease: A review. Journal of Dentistry 2009, 37, 413–423.
- Stoner, G.D.; Mukhtar, H. Polyphenols as cancer chemopreventive agents. Journal of Cellular Biochemistry. Supplement 1995, 22, 169–180.
- European pharmacopoeia, 7th Ed; Vol. 1. Council of Europe: Strasbourg, 2010; 01/2008:2.2.32.; p. 51.
- Kandaswami, C.; Lee, L.T.; Lee, P.P.; Hwang, J.J.; Ke, F.C.; Huang, Y.T.; Lee, M.T. The antitumor activities of flavonoids. In Vivo 2005, 19, 895–919.
- Hertog, M.G.; Feskens, E.J.; Hollman, P.C.; Katan, M.B.; Kromhout, D. Dietary antioxidant flavonoids and risk of coronary heart disease: The Zutphen Elderly Study. The Lancet 1993, 342, 1007–1011.
- Knekt, P.; Kumpulainen, J.; Järvinen, R.; Rissanen, H.; Heliövaara, M.; Reunanen, A.; Hakulinen, T.; Aromaa, A. Flavonoid intake and risk of chronic diseases. The American Journal of Clinical Nutrition 2002, 76, 560–568.
- Spencer, J.P. The impact of fruit flavonoids on memory and cognition. The British Journal of Nutrition 2010, 104, S40–S47.
- Price, K.R.; Prosser, T.; Richetin, A.M.F.; Rhodes, M.J.C. A comparison of the flavonol content and composition in dessert, cooking, and cider-making apples; distribution within the fruit and effect of juicing. Food Chemistry 1999, 66, 489–494.
- Schieber, A.; Keller, P.; Carle, R. Determination of phenolic acids and flavonoids of apple and pear by high-performance liquid chromatography. Journal of Chromatography A 2001, 910, 265–273.
- Marks, S.C.; Mullen, W.; Crozier, A. Flavonoid and chlorogenic acid profiles of English cider apples. Journal of the Science of Food and Agriculture 2007, 87, 719–728.
- Tsao, R.; Yang, R.; Young, J.C.; Zhu, H. Polyphenolic profiles in eight apple cultivars using high-performance liquid chromatography (HPLC). Journal of Agricultural and Food Chemistry 2003, 51, 6347–6353.
- Duda-Chodak, A.; Tarko, T.; Satora, P.; Sroka, P.; Tuszyński, T. The profile of polyphenols and antioxidant properties of selected apple cultivars grown in Poland. Journal of Fruit and Ornamental Plant Research 2010, 18, 39–50.
- Kähkönen, M.P.; Hopia, A.I.; Heinonen, M. Berry phenolics and their antioxidant activity. Journal of Agricultural and Food Chemistry 2001, 49, 4076–4082.
- Vasco, C.; Riihinen, K.; Ruales, J.; Kamal-Eldin, A. Phenolic compounds in Rosaceae fruits from Ecuador. Journal of Agricultural and Food Chemistry 2009, 57, 1204–1212.
- Escarpa, A.; González, M.C. High-performance liquid chromatography with diode-array detection for the determination of phenolic compounds in peel and pulp from different apple varieties. Journal of Chromatography A 1998, 823, 331–337.
- Hammerstone, J.F.; Lazarus, S.A.; Schmitz, H.H. Procyanidin content and variation in some commonly consumed foods. The Journal of Nutrition 2000, 130, 2086S–2092S.
- Carnésecchi, S.; Schneider, Y.; Lazarus, S.A.; Coehlo, D.; Gossé, F.; Raul, F. Flavanols and procyanidins of cocoa and chocolate inhibit growth and polyamine biosynthesis of human colonic cancer cells. Cancer Letters 2002, 175, 147–155.
- Lotito, S.B.; Actis-Goretta, L.; Renart, M.L.; Caligiuri, M.; Rein, D.; Schmitz, H.H.; … Fraga, C.G. Influence of oligomer chain length on the antioxidant activity of procyanidins. Biochemical and Biophysical Research Communications 2000, 276, 945–951.
- Terra, X.; Valls, J.; Vitrac, X.; Mérrillon, J.M.; Arola, L.; Ardèvol, … Blay, M. Grape-seed procyanidins act as antiinflammatory agents in endotoxin-stimulated RAW 264.7 macrophages by inhibiting NFkB signaling pathway. Journal of Agricultural and Food Chemistry 2007, 55, 4357–4365.
- Awad, M.A.; de Jager, A.; van Westing, L.M. Flavonoid and chlorogenic acid levels in apple fruit: Characterisation of variation. Scientia Horticulturae 2000, 83, 249–263.
- Alonso-Salces, R.M.; Ndjokob, K.; Queirozb, E.F.; Iosetb, J.R.; Hostettmannb, K.; Berruetaa, L.A.; … Vicentea, F. On-line characterisation of apple polyphenols by liquid chromatography coupled with mass spectrometry and ultraviolet absorbance detection. Journal of Chromatography A 2004, 1046, 89–100.
- Gosch, C.; Halbwirth, H.; Stich, K. Phloridzin: Biosynthesis, distribution, and physiological relevance in plants. Phytochemistry 2010, 71, 838–843.
- Masumoto, S.; Akimoto, Y.; Oike, H.; Kobori, M. Dietary phloridzin reduces blood glucose levels and reverses Sglt1 expression in the small intestine in streptozotocin-induced diabetic mice. Journal of Agricultural and Food Chemistry 2009, 57, 4651–4656.
- Kahle, K.; Kraus, M.; Richling, E. Polyphenol profiles of apple juices. Molecular Nutrition & Food Research 2005, 49, 797–806.