Abstract
A new polysaccharide (MEP-1) was isolated from fruit body of wide Morchella esculenta (L.) Pers with a stepwise procedure of hot water extraction, removal of the protein, dialysis against water, DEAE-Cellulose A52 and Sephadex G-100 columns. Physicochemical properties of the polysaccharide was determined by chemical methods, high-performance gel permeation chromatography, high performance liquid chromatography, paper chromatography, ultraviolet spectrum, and infrared spectrometry. The polysaccharides were primarily polymers of glucose, mannose, galactose, and arabinose. The average molecular mass was 43625 Da.
INTRODUCTION
Mushrooms contain a huge diversity of biomolecules with nutritional[Citation1] and/or bioactive properties.[Citation2] Interest in medicinal mushrooms has increased in recent years[Citation3] and some compounds extracted from mushrooms were potential immunomodulators or biological response modifiers (BRM).[Citation4−Citation6] Morel species were reported to minimize oxidative damage in organisms that occurred in several chronic diseases.[Citation7] Furthermore, these species could be used to find new antimicrobials overlapping the bacterial resistance to first choice antibiotics.[Citation8]
Morchella esculenta (L.) Pers. was one kind of precious medical food fungus, which had high nutritional value and contained many biological active materials, such as polysaccharides, protein, trace elements, dietary fiber, and vitamins, etc.[Citation9] Recently, it had been proven that morel possessed anti-inflammatory, antitumor, antioxidant, and antimicrobial activities,[Citation10−Citation12] especially, the polysaccharides from M. esculenta fruiting body had the functions of antitumor, immunoregulation, fatigue resistance, and antivirus.[Citation13−Citation15] In this study, we isolated water-soluble polysaccharides from wild Morchella esculenta (L.) Pers. from Yunnan province of China by extraction, dialysis, DEAE cellulose-52 chromatography, and Sephadex G-100 chromatography in order, meanwhile, we analyzed the elementary characterization of the polysaccharides fractions.
MATERIALS AND METHODS
Materials and Equipment
The wide Morchella esculenta (L.) Pers. fruit body was obtained from Institute of Edible Fungi of Kunming, China and then grounded to pass through 1 mm screen and stored at 4°C in a refrigerator. Anthrone and sulphuric acid were purchased from Sinopharm Chemical Reagent Co. (Congqing, China). A4-Molecular sieve was purchased from Municipality kemi’ou Chemical Reagent Co. (Tianjin, China). T-series Dextran, DEAE-Cellulose A52, and Sephadex G-100 were purchased from Pharmacia Co. (Sweden). The monosaccharide standards were purchased from Pharmacia (Sweden). All other reagents were of analytical grade.
Extraction and Purification of Polysaccharide
The dried wide M. esculenta powder (20 g) material was extracted with 1000 mL distilled water at 90℃ for 2 h and stirred regularly. Then the extraction (liquid fraction) and the residue were collected separately; the residue was also re-extracted twice more.[Citation16] The extracted liquid fraction was collected and enriched by vacuum concentration (RE52-98, purchased from Shanghai Yarong of China) at 40℃, precipitated with 95% ethanol (1:4, v/v) at 4℃ for 12 h. The precipitate was collected by centrifugation (TGL-16G, purchased from Shanghai Anting of China) at 4500 rpm, 10 min, and dried in vacuum to obtain the crude Morchella polysaccharide. The crude Morchella polysaccharide was dissolved in distilled water again, followed removal of the protein by the Sevag method[Citation17] and exhaustively dialyzed against water, then dried in vacuum. The brownish crude product was loaded onto a column (2.6 × 30 cm) of DEAE-Cellulose A52 and irrigated successively with distilled water, 0.1 mol/L NaOH solution and 0.2 mol/L NaOH solution stage gradient elution separately; it would obtain three kinds of elution products, MEP-1, MEP-2, and MEP-3. MEP-1, the largest water-eluted fraction, after concentration, was collected and loaded onto a gel filtration column (1.6 × 100 cm) of Sephadex G-100 and eluted with 0.05 M NaCl at a flow rate of 0.4 mL/min. The eluate was concentrated and lyophilized (FD-1, purchased from Beijing Boyikang of China) to get a pure Morchella polysaccharide.
Characterization of Polysaccharide Fraction
The polysaccharide of MEP-1
The physicochemical properties of MEP-1 were mainly through the following methods: Fehling reagent,[Citation18] DNS colorimetric method,[Citation19] Sulfuric acid-carbazole reaction,[Citation20,Citation21] Biuret reaction,[Citation22] Iodine-potassium iodide reaction,[Citation23−Citation25] and Ferric chloride reaction.[Citation26] MEP-1 was dissolved in distilled water (0.001 g/mL), and was loaded into the length of 10 cm polarimeter tube, then was detected specific rotation at 20℃ by the WZZ-2S digital automatic polarimeter.
Identification of MEP-1
MEP-1 was dissolved and diluted to 1 mg/mL, and then the solution was scanned from 190 to 400 nm with UV-2450FW ultraviolet scanner (Shimadzu Corporation). Paper chromatography (PC) and high performance liquid chromatography (HPLC) were used for identification. PC was performed on Xinhua No. 1 paper in the following solvent system: 5:5:1:3 EtOAc–Pyridine–HOAc–H2O, the sugars were identified by spraying with phthalic acid reagent (1.0 mL of aniline and 1.66 g of phthalic acid were dissolved in 100 mL of water-saturated n-butanol) and heating at 100℃ for about 15 min. Infrared radiation (IR) spectra were recorded using the KBr-disk method with a Perkin Elmer one infrared Fourier transform infrared spectroscopy (FTIR) spectrometer in the range 400–4000 cm−1. The average molecular weight of MEP-1 was determined by HPGFC using a gel filtration column (1.6 × 100 cm) of Sephadex G-100 eluted with double distilled water at a flow rate of 0.4 mL/min. The molecular weight was determined by comparison with retention times of Glucan standard (Dextran series, 10, 40, 50, 70, and 100 KDal).
Statistical Analysis
The polysaccharide was determined by sulfuric acid-anthrone method in the extraction and purification process.[Citation27] Statistical analysis of the data was subjected to a one way analysis of variance and the significant difference was determined by the least significance difference test (P < 0.05). All analyses were done in triplicate and data was expressed as means ± SD.
RESULTS AND DISCUSSION
Extraction and Purification of Polysaccharide
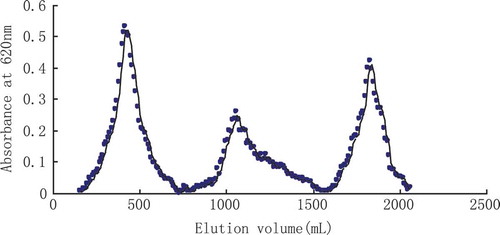
Neutral crude polysaccharide was obtained from fruit body of M. esculenta and the yield was about 4.96% by optimization of extraction process. The crude polysaccharide, after removal protein and dialysis against water, was carried on the DEAE-52 cellulose ionic exchange column chromatographic analysis after dissolved used distilled water. Using the distilled water, 0.1 mol/L NaOH solution and 0.2 mol/L NaOH solution stage gradient elution separately, we obtained three kinds of elution products: MEP-1 collected of 300 to 500 mL, MEP-2 collected of 1000 to 1200 mL, and MEP-3 collected of 1800 to 1900 mL (). Their yield rates were 12.40, 3.10, and 5.94%, respectively. The content of MEP-2 and MEP-3 were relatively small, so didn’t do research at temporary. The following research to MEP-1 as the object of study.
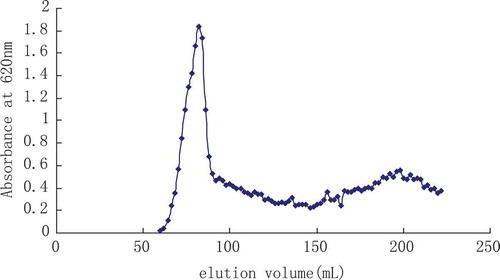
Then MEP-1 after double steams the water dissolution was carried on a glucosan gelatin SephadexG-100 molecular sieve column chromatographic analysis (16 × 1000 mm), on the quantity 20 mg, with double steams the water elution, the speed of flow was 0.4 mL/min, each tube collected 2 mL (collecting 35–48 eluents), dried and concentrated, the M. esculenta polysaccharide MEP-1 purely can be obtained (). And its yield rate was 1.58%.
Characterization of Polysaccharide Fraction
MEP-1 product assumed white powder with no smell, specific rotation [α]20D was + 257.0 (C = 0.001, H2O), water-solubility was easy and belonged to the neuter polysaccharide. The results of Fehling reagent, sulfuric acid carbazole reaction, biuret reaction, and ferric chloride reaction were negative, indicating that MEP-1 did not contain sugar, uronic acid, protein, polyphenol (). Mean molecule quality was 43.625 KD by HPGFC.
TABLE 1 Physico-chemical characteristics of the MEP-1
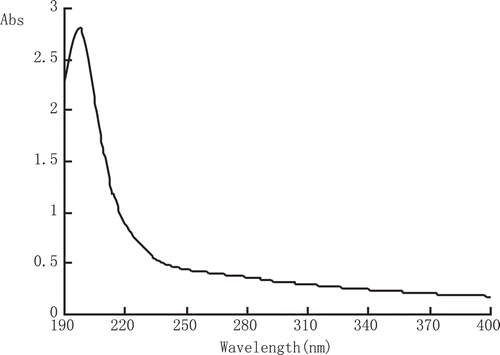
As shown in , MEP-1 had a maximum absorption peak only in the 199 nm; there was no characteristic absorption peak at 260 and 280 nm by ultraviolet scanning at 190–400 nm, indicating the absence of protein and nucleic acid. In addition, the single symmetry absorption peak of MEP-1 by HPLC indicated that it was a single component.
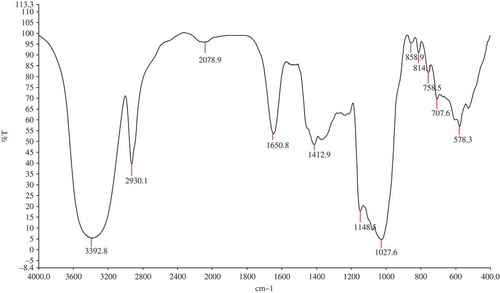
The FT-IR spectrum of MEP-1 was shown in . The band in the region of 3392 cm−1 was due to the hydroxyl stretching vibration of polysaccharide. The bands in the region of 2930 cm−1 was due to C–H stretching vibration and the bands in the region of 1670 cm−1 was due to C=O stretching vibration.[Citation28] Absorptions at 924.71 and 808.87 cm−1 were typical peak of D-glucose in pyranose form and mannose, respectively. MEP-1 exhibited the absorption at 878 cm−1, suggesting a D-galactose configuration. The absorptions at 1148 and 1027 cm−1 indicated a pyranose form of sugars. According to the color and the monosaccharide Rf value,[Citation29] it indicated that MEP-1 did not contain glucuronic acid, might contain mannose, galactose, arabinose, and glucose by PC.
CONCLUSION
According to our experiment, three M. esculenta polysaccharide fractions MEP-1, MEP-2, and MEP-3, were obtained. The results indicated that MEP-1 was a water-soluble polysaccharide, which was white powder and no smell, specific rotation [α]20D was + 257.0. And we preliminary demonstrated that MEP-1 contained glucose, mannose, galactose, and arabinoser. The polysaccharide was a heteropolysaccharide connected with β type of glycosidic bond and the mean molecule quality was 43.625 kDa.
FUNDING
The authors gratefully acknowledge the financial supports by the cooperation project of Yunnan Province and Southwest University (2012yx24).
Additional information
Funding
REFERENCES
- Kalac, P. Chemical composition and nutritional value of European species of wild growing mushrooms: A review. Food Chemistry 2009, 113, 9–16.
- Ferreira, I.C.F.R.; Vaz, J.A; Vasconcelos, M.H.; Martins, A. Compounds from wild mushrooms with antitumour potential. Anti-cancer Agents in Medicinal Chemistry 2010, 10, 424–436.
- Wang, J.; Zhang, L.; Yu, Y.; Cheung, P.C. Enhancement of antitumor activities in sulfated and carboxymethylated polysaccharides of Ganoderma lucidum. Journal of Agricultural and Food Chemistry 2009, 57, 10565–10572.
- Malek, S.N.A.; Kanagasabapathy, G.; Sabaratnam, V.; Abdullah, N.; Yaacob, H. Lipid components of a Malaysian edible mushroom, termitomyces heimii natarajan. International Journal of Food Properties 2012, 4, 809–814.
- Lee, H.H.; Lee, J.S.; Cho, J.Y.; Kim, Y.E.; Hong, E.K. Study on immunostimulating activity of macrophage treated with purified polysaccharides from liquid culture and fruiting body of Lentinus edodes. Journal of Microbiology and Biotechnology 2009, 19, 566–572.
- Zaidman, B.Z.; Yassin, M.; Mahajna, J.; Wasser, S.P. Medicinal mushroom modulators of molecular targets as cancer therapeutics. Applied Microbiology and Biotechnology 2005, 67, 453–468.
- Ferreira, I.C.F.R.; Barros, L.; Abreu, R.M.V. Antioxidants in wild mushrooms. Current Medicinal Chemistry 2009, 16, 1543–1560.
- Alves, M.J.; Ferreira, I.C.F.R.; Dias, J.; Teixeira, V.; Martins, A.; Pintado, M.A. Review on antimicrobial activity of mushroom (Basidiomycetes) extracts and isolated compounds. Planta Medica 2012, 78, 1707–1718.
- Litchfteld, J.H.; Vely, V.G.; Overbeck, R.C. Nutrient content of morel mushroom mycelium: Amino acid composition of the protein. Journal of Food Science 2006, 28, 741–743.
- Mau, J.-L.; Chang, C.-N.; Huang, S.-J.; Chen, C.-C. Antioxidant properties of methanolic extracts from Grifola frondosa, Morchella esculenta, and Termitomyces alduminosus mycelia. Food Chemistry 2004, 87, 111–118.
- Nitha, D.; Fijesh, P.V.; Janardhanan, K.K. Hepatoprotective activity of cultured mycelium of morel mushroom, Morchella esculenta. Experimental Toxicology and Pathology 2011, 6, 6–7.
- Nitha, D.; Meera, C.R.; Janardhanan, K.K. Anti-inflammatory and antitumour activities of cultured mycelium of morel mushroom, Morchella esculenta. Current Science 2007, 92, 235–239.
- Nitha, D.; Janardhanan, K.K. Aquouse-ethanolic extract of morel mushroom mycelium Morchella esculenta, protects cisplatin, and gentamicin induced nefrotoxicity in mice. Food and Chemical Toxicology 2008, 46, 3193–3199.
- Rotaoll, N.; Dunkel, A.; Hofmann, T. Activity-guided identification of (S)-malic acid 1-O-D-glucopyranoside (morelid) and gamma-aminobutyric acid as contributors to umami taste and mouth-drying oral sensation of morel mushrooms (Morchella deliciosa Fr.). Journal of Agricultural and Food Chemistry 2005, 53, 4149–4156.
- Wasser, S.P. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Applied Microbiology and Biotechnology 2002, 60, 258–274.
- Wiboonsirikul, J.; Mori, M.; Khuwijitjaru, P.; Adachi, S. Properties of extract from Okara by its subcritical water treatment. International Journal of Food Properties 2013, 5, 974–982.
- Staub, A.M. Removal of proteins Sevag-method. Methods in CarbohydrChem 1965, 5, 5–6.
- Huang, J. Improvement of Fehling reagent for determination of reducing sugar. Journal of Yuncheng University 1995, 4, 7–10.
- Qi, X.J.; Gou, J.X.; Han, X.J. Study on measuring reducing sugar by DNS reagent. Journal of Cellulose Science and Technology 2004, 3, 17–20.
- Lin, Q.B.; Gao, D.W.; Yu, S.J; Liu, S.H. Study on monosaccharide composition of polysaccharides from Chinese red dates by HPLC. Journal of Zhengzhou Grain College 1998, 3, 57–60.
- Liu, G.H.; Zao, X.M.; Chen, J.D. Research on the determination method and stability of acid mucopolysaccharide in stichopus japonicus sekenka. Preventive Medicine of Chinese People’s Liberation Army 2004, 2, 107–109.
- Zhang, S.H. Food Analysis. China Light Industry Press: Beijing, 2004, p. 286.
- Meng, Q.Y.; Liu, Z.H.; Xu, M.Y.; DongYe, G.Z. Extraction and analysis of sargassum hemiphyllum polysaccharides. Spectroscopy and Spectral Analysis 2004, 12, 156–152.
- Jiang, P.H.; Lu, H.; He, Z.P.; Wu, Z.L. On the relationship between color reaction of starch with I2 and content of reducing sugar. Research and Exploration in Laboratory 2005, 3, 31–32.
- OuYang, T.Z.; Xie; J.G. Isolation, purification, and characterization of alkalisoluble polysaccharides of “kikurage,” fruit body of auricularia auriadajudae. Journal of HuaZhong Agricultural 1999, 1, 46–48.
- Liu, Z.W.; Jiang, H.N. Study on extraction, purification identification, and physical and chemical characteristics assay of rhodiola henryi polysaccharides. Food Science 2005, 3, 60–63.
- Zhang, Y.S. Food Analysis. China Light Industry Press: Beijing, 1983, p. 163.
- Zhao, G.H.; Kan, J.Q; Li, Z.X.; Chen, Z.D. Structural features and immunological activity of a polysaccharide from Dioscorea opposita Thunb roots. Carbohydrate Polymers 2005, 61, 125–131.
- Tan, Z.J.; Xie, D.P.; Wang, Z.; Xiao, Q.M.; Li, L.H. Studies on isolation, purification, and some properties of polysaccharide from armillaria mellea. Food Science 2002, 9, 49–53.