Abstract
Sodium bis (2-ethylhexyl) sulfosuccinate (AOT)-sodium dodecyl sulfate (SDS)/isooctane-octanol reverse micelle extraction was tested an efficient and effective approach to separate peanut protein from full-fat peanut powder. Here, important kinetic factors including pH, ion strength, and temperature were studied during reverse micelle backward extraction. The extraction conditions were obtained by response surface experiments as follows: pH 7.5, ion concentration 1.1 mol/L at temperature 35°C. Under these optimum extraction conditions, the extraction rate of protein reached 79.03%. A model on the kinetic partitioning of peanut protein was also developed. The backward extraction in this reverse micelle system was controlled by interfacial resistance instead of diffusion resistance in reverse micelle and aqueous phase with the total mass transfer rate of 0.8×10−5 m3·s−1. A two-film theory may be the mechanism for flat interface. Results of mass transfer process are helpful for creating an reverse micelle extraction process, and used for purification of peanut proteins, promoting the development of food industry.
INTRODUCTION
Peanuts contain 40–50% oil, 25–29% protein, 20–23% carbohydrates. It is the world’s third largest source for vegetable oil with a yield of around 37 million tons in 2013.[Citation1] Peanut protein has multiple amino acids. It is an important component in many food products serving as auxiliary material and is added to breakfast food, baby food, and even patients’ food as a kind of protein resource to improve foods’ nutritional and functional properties.[Citation2] On the other hand, due to its oil component, most peanuts are processed for producing oil and peanut meal is generated as a byproduct during this processing.[Citation3,Citation4] Though the peanut meal has appropriate amino acid profile, the application of this meal in the food industry is still limited due to some limitation of its functional properties.[Citation5] Currently, a majority of the meal is applied as animal feed and this application has very low economic value.[Citation6] Thus, the development and application of peanut protein is necessary because it can be applied as a high protein food ingredient for protein fortification and product formulation.[Citation7] As animal protein is pretty expensive and not affordable for some people in some developing countries, to maximize the use of this peanut protein, a suitable separation and purification technique for peanut protein is critically needed.
Reverse micelle extraction has been widely studied in the food industry, pharmaceutical industry, and nano-materials. Because of its multiple advantages including the easily reuse of corresponding surfactant and solvent, high efficiency of extraction, being easy to scale-up and continuous technology separating target molecules from mixtures, etc.[Citation8−Citation11] In reverse micelles, the non-polar tail parts of surfactants turn outward and confront with organic solvent, while the polar head parts turn inward and form “water pools,” which dissolve biological active molecules including protein and enzymes without denaturing them when extraction parameters are adjusted.[Citation12]
More recently, much research effort went to the mass transfer characterization of reverse micelles. Many articles reported the mass transfer process and coefficients of biomolecules such as amino acid and DNA between an aqueous phase and a reverse micelle phase.[Citation13−Citation15] Mohd-Setapar et al.[Citation9] studied the effect of several important parameters on the kinetic transfer of penicillin G and investigated the mechanism controlling reverse micelle system via forward and backward transfers. They predicted that the extraction was governed by interface solubilization and the diffusion of penicillin G at boundary layer within the aqueous phase. Similar results were obtained from L-isoleucine extraction to a reverse micelle from aqueous phase.[Citation13] In another study, the glutathione (GSH) extraction using reverse micelles from yeast fermentation broth was investigated and the effects of surfactant concentration, pH, and ions were discussed.[Citation11] Overall, the current results of extraction kinetics revealed that the volumetric mass transfer rates of the reverse micelle forward and backward extraction were around 0.209 s−1 and 0.054 s−1, respectively. In general, the backward extraction has a lower mass transfer rate than forward extraction.
The effect of different conditions on kinetics of reverse micelle backward extraction has been studied widely.[Citation11,Citation13,Citation16] However, to the best of our knowledge, limited information is available on the kinetic of plant protein extraction from reverse micelle so far. Understanding the kinetic partitioning of peanut protein in reverse micelle system will help understand the mechanism of mass transfer underlying the protein extraction and facilitate the utilization of the extraction as a practical approach for purifying and concentrating proteins.
The purpose of this study was to understand the influence of different conditions on mass transfer resistance of peanut proteins extraction from sodium bis (2-ethylhexyl) sulfosuccinate (AOT)-sodium dodecyl sulfate (SDS)/isooctane-octanol reverse micelles to an aqueous phase and optimize the extraction parameters. The kinetics of backward extraction was also investigated for getting the mass transfer rate via the approach of fitting the experimental data to theoretical equations.
MATERIALS AND METHODS
Materials
AOT of 99% purity was purchased from Shanghai Haiqu Chemical Factory (Shanghai, China). SDS was obtained from Tianjin Kermel Chemical Reagent Co., Ltd. (Tianjin, China), isooctane and n-octanol were purchased from Tianjin Bodi Chemical Co., Ltd (Tianjin, China). All of these chemicals were of analytical grade. Full-fat peanut powder was purchased from Henan Di Xin Food Co., Ltd (Kaifeng, Henan, China).
Forward Extraction
Forward extraction is a solid-liquid extraction process. Reverse micelle containing 80 g/L AOT/SDS in isooctane and octanol was employed throughout this work. Forward extraction was performed by contacting the reverse micelle phase with full-fat peanut powder (typically 10 g/L) in 10 mmol/L pH 8.0 sodium phosphate buffer in an Erlenmeyer flask which was treated by a shaking table. The temperature was controlled at 35 ± 2°C. After 20 min, the mixture was centrifuged at 2433 g for 5 min. The clear supernatant solution was utilized for backward extraction.
Backward Extraction
For backward extraction, known volumes of the clear supernatant solution and the aqueous phase with adjusted pH and ionic strength were contacted in a magnetically agitated Erlenmeyer flask under different temperature conditions. The volume ratio of reverse micelle phase to aqueous phase was 1:1. The two phases were mixed for 1 h. Sample of 5 mL was taken out for analysis at different time intervals. Then the mixture was centrifuged at 1026 g for 20 min to separate the phases. The peanut protein concentrations in both phases were determined by absorbance at 280 nm using an UV spectrophotometer (Beijing Purkinje General Instrument Co., Ltd. UV–1901 spectrophotometer). The extraction partition coefficient in the two phases was then calculated.
Mass Transfer Model for Backward Extraction
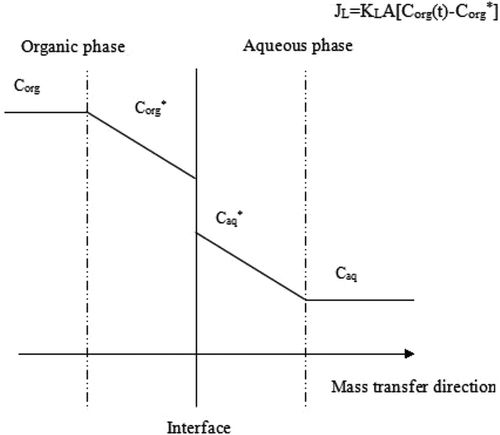
The two-film theory was successfully applied for characterizing the mass transfer coefficient during reverse micelle extraction.[Citation16−Citation19] In the current study, the two-film theory describing flat interface was applied to determine the mass transfer coefficient. For protein, the mass transfer coefficients from organic phase to aqueous phase via interface were determined at given pH and ionic strength by assaying the peanut protein concentration. The rates were obtained based on the time-dependent data.
During the backward extraction, the reverse micelle aggregates containing peanut protein molecules diffused from organic phase to the interface, where peanut proteins escaped from this organic phase to the bulk of aqueous phase, The interfacial resistance was resulted from the formation of the reverse micelles filling with solvent and the release of protein from the micelles.[Citation11]
The overall resistance to peanut protein transfer using the two-film theory can be defined as the total of three individual resistances.[Citation20,Citation21] The overall mass transfer coefficient, KL, can be expressed as Eq. (1).[Citation13,Citation19,Citation22]
where kaq and korg are the mass transfer coefficient in the aqueous film and in the organic film, respectively. kS is the interfacial transfer coefficient arising from the formation of peanut protein-filled reverse micelles, and protein release from the micelles into the interface. During the backward extraction in the system, the mass balance of peanut protein at any given time is:
where Corg(0) is the initial concentration of peanut protein in reverse micelle phase. Corg(t) and Caq(t) are the protein concentration in organic phase and aqueous phase at time t, respectively. At the beginning of backward extraction, all peanut protein resided in reverse micelle phase, thus the concentration of the aqueous phase Caq(0) equaled 0. Vorg and Vaq are the volume of organic and aqueous phase, respectively. They are equal, namely:
During the backward extraction, the overall mass transfer rate can be defined as follows:
where the KLA is a combined mass transfer coefficient with units of m3·s−1 and A is the total interfacial area between organic and aqueous phases. Corg* is the concentration of peanut protein in organic phase at equilibrium. The value of m*, an equilibrium partition coefficient, equals to Corg* divided by Caq*, shown as follows,
According to the above Eqs. (2–5), a new equation was drawn:
when Eq. (6) was integrated between the limits Corg and rearranged, new equation was generated:
where
Equations (7–10) described the protein transfer between the two phases. E’ reflects the organic phase backward extraction rate. The aqueous phase backward extraction rate can be described by
In Eq. (9), the β value was calculated from the equilibrium partition coefficient (m*). The value of α can be calculated by substituting β value into Eq. (7) by an iterative procedure with computer based. The model was fitted to Corg(t) versus t data and recalculation was performed until the value of a fitted data point allowed a maximum error limitation of approximately 10%.[Citation9] The overall mass transfer coefficient KLA can be obtained from the definition of parameters, Eq. (8). Dynamics equation is based on the two-film theory, thus the suitability of using this model to reverse micelle system can be obtained by rearranging Eqs. (7–10):
The figure of time t for ln([E’-β]/[1-β]) should be a line passing via the origin with the slope of –α if the two-film model is suitable.
RESULTS AND DISCUSSION
Model Validation
The two-film model for reverse micelle extraction is shown in . Validation results are shown in . As shows, there existed fairly good linearity between the time and ln([E’-β]/[1-β]) (R2 = 0.9). Thus, we conclude that the two-film theory is appropriate for the mass transfer kinetic of peanut protein in backward extraction. The two-film theory assumes the overall mass transfer coefficient KLA and the equilibrium constants m* is constant, and there is a certain deviation between the actual data and the fitted line. This is consistent with other studies, for example, Lye et al.[Citation19] investigated the kinetics of AOT/isooctane reverse micelles on extracting ribonuclease-A and lysozyme. When applied two-film theory, they found that there were some deviations between the actual data and the model as well.
Effect of Aqueous Phase pH on the Kinetic of Backward Extraction
and , respectively, show the protein backward extraction efficiency in aqueous phase and organic phase (E and E’) versus time at different pH. While the ion strength was 1.0 mol/L, temperature was 35°C. Throughout the backward extraction, the pH was optimized for preventing re-extraction of peanut protein back into the reverse micelles after the backward extraction process. Modeling was performed using the two-film theory to study the combined mass transfer coefficients, KLA, and the equilibrium partition coefficients m* (). Mass transfer of protein between organic and aqueous phases will be difficult when unfavorable pH is adopted. As the electrostatic interaction between the peanut protein molecules and ionic surfactants is mainly controlled by the aqueous phase pH, the interfacial resistance may play a more important role in the mass transfer process than diffusion resistance.
FIGURE 3 The protein backward extraction rate versus time for the effect of pH, [KCl] on the aqueous phase containing 1 mol/L, temperature 35°C. (a) Effect of pH on the protein backward extraction rate in water phase (E) versus time. (b) Effect of pH on the protein backward extraction rate in organic phase (E’) versus time.
![FIGURE 3 The protein backward extraction rate versus time for the effect of pH, [KCl] on the aqueous phase containing 1 mol/L, temperature 35°C. (a) Effect of pH on the protein backward extraction rate in water phase (E) versus time. (b) Effect of pH on the protein backward extraction rate in organic phase (E’) versus time.](/cms/asset/547b57a3-051d-4790-8d49-8a4871d1525b/ljfp_a_919318_f0003_b.gif)
The highest mass transfer coefficient was obtained at pH 7. The reason for this could be due to the effect of aqueous phase pH on the charge distribution of solute surface. It was suggested that the charge distribution depends on the initial chemical properties of the solute and it remains constant in the whole pH range of the solution.[Citation9] Thus, altering electrostatic interaction between solute surface and the surfactant polar head greatly affected the backward extraction process.[Citation23]
Isoelectric point of peanut protein is 4.5 and surfactant is anionic. When the protein net charge is the same as that of the surfactant head-groups, protein can easily release from the reverse micelle to the aqueous phase.[Citation24,Citation25] Here, the results showed that the optimum extraction rate attained when pH was 7. Lower or higher pH led to decreased extraction rate. With higher pH, more peanut protein molecules were negatively charged so that the charge density increases, which weakened the electrostatic interaction between protein and surfactants, and promoted the backward extraction process, that was, the releasing rate of protein from the reverse micelles increased. On the other hand, when the pH value reached 8, the value of KLA decreased from 0.84×10−5 m3·s−1 to 0.60×10−5 m3·s−1. This may be because the higher pH promoted the formation of aggregates between protein and surfactants, thus the releasing process was slowed down.
Effect of Aqueous Phase Ion Strength on the Kinetic of Backward Extraction
The effect of aqueous phase salt concentration on the kinetic partitioning of peanut protein during backward extraction is shown in and . While the value of pH was 7, temperature was 35°C The maximal concentration of peanut protein potentially be extracted during backward extraction was achieved when initial ion strength was obtained with 1.0 mol/L KCl. shows the value of KLA and the equilibrium partition coefficients m*. The results that mass transfer coefficient changed as the salt concentration changed revealed that there was electrostatic interaction between peanut protein and surfactant.[Citation26]
TABLE 1 Values of overall mass transfer coefficient (KLA) and equilibrium partition coefficient (m*) for the effects of pH, ion strength, and temperature on the backward extraction
FIGURE 4 The protein backward extraction rate versus time for the effect of [KCl], pH on the aqueous phase 7, temperature 35°C. (a) Effect of [KCl] on the protein backward extraction rate in water phase with variation of the time. (b) Effect of [KCl] on the protein backward extraction rate in organic phase with variation of the time.
![FIGURE 4 The protein backward extraction rate versus time for the effect of [KCl], pH on the aqueous phase 7, temperature 35°C. (a) Effect of [KCl] on the protein backward extraction rate in water phase with variation of the time. (b) Effect of [KCl] on the protein backward extraction rate in organic phase with variation of the time.](/cms/asset/a370e995-83de-4891-a971-e05b562db9fa/ljfp_a_919318_f0004_b.gif)
Adding salt to the aqueous phase is pretty important for avoiding forming a stable reverse micelle. According to the electrostatic shielding effect theory of Debye-Hückel, when the concentration of KCl increased to 1 mol/L, the electric double layer in the inner surface of reverse micelles became thinner, reducing the electrostatic interaction between surfactants and peanut protein. Besides, the thinner electric layer results in mutual repulsion among the polar head of surfactants. Space resistance reduced when water pool size reached the size of solute resulting in peanut protein release from the phase of reverse micelle via size exclusion.[Citation27] However, when salt concentration increases, the salinity tends to migrate in the reverse micelle “pool” and replaces proteins to make proteins salt out from the reverse micelles. All these effects decreased the solubility of proteins so that proteins can be easily extracted from the organic phase. Overall mass transfer coefficient varies by differed ionic strength. For example, the KLA increased from 0.51×10−5 m3·s−1 to 0.78×10−5 m3·s−1 when the value of ion strength increased from 0.5 mol/L to 1 mol/L. However, when the ion strength increased to 1.5 mol/L, the transfer rate decreased. This result may be because high ion strength caused protein denaturation and aggregation formation between protein and surfactants.
Effect of Temperature on the Backward Extraction
During the backward extraction, peanut proteins transferred from the organic phase of reverse micelle to the aqueous phase are conventionally conducted by adjusting the operating parameters. In the current study, the effect of operating temperature was also investigated. While the ion strength was 1.0 mol/L, the value of pH was 7. and showed the back extraction efficiency versus time with operating temperature ranging from 25 to 45°C. The result showed that the backward extraction rate in the aqueous phase reached maximum when operating temperature rose to 35°C with a maximum KLA of 0.86×10−5 m3·s−1. Backward extraction process of peanut protein belongs to the type of interfacial resistance control. Thus, interfacial resistance between organic and aqueous phase affects significantly on the overall mass transfer coefficient under different extraction temperatures.
FIGURE 5 The protein backward extraction rate versus time for the effect of temperature, pH on the aqueous phase 7, [KCl] 1 mol/L. (a) Effect of temperature on the protein backward extraction rate in water phase (E) with variation of the time. (b) Effect of temperature on the protein backward extraction rate in organic phase (E’) with variation of the time.
![FIGURE 5 The protein backward extraction rate versus time for the effect of temperature, pH on the aqueous phase 7, [KCl] 1 mol/L. (a) Effect of temperature on the protein backward extraction rate in water phase (E) with variation of the time. (b) Effect of temperature on the protein backward extraction rate in organic phase (E’) with variation of the time.](/cms/asset/19a7338d-c5df-4a00-82a7-d75c720aaa9b/ljfp_a_919318_f0005_b.gif)
Temperature effects on the mass transfer coefficient are shown in . It was obtained that the mass transfer coefficient increased with temperature increased from 25 to 35°C. Since the effect of temperature on the kinetic of backward extraction was rarely studied before, the underlying mechanism of temperature on backward extraction of macromolecular proteins has not been totally understood. Some possible explanations can be suggested based on the data in and . First of all, higher temperature might increase the collision probability between the reverse micelle and phase interface, thus it is easier for the protein to release from the reverse micelles “pool,” resulting in increased mass transfer coefficient and the backward extraction rate. Second, higher temperature caused reverse micelle instable and may rupture more easily, hence the protein could release from the reverse micelle phase easily. However, too high of a temperature will lead to protein denaturation. The above deduction explained the current results that initially the partition coefficient increased until maximum when temperature increased, which then decreased with a further increased temperature.
Optimum Experimental Conditions
Based on single-factor experiments, the optimal conditions for backward extraction of peanut protein was investigated via a series of response surface experiments.[Citation28] The optimum conducting conditions obtained for backward extraction are: pH value of the aqueous phase of 7.5, ion concentration of 1.1 mol/L, and extraction temperature of 35°C. Under the optimum conditions, the single backward extraction efficiency reached 79.03%. After backward extraction, the backward extraction liquid was performed and freeze dried to obtain the final peanut protein products for the purpose of the food industry.
CONCLUSIONS
In this study, the backward extraction of peanut protein and the kinetic of it releasing from AOT-SDS/isooctane-octanol reverse micelle phase to aqueous phase were investigated. The effects of some critical factors including pH, ion strength, and operating temperature on peanut protein extraction were studied. In addition, the optimum operating conditions for backward extraction was acquired by response surface experiments, which were pH 7.5, ion concentration 1.1 mol/L, with temperature at 35°C. Under these optimum operating conditions, the extraction rate of peanut protein could reach 79.03%. Furthermore, verification experiment was performed, which indicated two-film model was suitable for the peanut protein backward extraction. By fitting our experimental kinetic results to theoretical equations, the overall mass transfer coefficient (KLA) and the equilibrium partition coefficient (m*) were obtained. Results indicated that the overall mass transfer resistance was composed of the interfacial resistance and the diffusion resistance in both reverse micelle and the aqueous phases. And the mass transfer coefficient could be largely impacted by pH, ion concentration, and temperature, indicating that in the current system, the interfacial resistance more affected the mass transfer process than diffusion resistance. This study revealed that the appropriate of reverse micelle extraction as a technique in protein purification and provided theoretical basis for the industrialization production of peanut protein.
FUNDING
Projects 21176058, 31171790, and 31371851 supported by National Natural Science Foundation of China contributed to this study.
REFERENCES
- Latif, S.; Pfannstiel, J.; Makkar, H.; Becker, K. Amino acid composition, antinutrients, and allergens in the peanut protein fraction obtained by an aqueous enzymatic process. Food Chemistry 2012, 136 (1), 213–217.
- Wang, L.; Liu, H.; Liu, L.; Wang, Q.; Li, Q.; Du, Y.; Zhang, J. Protein contents in different peanut varieties and their relationship to gel property. International Journal of Food Properties 2014, 17, 1560–1576.
- Zhang, S.B.; Lu, Q.Y.; Yang, H.; Li, Y.; Wang, S. Aqueous enzymatic extraction of oil and protein hydrolysates from roasted peanut seeds. Journal of the American Oil Chemists’ Society 2011, 88 (5), 727–732.
- Zhang, Y.; Liu, J.; Lu, X.; Zhang, H.; Wang, L.; Guo, X.; Qi, X.; Qian, H. Isolation and identification of an antioxidant peptide prepared from fermented peanut meal using bacillus subtilis fermentation. International Journal of Food Properties 2014, 17, 1237–1253.
- Beuchat, L.R.; Cherry, J.P.; Quinn, M.R. Physicochemical properties of peanut flour as affected by proteolysis. Journal of Agricultural and Food Chemistry 1975, 23 (4), 616–620.
- Batal, A.; Dale, N.; Café, M. Nutrient composition of peanut meal. The Journal of Applied Poultry Research 2005, 14 (2), 254–257.
- Wu, H.W.; Wang, Q.; Zhou, S.M. Research progress on peanut protein and its functional properties. China Oils and Fats 2007, 32 (9), 7–11.
- Naoe, K.; Yoshimoto, S.; Naito, N.; Kawagoe, M.; Imai, M. Preparation of protein nanoparticles using AOT reverse micelles. Biochemical Engineering Journal 2011, 55 (2), 140–143.
- Mohd-Setapar, S.H.; Mat, H.; Mohamad-Aziz, S.N. Kinetic study of antibiotic by reverse micelle extraction technique. Journal of the Taiwan Institute of Chemical Engineers 2012, 43 (5), 685–695.
- Kafshgari, M.H.; Khorram, M.; Mansouri, M.; Samimi, A.; Osfouri, S. Preparation of alginate and chitosan nanoparticles using a new reverse micellar system. Iranian Polymer Journal 2012, 21 (2), 99–107.
- Dong, L.; Huang, S.; Luo, Q.; Zhou, X.; Zheng, S. Glutathione extraction and mass transfer in di-(2-ethylhexyl) ammonium phosphate/octanol reverse micelles. Biochemical Engineering Journal 2009, 46 (2), 210–216.
- Shen, C.W.; Yu, T. Protein separation and enrichment by counter-current chromatography using reverse micelle solvent systems. Journal of Chromatography A 2007, 1151 (1), 164–168.
- Dövyap, Z.; Bayraktar, E.; Mehmetoğlu, Ü. Amino acid extraction and mass transfer rate in the reverse micelle system. Enzyme and Microbial Technology 2006, 38 (3), 557–562.
- Pires, M.J.; Cabral, J. Liquid-liquid extraction of a recombinant protein with reverse micelles. Journal of Chemical Technology and Biotechnology 1994, 61 (3), 219–224.
- Song, X.; Sun, S.; Yin, Z.; Zhang, W.; Yang, Y. The study on formation of the reversed micelle in extraction system of primary amine N1923 sulfate. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2002, 209 (1), 57–63.
- Adachi, M.; Harada, M.; Nishita, R.; Shioi, A. Extraction kinetics of small charged molecules in water-in-oil microemulsion/brine system. The Journal of Physical Chemistry 1995, 99 (21), 8722–8729.
- Nishiki, T.; Sato, I.; Muto, A.; Kataoka, T. Mass transfer characterization in forward and back extractions of lysozyme by AOT-isooctane reverse micelles across a flat liquid-liquid interface. Biochemical Engineering Journal 1998, 1 (2), 91–97.
- Liu, Y.; Dong, X.-Y.; Sun, Y. Equilibria and kinetics of protein transfer to and from affinity-based reverse micelles of Span 85 modified with Cibacron Blue F-3GA. Biochemical Engineering Journal 2006, 28 (3), 281–288.
- Lye, G.; Asenjo, J.; Pyle, D. Protein extraction using reverse micelles: Kinetics of protein partitioning. Chemical Engineering Science 1994, 49 (19), 3195–3204.
- McCabe, W.L.; Smith, J.C.; Harriott, P. Unit Operations of Chemical Engineering. McGraw-Hill: New York, NY, 1956.
- Cardoso, M.; Viegas, R.; Crespo, J. Extraction and re-extraction of phenylalanine by cationic reversed micelles in hollow fibre contactors. Journal of Membrane Science 1999, 156 (2), 303–319.
- Sun, Y.; Bai, S.; Gu, L.; Tong, X.-D.; Ichikawa, S.; Furusaki, S. Effect of hexanol as a cosolvent on partitioning and mass transfer rate of protein extraction using reversed micelles of CB-modified lecithin. Biochemical Engineering Journal 1999, 3 (1), 9–16.
- Naoe, K.; Ura, O.; Hattori, M.; Kawagoe, M.; Imai, M. Protein extraction using non-ionic reverse micelles of Span 60. Biochemical Engineering Journal 1998, 2 (2), 113–119.
- Luisi, P.L.; Bonner, F.J.; Pellegrini, A.; Wiget, P.; Wolf, R. Micellar solubilization of proteins in aprotic solvents and their spectroscopic characterisation. Helvetica Chimica Acta 1979, 62 (3), 740–753.
- Kinugasa, T.; Tanahashi, S.; Takeuchi, H. Extraction of lysozyme using reversed micellar solution: Distribution equilibrium and extraction rates. Industrial & Engineering Chemistry Research 1991, 30 (11), 2470–2476.
- Hebbar, H.U.; Raghavarao, K. Extraction of bovine serum albumin using nanoparticulate reverse micelles. Process Biochemistry 2007, 42 (12), 1602–1608.
- Carneiro-da-Cunha, M.; Cabral, J.; Aires-Barros, M. Studies on the extraction and back-extraction of a recombinant cutinase in a reversed micellar extraction process. Bioprocess Engineering 1994, 11 (5), 203–208.
- Deng, J.; Yang, H.; Cao, W.; Fan, D.; Cheng, N.; Wang, B.; Gao, H. Extraction optimization and functional properties of proteins from kiwi fruit (Actinidia chinensis Planch) seeds. International Journal of Food Properties 2014, 17, 1612–1625.

![FIGURE 2 The value of ln([E’–β]/[1-β]) versus time of different pH for the backward extraction process. The regression coefficients were 0.9856, 0.9127, and 0.9658, when pH were 6, 7, and 8, respectively.](/cms/asset/1179b893-8122-4a94-9dba-c415ec60de2e/ljfp_a_919318_f0002_b.gif)