Abstract
Edible shellfish Mytilus galloprovincialis and Crassostrea gigas have been investigated for the paralytic shellfish poisons using mouse bioassay and high performance liquid chromatography with fluorescence detection. Paralytic shellfish poisons toxins were detected in mussels and oysters from September 2007 to May 2008. The level of paralytic shellfish poisons toxins in mussels reached the maximum in November with 832.9 μg saxitoxin-eq/100 g tissue. In oysters, toxins were detected with a maximum of 11.2 μg saxitoxin-eq/100 g tissue. The toxin high performance liquid chromatography profiles in mussels and oysters revealed the dominance of gonyautoxin 5 and N-sulfocarbamoyl-gonyautoxin-2 and -3 (C1-2), whereas GTX1-4, saxitoxin, and neosaxitoxin were found at low amounts. Overall, levels of paralytic shellfish poisons toxins were 20–70 times greater in mussels than in oysters. This is the first report on the qualitative and quantitative paralytic shellfish poisons content of M. galloprovincialis and C. gigas from a shellfish farming lagoon in Tunisia.
INTRODUCTION
Paralytic shellfish poisoning (PSP) toxins are potent marine neurotoxins, which block sodium channels of the neuronal cell membrane. They consist of a family of over 20 toxins which are produced by temperate Alexandrium species and Gymnodinium catenatum and by tropical Pyrodinium bahamense var. compressum.[Citation1] Toxic algae of the genus Alexandrium are the dominant sources of PSP toxins in contaminated bivalves.[Citation2] It is a widespread food toxicological problem which occurs frequently, and in some cases chronically, along the Pacific and Atlantic coasts of North America, in Central and South America, on the western European coasts, in Japan, Southeast Asia, South Africa, New Zealand, and Australia.[Citation3]
Bivalves are plankton feeders and can accumulate high levels of PSP toxins,[Citation4] becoming a worldwide food safety concern. Current European legislation (EC) N° 2074/2005[Citation5] recommends shellfish harvesting areas to be monitored for PSP using the mouse bioassay (MBA) as described by the Association of Analytical Chemists[Citation6,Citation7] or by means of the high performance liquid chromatography (HPLC) method following a pre-column oxidation step. Amounts over 80 μg saxitoxin (STX) equivalents/100 g edible tissue are considered potentially harmful to humans; and this concentration has been set as the legal limit for the sale of shellfish in Canada, Europe (EC, 853/2004)[Citation8] and many other countries. Therefore, when PSP level exceeds 80 μg STX-eq/100 g shellfish flesh, this leads to temporary closing the harvesting area.
To date, MBA was the most widely used analytical method, first developed by Sommer and Meyer[Citation9] and later standardized to become an AOAC “official method”[Citation10] widely applied in most countries. Later, alternative methods have been used including HPLC with fluorimetric detection.[Citation11] The MBA measures total toxicity, but not the concentrations of specific toxins, whereas the chromatographic HPLC method identifies individual toxin peaks on the basis of their retention times. The main purpose of this study is to analyze qualitatively and quantitatively, for the first time, PSP toxins in Mytilus galloprovincialis and Crassostrea gigas collected at the lagoon of Bizerte (North Tunisia) from September 2007 to May 2008 using the MBA and the high performance liquid chromatography with fluorescence detector (HPLC-FLD). The study is conducted in a comparative manner to evaluate PSP toxins distributions and profiles in these two bivalve species collected from the same area and during the same period.
MATERIALS AND METHODS
Reagents
All organic solvents were of HPLC grade (Carlo Erba, Milan, Italy). Water was distilled and passed through a MilliQ water purification system (Millipore Ltd, Bedford, MA, USA). Certified standard solutions for STX, neosaxitoxin (NEO), GTX2&3, GTX1&4, dcSTX, dcGTX2-3, gonyautoxin 5 (GTX5), C1-2, and dcNEO were purchased from the Certified Reference Materials Program (CRMP) of the Institute for Marine Biosciences (National Research Council, Halifax, Canada).
Investigated Area and Sampling Stations
Bizerte lagoon is a semi-enclosed area of the southern Mediterranean Sea located in North Tunisia (37º 8ʹ-37º14ʹ N, 9º46ʹ-9º56ʹ E). The surface area is about 128 km2, maximum width 11 km, and maximum length 13 km; the mean depth is 7 m. The lagoon is connected to the east of the Mediterranean Sea via a 6 km long canal, and to the West to Ichkeul Lake via a 5 km long canal Tinja (). In Tunisia, mussels and oysters farming is developed only in this lagoon. Samples of M. galloprovincialis and C. gigas were harvested monthly from Menzel Jmil station at 0.5 m depth from September 2007 to May 2008. For phytoplankton, water samples were harvested from the water surface of the lagoon.
Phytoplankton Sampling and Identification
Field samples containing Alexandrium cells were collected weekly at the surface during an A. catenella bloom, in November 2007. The seawater samples were fixed with Lugol’s iodine solution. A sub-volume (2–5 mL) of fixed seawater samples was settled in sedimentation chambers for 24 h and counted using an inverted microscope following the Utermöhl method. The dinoflagellate species were identified using universally accepted taxonomic keys based specially on the morphology of the cells and the thecal plate patterns.[Citation12]
Extraction Procedures
Collected samples of M. galloprovincialis and C. gigas were stored frozen at –20°C until extraction. The whole flesh tissue was removed from the shell and rinsed with freshwater and then drained for 5 min in a sieve to remove salt water and sediment. Mussel and oyster samples were then used for toxin extraction. Briefly, 100 g of homogenized mussel or oyster tissues was mixed with an equal volume of 0.1 M HCl, and heated in boiling water for 5 min after adjusting the pH to 2–3. The mixture was diluted to 200 mL with distilled water and centrifuged at 3000 × g for 15 min. The supernatant was then stored at –20°C until analysis.
Toxin Analysis
MBA
The toxicity of the oyster and mussel extracts was assessed by the MBA according to the AOAC method.[Citation6] The MBA was carried out at the Centro Ricerche Marine Laboratory (Cesenato, Italy). Three mice (20 ± 2 g) were injected with 1 mL of the shellfish extract. Toxicity was correlated with death time and converted to μg STXeq 100 g−1 shellfish flesh using standard toxicity curves.
HPLC analysis
Extracts obtained from the samples collected during the period of September 2007 to May 2008 were analyzed according to the AOAC-approved HPLC-FLD pre-column oxidation method for PSP toxins determination in shellfish.[Citation13] After solid-phase extraction (SPE) clean-up steps, periodate, and peroxide oxidation of PSPs were carried out prior to toxins analysis. Chromatographic separations were performed with an UPLC Acquity (Waters, Milford, MA, USA) on a reversed phase column C18 Kinetex (100 × 2.1 mm, 2.6 μm; Phenomenex, Torrance, CA, USA) equipped with multi wavelength fluorescence detector 2475 (Waters) with excitation set to 340 nm and emission to 395 nm. The mobile phase gradient used to elute the PSP oxidation products consisted of two mobile phases: A (0.1 M ammonium formate) and B (0.1 M ammonium formate with 5% acetonitrile), both adjusted to pH 6 by adding 6 ml 0.1 M acetic acid. HPLC conditions were: 0–5% mobile phase B during the first 5 min, 5–70% B for the next 4 min and back to 0% B over the next 2 min; then at 0% B for another 5 min before the next injection. The flow rate was 0.2 mL/min and the temperature was 30°C. Standard curves were run in each session and generated with a minimum of five concentrations for each reference compound. The toxicity factors stated in the ‘‘Supplemental Information for PSP toxins CRMs’’ (Quilliam, 2004) were used for calculation of PSTs in terms of STX equivalents based on those reported by Oshima.[Citation14] For toxins that co-elute using the method described by Lawrence et al.[Citation11] and were determined together (C1&2; dcGTX2&3; GTX1&4; and GTX2&3), the value of the compound with the highest toxicity factor was used.
Statistical Analyses
Statistica for Windows, Version7.0 (StatSoft Inc., Tulsa, OK) was used to calculate the means of three independent experiments involving triplicate analyses for each sample/condition. Evaluation of statistical significance of differences was performed by one-way analysis of variance and differences of P < 0.05 were considered significant.
RESULTS
Phytoplankton Investigation
Qualitative analysis of the phytoplankton in the bloom samples revealed that the samples collected from the lagoon of Bizerte (Tunisia) were dominated by a dinoflagellate belonging to the genus Alexandrium, which occurred along with a few other dinoflagellate and flagellate species. Preliminary examinations of the Alexandrium cells led to the subsequent identification of the species as A. catenella. Quantitative analyses found that the highest cell concentration of this species in the bloom was reached in November 2007 at a level of 7.6 105 cells l−1. One month after this peak, the cell density decreased to 1.8 105 cells l−1, and then disappeared in January 2008.
PSP Toxins Prevalence
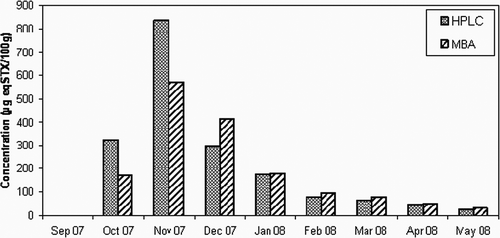
The seasonal variation of mussels toxicity estimated by the MBA and HPLC-FLD is shown in . A high correlation (R2 = 0.90) was observed between the toxicities of the shellfish in MBA and those calculated from HPLC-FLD analysis. Considerable toxicity was recorded in the mussel collected since October 2007 (273 μg STX-eq/100 g tissue) reaching a maximum of 569.6 μg STX-eq/100 g tissue in November 2007 and then gradually decreasing until May 2008 with 51 μg STX-eq/100 g tissue (). It was noticed that the toxicity in the investigated mussels increased concomitantly with the increase of A. catenella; however, high toxicity above regulatory limit (80 μg STX-eq/100 g tissue) was still observed for three months after complete disappearance of the dinoflagellate. PSP toxins in oysters were detected only during the period of November–December 2007 with the highest concentration of 11.2 μg STX-eq/100 g tissue as determined by HPLC was observed in November. The obtained data show that the toxicity level of bivalve exposed to a bloom of A. catenella density in the water is different among bivalve species, which is in accordance with previous results reported by Sakamoto et al.[Citation15] Among the bivalve species examined in the present study, the mussel M. galloprovincialis was found to accumulate much more toxins than the oyster C. gigas. In addition, the mussel seems to maintain the toxins for a long period.
Analysis of PSP Specific Profiles
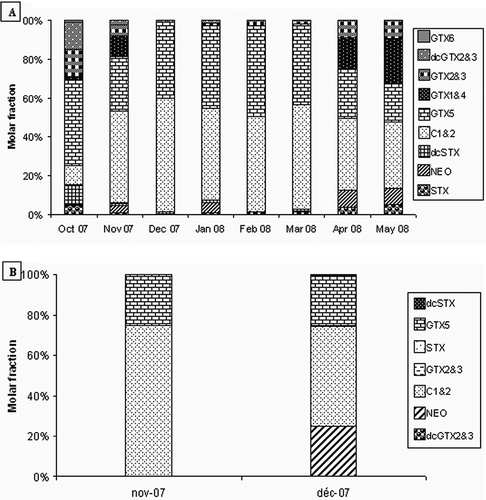
The contents of PSP toxins in the two bivalves were analyzed by HPLC-FLD and illustrates typical chromatograms. summarizes the toxin qualitative and quantitative profiles. In October 2007, one month before the peak of A. catenella, the toxin profile indicates the presence of several PSP toxins (GTX1-6, C1-2, STX, NEO, dcSTX, dcGTX2-3) with predominance of GTX5 which constitutes around 44% of total toxin burden in the mussel extract (). However, during the bloom period (November 2007), the toxin profile shows a slow decrease of the proportion of the GTX5 with an increase in C1-2 which becomes the main toxin representing around 48% of the total toxin proportion. Temporal evolution of toxin profile in mussels showed a relative increase in GTX5 and C1-2 proportions until March 2008. From April 2008, a relative increase in GTX1-4, GTX2-3, NEO and STX proportions was observed with a relatively slow decrease in C1-2 and GTX5 toxins (). On the other hand, in the oyster extract prepared during the peak of A. catenella (November 2007), results showed the presence of a high level of C1-2 and GTX 5 with a proportion of 72 and 27%, respectively. However, in the extract of oyster collected in December 2007 the most dominant toxins present were C1-2, GTX5, and NEO. The quantitative analysis showed that C1-2 was the main toxin (49.5%), whereas NEO and GTX5 were present at relatively equivalent amounts (≈24.75%). Compared to the toxic profile of mussels, it was noted that GTX1-4, dcGTX2-3, STX, and GTX6 have not been detected in the oyster extract. The obtained results showed also the absence of decarbamoyl NEO (dcNEO) in the mussel M. galloprovincialis and the oyster C. gigas collected from the Bizerte lagoon.
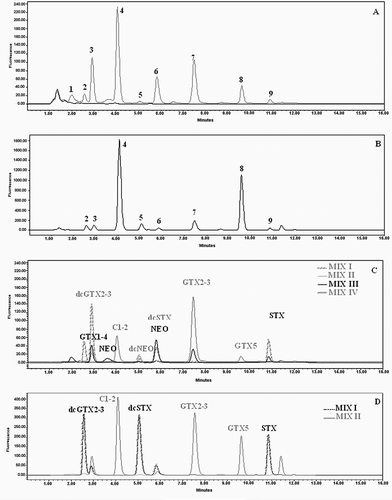
FIGURE 3 Chromatograms of PSP toxins from whole edible parts of mussels harvested November 25, 2007 after Alexandrium catenella bloom, (a) after periodate oxidation; (b) same sample after peroxide oxidation; (c) chromatograms of PSP standard after periodate oxidation; and (d) PSP standard after peroxide oxidation. After periodate oxidation compound 1 correspond mainly to gonyautxines 1+4 (GTX1/4); compounds 2 and 3 correspond to decarbamoyl-gonyautoxins 2+3 (dcGTX2+dcGTX3); compound 4 to N-sulfocarbamoyl 1+2 (C1+C2); compounds 5 and 6 to decarbamoyl-saxitoxin (dcSTX); 7 to gonyautoxins 2+3 (GTX2/3); 8 to N-sulfocarbamoyl B1 (GTX5), and 9 to saxitoxin (STX) and/or neosaxitoxin (NeoSTX).
DISCUSSION
The particular toxin mixture retained in the soft tissues of the shellfish is mainly determined by the species and strains of the dinoflagellates and shellfish themselves. Additional factors including environmental conditions are also involved in the qualitative and quantitative distribution of individual and overall PSP toxins.[Citation16] The dinoflagellate genus Alexandrium includes about 30 species, and at least nine of them produce a number of neurotoxins that can lead to PSP events. Some of these species, Gymnodinium catenatum, A. tamarense, A. minutum, and A. catenella are widely distributed in the Mediterranean Sea. Events of PSP have been frequently associated with these species in different basins.[Citation17] The herein conducted plankton research revealed, for the first time, the occurrence of the species A. catenella in the lagoon of Bizerte (North Tunisia). This work showed also that this species is a causative organism for PSP toxins accumulation in the mussel M. galloprovincialis and the pacific oyster C. gigas during a bloom of this species, occurring in November 2007, which coincides with the beginning of the rainy season in Tunisia. The species A. catenella has been reported over a number of geographical areas in the Mediterranean Sea.[Citation17] In the lagoon of Bizerte, the highest cell concentration was 7.6 105 cells l−1 (November 2007), a concentration which far exceeds acceptable concentrations (<103 cells l−1) of PSP-toxin producing dinoflagellate in several Mediterranean countries including France, Italy, and Spain.
PSP is a public health concern worldwide.[Citation18] The main cause of PSP is consumption of bivalves contaminated with PSP-toxins through accumulation of toxinogenic dinoflagellate in their digestive glands. Since the mussel M. galloprovincialis and the pacific oyster C. gigas are a major fishery product in the lagoon of Bizerte (North Tunisia), a surveillance of seasonal appearance of PSP-toxins in bivalves as well as its causative plankton is conducted periodically. With the aim of preventing food poisoning caused by PSP-toxin contaminated shellfish, such as mussels or oysters, their toxicity is officially monitored in Tunisia using the MBA. In the present study, the toxicity and toxin elementary composition of two bivalves collected from Bizerte lagoon between September 2007 and May 2008 were compared according to MBA and HPLC-FLD with pre-column derivatization methods, respectively. A comparison of these two methods revealed that despite some differences, the overall values generated by both methods were highly correlated (R2 = 0.90). Previous studies have also shown that results from the MBA and pre and/or post-column oxidation HPLC methods were relatively well correlated.[Citation19] Additionally, the extraction solvent, HCl versus acetic acid, may impact the overall toxicities. Some toxins can be converted into more or less toxic derivatives during HCl boiling extraction for the MBA, which would make the MBA results slightly higher or lower in some cases. The herein obtained results showed a large variation of accumulated PSP toxins in both studied species collected from the same area. The maximal PSP toxins contents were recorded in mussel and oyster tissues in November 2007, and coincided with the bloom of A. catenella (7.6 105 cells l−1). The level of PSP toxins in mussels exceeded the regulatory limit from October 2007 to January 2008 with a maximum reached in November at 569.6 μg and 832.9 μg STX-eq/100 g tissue as determined by MBA and HPLC method, respectively. However, PSP toxins in oysters were detected only from November to December 2007 with a peak of 11.2 μg STX-eq/100 g tissue determined by HPLC. Data shows that mussel M. galloprovincialis sequestered PSP toxins in its tissues even when the potentially toxin producing microalgae are not present, a finding in accordance with previous reports.[Citation20]
Several studies reported that bivalves exhibit marked (up to 100-fold) interspecific differences in their capacity to accumulate phycotoxins such as diarrhetic shellfish toxins (DSTs)[Citation21] and paralytic shellfish toxins (PSTs).[Citation22] It is reported in temperate areas that toxin level of bivalve exposed to a bloom of toxic dinoflagellate such as A. tamarense and G. catenatum is different among bivalve species.[Citation14] In a recent study, Kacem et al.[Citation20] showed that the mussel M. galloprovincialis accumulates extremely higher level of okadaic acid than the pacific oyster C. gigas harvested at the same period and from the same area, the lagoon of Bizerte. Mafra, Bricelj, and Fennel[Citation23] demonstrated, under controlled laboratory experimental conditions, that feeding physiology, e.g., reduced clearance rate and selective rejection of toxic Pseudo-nitzschia multiseries cells, plays a major role in explaining the relatively low capacity of oysters, C. virginica, to accumulate domoic acid during toxic blooms relative to other bivalves, most notably the mussel M. edulis. On the other hand, Montojo et al.[Citation24] reported, in a laboratory study, that thorny oyster Spondylus squamosus accumulate extremely higher level of toxin than other bivalve species when exposed to a bloom of P. bahamense var. compressum. In Alaska, the mussel, Mytilus edulis, can accumulate in excess of 20,000 μg of STX per 100 g of tissue, an extremely dangerous level.[Citation25] In the Kodiak area during the summer of 1993, one death and several illnesses were attributed to mussels containing 19,600 μg of STX per 100 g of tissue.[Citation25] The extreme toxicity of mussels is due primarily to their relatively insensitivity to high toxin accumulations that enables them to continue feeding. Their high tolerance to STXs and continued feeding on toxic algae can result in initially toxin-free mussels exceeded the European regulatory limit of PSP toxins in less than a one hour.[Citation16]
Inter-specific differences in toxin accumulation in mussels and oysters can be also explained by the difference in feeding behavior of the animals.[Citation26] The pacific oyster C. gigas tends to consume toxic algae readily during initial contact but decreases and eventually stops feeding when tissue toxin levels become high.[Citation27] In accordance, Lassus et al.[Citation17] supports the hypothesis of a threshold toxic alga concentration that would cause inhibition of filtration activity of oysters and subsequently a decrease in toxin uptake. The different contamination level of PSP toxins between oysters and mussels can also be explained by the significant effect of temperature upon paralytic toxin accumulation rates in oysters.[Citation17] In agreement, Bougrier et al.[Citation28] mentioned the temperature of 19°C as optimal value for C. gigas clearance activity, whereas no significant effect of temperature on clearance rate of mussels was reported.
The toxin profiles indicate that GTX5 and C1-2 were the most abundant toxins quantified by HPLC in mussels during all the considered period in this work (). Several other toxins, namely GTX1-4, NEO, STX, dcSTX, dcGTX2-3, and GTX6 were detected especially in the beginning of the A. catenella bloom, and later, in smaller amounts, following the complete disappearance of this toxin-producing species (April 2007). Temporal evolution of toxin profile in mussel extracts showed an increase in GTX5 and C1-2 proportions until March 2008. Although the levels of PSP toxins were 20–70 times greater in mussels than in oysters, their toxin profiles investigated by HPLC were similar and revealed the dominance of GTX5 and N-sulfocarbamoyl-gonyautoxin-2 and -3 (C1-2) toxins.
PSP toxin compositions of bivalves are not necessarily the same as those of the causative dinoflagellates, and interspecific differences are also observed among bivalves collected from the same area at the same time. However, several studies reported that mussels and oysters better reflect the toxin compositions of the causative planktons.[Citation16] In this study, the crude extract of the A. catenella harvested during the bloom period was not conserved and consequently, the toxin profile of this species was not determined. Krock et al.[Citation29] reported that the PSP toxin LC-FD profile of an A. catenella strain from the Chilean coast revealed the presence of the following toxins in decreasing order of molar percentage of total content, C1/C2 (57%), GTX1/4 (24%), GTX5 (13%), GTX2/3 (3%), NEO (2%), GTX6 (1%), and in trace amounts (<1%) STX, dcSTX, dcGTX2/3, C3, and C4. Therefore, in the A. catenella strain from Chile, the N-sulfocarbamoyl derivatives (C1/C2, GTX5) and the carbamoyl gonyautoxins GTX1/4 comprise about 94% (>90%) of the PSP toxin content on a molar basis. In this study a similar toxin profile was observed in mussels collected during the bloom period of A. catenella in the Bizerte lagoon with the dominance of C1/2 (47%), GTX5 (28%), GTX1/4 (11%), and GTX2/3 (5.5%) representing 91.5% (>90%) of the PSP toxin content on a molar basis. However, in oysters collected at the same area and at the same time the congeners C1/2 (72%) and GTX5 (27%) represent 99% of the PSP toxin; whereas GTX1-4, dcGTX2-3, STX, and GTX6 were not detected. Although the differences in toxin profiles between bivalves are considered to come from the enzymatic transformation of toxins in bivalve tissues,[Citation22,Citation30] possible participation of selective uptake or excretion[Citation22] of specific toxin components in bivalves cannot be excluded. This study bring to light new insights to understand the prevalence of the toxins in the shellfish studied herein to help the quality control this food as well as its derivatives[Citation31] in order to have healthy products for the consumers.
CONCLUSION
In this study, the seasonal fluctuations of PSP toxin concentrations were investigated for the first time in the lagoon of Bizerte (North Tunisia). The microscopic examination of the phytoplankton samples showed the dominance of the species Alexandrium catenella in autumn, producing a bloom in November 2007. While low amounts of PSP toxins were detected in oysters, the levels in mussels exceeded the European regulatory limit from October 2007 to January 2008 and reached a maximum in November. Data reinforce the evidence that the mussel retained its toxicity for slightly longer period than oyster. The toxin profiles in the both bivalves investigated by HPLC-FD revealed the relative dominance of the GTX 5 and the C toxins (C1 and C2). The observed differences in toxin profiles may emphasize differential enzymatic bioconversion and/or selective uptake or excretion of specific toxin components between the studied bivalves, a track that should be worthy of deep investigation.
ACKNOWLEDGMENTS
The authors would like to thank Margarita Fernández (Unitat de Seguiment del Medi Marí, Institut de Recerca i Technologia Agroalimentàries, Spain) for her assistance on dinoflagellates identification and to A. Milandri (Laboratoire National de Référence en Biotoxines Marines, Centre de Recherche Marine, Cesenatico; Italie) for MBAs (The MBA was completed with the approval of the National Animal Ethics Committee). Thanks also to Société Tunisie Lagune (Menzel Jmile, Bizerte, Tunisia) for accommodation during the fieldwork phase and harvesting of shellfish.
REFERENCES
- Vale, P. Hydrolysis of hydroxybenzoate saxitoxin analogues originating from Gymnodinium catenatum. Food Chemistry 2010, 125, 1160–1165.
- Li, S.C.; Wang, W.X. Radiotracer studies on the feeding of two marine bivalves on the toxic and non-toxic dinoflagellate Alexandrium tamarense. Journal of Experimental Marine Biology and Ecology 2001, 263, 65–75.
- Sagou, R.; Taleb, H.; Vale, P.; Blaghen, M.; Loutfi, M. Comparative study on differential accumulation of PSP toxins between cockle (Acanthocardia tuberculatum) and sweet clam (Callista chione). Toxicon 2005, 46, 612–618.
- Gutiérrez-Praena, D.; Jos, A.; Pichardo, S.; Moreno, I.M.; Cameán, A.M. Presence and bioaccumulation of microcystins and cylindrospermopsin in food and the effectiveness of some cooking techniques at decreasing their concentrations: A review. Food and Chemical Toxicology 2013, 53, 139–152.
- Commission Regulation (EC) No 2074/2005 of 5 December 2005 laying down implementing measures for certain products under Regulation (EC) No 853/2004 of the European Parliament and of the Council and for the organisation of official controls under Regulation (EC) No 854/2004 of the European Parliament and of the Council and Regulation (EC) N° 882/2004 of the European Parliament and of the Council, derogating from Regulation (EC) No 852/2004 of the European Parliament and of the Council and amending Regulations (EC) No 853/2004 and (EC) No 854/2004 OJ L 338, 22.12.2005, pp. 27–59.
- AOAC (Association of Official Analytical Chemists), 2000. International, official method 959.08. Paralytic Shellfish poison, biological method. In: Official Method of Analysis of AOAC International, (17th ed.) Gaithersburg, Maryland: AOAC International. pp. 59–61.
- Riobó, P.; Paz, B.; Franco, J.M.; Vázquez, J.A.; Murado, M.A.; Cacho, E. Mouse bioassay for palytoxin. Specific symptoms and dose-response against dose-death time relationships. Food and Chemical Toxicology 2008, 46, 2639–2647.
- European Parliament Regulation (EC) 853/2004 of the European Parliament and of the Council of 29 April 2004 laying down specific hygiene rules for food of animal origin. Official Journal of the European Union 2004, 139, 55–205.
- Sommer, H.; Meyer, K.F. Paralytic shellfish poisoning. Archives of Pathology 1937, 24, 560–598.
- AOAC, 1990. In: Official Methods of Analysis (Association of Official Analytical Chemists. Method 959.08: Paralytic Shellfish poison, 15th ed., Williams (Arlington, USA), pp. 881–882.
- Lawrence, J.F.; Menard, C.; Cleroux, C. Evaluation of prechromatographic oxidation for liquid chromatographic determination of paralytic shellfish poisons in shellfish. Journal of AOAC International 1995, 78, 514–520.
- Faust, M.A.; Larsen, J.; Moestrup, O. Potentally toxic phytoplancton. 3. Genus Prorocentrum (Dinophyceae). ICES Identification leaflets for Plancton. International council for the exploration of the sea. Leaflet 1999, 184, 24.
- Lawrence, J.F.; Niedzwiadek, B.; Menard, C. Quantitative determination of paralytic shellfish poisoning toxins in shellfish using prechromatographic oxidation and liquid chromatography with fluorescence detection: Collaborative study. Journal of AOAC International 2005, 88, 1714–1732.
- Oshima, Y. Postcolumn derivatization liquid-chromatography method for paralytic shellfish toxins. Journal of AOAC International 1995, 78, 528–532.
- Sakamoto, S.; Nagasaki, K.; Matsuyama, Y.; Kotani, Y. Comparison of toxicity and toxin composition among bivalves affected with PSP toxins by the red tide of Alexandrium catenella in Tokuyama Bay. Bulletin of Fisheries and Environment of Inland Sea 1999, 1, 55–61.
- Bricelj, V.M.; Lee, J. H.; Cembella, A.D.; Anderson, D.M. Uptake kinetics of paralytic shellfish toxins from the dinoflagellate Alexandrium fundyense in the mussel Mytilus edulis. Marine Ecology Progress Series 1990, 63, 177–188.
- Lassus, P.; Amzil, Z.; Baron, R.; Sechet, V.; Barille, L.; Abadie, E.; … Gueguen, M. Modelling the accumulation of PSP toxins in Thau Lagoon oysters (Crassostrea gigas) from trials using mixed cultures of Alexandrium catenella and Thalassiosira weissflogii. Aquatic Living Resources 2007, 20, 59–67.
- Martin, J.L.; Richard, D. Shellfish toxicity from the Bay of Fundy Eastern Canada: 50 years in retrospect. In: Harmful and Toxic Algal Blooms; Yasumoto, T.; Ed.; IOC/UNESCO: Sendai, Japan, 1996; 3–6.
- Reis Costa, P.; Baugh, A.K.; Wright, B.; Ralonde, R.; Nance, S.L.; Tatarenkova, N.; … Lefebre, K. Comparative determination of paralytic shellfish toxins (PSTs) using five different toxin detection methods in shellfish species collected in the Aleutian Islands, Alaska. Toxicon 2009, 54, 313–320.
- Kacem, I.; Bouaicha, N.; Hajjem, B. Comparison of okadaic acid profiles in mussels and oysters collected in mediterranean lagoon, Tunisia. International Journal of Biology 2010, 2, 238–245.
- Reizopoulou, S.; Strogyloudi, E.; Giannakourou, A.; Pagou, K.; Hatzianestis, I.; Pyrgaki, C.; Graneli, E. Okadaic acid accumulation in macrofilter feeders subjected to natural blooms of Dinophysis acuminata. Harmful Algae 2008, 7, 228–234.
- Bricelj, V.M.; Shumway, S.E. Paralytic shellfish toxins in bivalve molluscs: Occurence, transfert kinetics, and biotransformation. Fisheries Science 1998, 6, 315–383.
- Mafra, L.L. Jr.; Bricelj, V.M.; Fennel, K. Domoic acid uptake and elimination kinetics in oysters and mussels in relation to body size and anatomical distribution of toxin. Aquatic Toxicology 2010, 100, 17–29.
- Montojo, U.M.; Sakamoto, S.; Cayme, M.F.; Gatdula, N.C.; Furio, E.F.; Relox, J.R. Jr.; … Kodama, M. Remarkable difference in toxin accumulation of paralytic shellfish poisoning toxins among bivalve species exposed to Pyrodinium bahamense var. compressum bloom in Masinloc bay, Philippines. Toxicon 2006, 48, 85–92.
- RaLonde, R. Paralytic shellfish poisoning: The Alaska problem. Alaska’s Marine Resources 1996, 8, 20.
- Sekiguchi, K.; Sato, S.; Ogata, T.; Kaga, S.; Kodama, M. Accumulation and depuration kinetics of paralytic shellfish toxins in the scallop Patinopectin yessoensis fed Alexandrium tamarense. Marine Ecology Progress Series 2001, 220, 213–218.
- Bardouil, M.; Bohec, M.; Cormerais, M.; Bougrier, S.; Lassus., P. Experimental study of the effects of toxic microalgal diet on feeding of the oyster Crassostrea gigas Thunberg. Journal of Shellfish Research 1993, 12, 417–422.
- Bougrier, S.; Geairon, P.; Deslous-Paoli, J.M.; Bacher, C.; Jonquières, G. Allometric relationships and effects of temperature on clearance and oxygen consumption rates of Crassostrea gigas (Thunberg). Aquaculture 1995, 134, 143–154.
- Krock, B.L.; Bilotta, J.; Perkins, B.D. Non-cell-autonomous photoreceptor degeneration in a zebrafish model of choroideremia. Proceedings of the National Academy of Sciences 2007, 104, 4600–4605.
- Fast, M.D.; Cembella, A.D.; Ross, N.W. In vitro transformation of paralytic shellfish toxins in the clams Mya arenaria and Protothaca staminea. Harmful Algae 2006, 2, 201–206.
- Tang, N.H.D.; Xi C.W. Volatile, taste components, and sensory characteristics of commercial brand oyster sauces: Comparisons and relationships. International Journal of Food Properties 2012, 15 (3), 518–535.