Abstract
Rice bran contains health-beneficial dietary fiber, but its secondary applications are limited by its poor water solubility. The water solubility of polysaccharides in rice bran was increased through the chemoselective oxidation of primary hydroxyl groups by 2,2,6,6-tetramethyl-1-piperidinyl oxoammonium ion and sodium hypochlorite. Response surface methodology was employed to optimize chemoselective conditions in terms of tetramethyl-1-piperidinyl oxoammonium concentration, sodium hypochlorite concentration, and reaction pH. The maximum degree of oxidation reached 98.77% under the optimum conditions of 0.16 mmol tetramethyl-1-piperidinyl oxoammonium, 8.52 mmol sodium hypochlorite, and pH 10.85. The introduction of C6 carboxyl group was confirmed by 13C-NMR and Fourier transform infrared. Water solubility of the oxidized bran (84.65%) was higher than native rice bran (30.65%). Sensory evaluation indicated that high-quality cookies and bread could be prepared by partially replacing wheat flour with oxidized rice bran, demonstrating that oxidized rice bran could be used as a new water-soluble dietary fiber in the food industry.
INTRODUCTION
Rice, a staple food of the Orient, is usually dehulled before consumption. Rice bran, the outer layer of brown rice, is thus a byproduct of the rice-milling industry.[Citation1] Due to the abundance of various chemically functional compounds, rice bran has been extensively studied.[Citation2−Citation4] Rice bran contains high levels of several phytochemicals that have beneficial effects on human health.[Citation5,Citation6] Specifically, rice bran is a good source of dietary fibers, such as β-glucan, pectin, and gums.[Citation2] These fibers can increase food volume without increasing caloric content, and they promote satiety and reduce low-density lipoprotein cholesterol.[Citation7] Dietary fibers also have beneficial effects against certain chronic diseases, such as cardiovascular disease, diverticulosis, diabetes, and colon cancer.[Citation8] For these reasons, these polysaccharides have received attention as new functional food components.
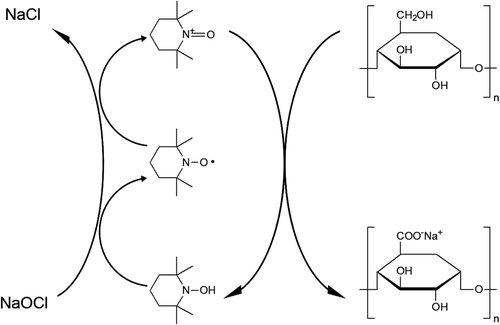
However, poor water solubility of dietary fibers in rice bran has limited their application in the food industry. Therefore, many researchers have attempted to physically, chemically, and/or enzymatically modify dietary fibers to increase their water solubility.[Citation9] Micronizing and nanosizing are the widely accepted physical methods to increase solubility and the stability of solution.[Citation10] There are some studies that the solubility of rice bran could be increased by reducing its size to sub-micron size, ultrafine particles.[Citation11] However, strictly speaking, since a smaller particles have the tendency to be precipitated slower and to make the suspension stable, the particle size reduction would increase the dispersibility and the stability of solution, rather than the solubility. Also, small particles tend to form secondary agglomerates to minimize their surface energy in aqueous phase.[Citation12,Citation13] The chemical modification of polysaccharides is generally used to develop new polymers with improved physicochemical properties.[Citation14] Chemical methods are is generally based on the surface modification, such as the increase of hydrophilicity or the introduction of hydrophilic groups on the surface.[Citation15] Carboxymethylation, methylation, and hydroxypropylation are the most widely used procedures that have been used to increase the dietary fibers such as cellulose, starch, seed gums, corn cob, and rice bran.[Citation15,Citation16] It makes the dietary fibers more soluble in aqueous solutions since it introduces hydrophilic residues (carboxymethyl, methyl, or hydroxypropyl groups) in on the surface of dietary fibers. Compared with the chemical methods used in the past, the catalytic and chemoselective oxidation of primary hydroxyl groups in the polysaccharides by means of a stable radical, such as the 2,2,6,6-tetramethyl-1-piperidinyl oxoammonium ion (TEMPO) is one of the new generation of chemical modification.[Citation17] The water solubilities of cellulose and chitin, both of which are normally insoluble in water, have been increased through TEMPO/sodium hypobromite (NaOBr)-mediated selective oxidation of primary hydroxyl groups.[Citation18] Bromide-free TEMPO-mediated oxidation of potato starch has also been reported.[Citation19] A schematic representation of bromide-free TEMPO-mediated oxidation process is depicted in . TEMPO is reduced to TEMP by the selective oxidation of primary alcohol into carboxylic acid, and then, TEMPO is regenerated from TEMP by the reduction of sodium hypochlorite (NaOCl). Although it could varied dependent on their molecular structures and weights, most of no charged polysaccharides are rarely dissolved into the aqueous solutions. Through the TEMPO-mediated oxidation of polysaccharides, the primary alcohol groups in such polysaccharides could be transformed to the corresponding polyuronic compounds which are called with polyelectrolytes, especially polyanions. These groups are charged in the aqueous solutions, the oxidized polysaccharides would be easily solubilized in the aqueous solutions.
SCHEME 1 TEMPO/NaOCl-mediated oxidation of primary alcohol groups in carbohydrates to C6 carboxyl groups.
The aim of this study was to optimize the TEMPO/NaOCl-mediated chemoselective oxidation of rice bran by finding the TEMPO concentration, NaOCl concentration, and reaction mixture pH that yielded the maximum degree of oxidation (DO), using response surface methodology (RSM). We also developed acceptable cookies and bread, in which some wheat flour was replaced with solubilized functional dietary fibers obtained by the chemoselective oxidation of rice bran, and compared the sensory qualities of these cookies and bread with their control counterparts. Thus, we investigated the applicability of these solubilized dietary fibers as new functional food ingredients.
MATERIALS AND METHODS
Materials
Rice bran was obtained from the National Crop Experiment Station of the Rural Development Administration (Suwon, Republic of Korea). Before the oxidation of rice bran, the most of human health promoting compounds in rice bran, such as oryzanol, phytochemicals, and phenolic compounds, were extracted for other study about the functionality determination. Since the solubility of functional compounds in rice bran were dependent on their polarity, water, methanol, and acetone were used as an extraction solvent. Also, if needed, the extraction solvents with the various polarity were prepared by mixing solvents mentioned above. Therefore, the water-insoluble polysaccharides were the most major components of the rice bran used in this study. Its polysaccharides and dietary fiber contents were approximately 71 and 48%, respectively. TEMPO was purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). NaOCl and sodium hydroxide (NaOH) were obtained from Daejung Chemicals & Metals Co., Ltd. (Shihueng, Republic of Korea). Deuterium oxide (D2O), and dimethyl sulfoxide, which were used as the solvents for 13C nuclear magnetic resonance (NMR), were purchased from Sigma-Aldrich Co. All other chemicals were of analytical reagent grade.
TEMPO/NaOCl-Mediated Chemoselective Oxidation
The chemoselective oxidation of rice bran was conducted using a previously described procedure.[Citation18] with some modifications. Rice bran containing 10 mmol anhydroglucose units (1.62 g) was suspended in 200 mL distilled and deionized water (DDW). The pH of the suspended rice bran solution was adjusted to the appropriate value using 4 N HCl or NaOH before starting the oxidation reaction. The oxidation reaction was initiated by adding an appropriate volume of 10% NaOCl solution containing an appropriate concentration of TEMPO to the rice bran solution at 10°C. The final concentrations of NaOCl and TEMPO varied, according to the experiment. The pH of the reaction mixture was monitored and maintained at the desired value by using an autotitrator (pH Stat; Metrohm, Herisau, Switzerland) to add 0.5 N NaOH as needed. After 20 mL of 0.5 N NaOH (i.e., 10 mmol) was consumed, the oxidation reaction was stopped by adjusting the pH of the mixture to 7.0. The resulting solution was centrifuged at 8,800 × g for 30 min to remove residual insoluble material, and the oxidized rice bran in the supernatant was precipitated by the addition of three volumes of ethanol. The pellet was washed three times with 600 mL ethanol, followed by centrifugation at 8,800 × g for 30 min. The washed pellet was dried at 60°C in a vacuum oven for 24 h and then stored at –20°C.
Determination of DO
The DO of the primary hydroxyl groups in the polysaccharides was calculated using Eq. (1) after determining the amount of 0.5 N NaOH consumed to maintain the initial pH of the reaction mixture:
Experimental Design and Statistical Analysis
RSM was used to optimize the conditions for chemoselective oxidation to obtain a maximum DO. Central composite design was used to determine the effects of TEMPO concentration, NaOCl concentration, and pH of the reaction mixture on the DO. The independent variables (Xi) and their levels are presented in , and the response (Y) was the DO (%). The design scheme consisted of 20 experiments with 23 factorial points and six axial points (α = 2.0), and the central point was replicated six times (). A quadratic polynomial model was assumed to predict the response Y (DO, %), using Eq. (2):
TABLE 1 Experimental range and levels of the independent variables used in RSM
TABLE 2 Central composite design and the response for degree of oxidation
Measurement of Water Solubility
The water solubilities (%) of native and oxidized products were determined according to the method of Chang et al.[Citation18] Samples (100 mg) were dispersed in 30 mL of DDW and stirred with a magnetic stirrer running at 300 rpm for 12 h at 25°C. Then, the dispersion was centrifuged at 8800 × g for 15 min at 4°C, and the pellet was dried at 60°C in a vacuum oven for 24 h. Water solubility (%) was calculated using Eq. (3):
Structural Analysis of Chemoselectively Oxidized Polysaccharides
A liquid-state 13C-NMR experiment was conducted using an AVANCE 600 instrument (Bruker, Ettlingen, Germany) to estimate the conversion of primary hydroxyl groups to carboxyl groups by TEMPO/NaOCl-mediated chemoselective oxidation. The samples were spun at a rate of 20 Hz at 25°C with a spectral width of 42 kHz. The acquisition time was 0.773 s and the line broadening was 3 Hz. The native rice bran (60 mg/mL) was dissolved in deuterated dimethyl sulfoxide, and the oxidized rice bran (60 mg/mL) was dissolved in D2O. Tetramethylsilane was used as an internal standard. Fourier transform infrared (FT-IR) spectra were obtained using a Nicolet 6700 FT-IR spectrophotometer (Thermo Scientific, Waltham, MA, USA) equipped with an attenuated total reflectance accessory. The spectra were recorded at 25°C in transmission mode from 650 to 4,500 cm−1 at a resolution of 8 cm−1.
Cookie and Bread Preparation
The formulation and ingredients used for the experimental cookies and bread are presented in supplementary data (Table S1). All ingredients used for this study were commercially available in the market and the oxidized rice bran was finely ground through a 150-μm sieve. Butter and sugar were creamed together with a KitchenAid mixer (5K5SSWH; KitchenAid, St. Joseph, MI, USA) running at speed four. Eggs, baking powder, and wheat flour were then mixed in at speed four. The cookie dough was sheeted to a thickness of 0.3 cm and cut using a 5-cm diameter cutter, prior to baking on greased aluminum cookie sheets. The cookies were baked at 180°C for 10–15 min. After cooling for 10 min, the cookies were removed from the sheet pan and allowed to cool to room temperature on wire racks. The cookies were prepared 1 day ahead of the sensory evaluation.
For the bread, all dry ingredients, milk, and water were mixed at speed four with a KitchenAid mixer equipped with a flat blade. After mixing, the dough was fermented at 28–30°C for 90 min in an enclosed chamber. The dough was divided into 120-g pieces, manually rounded, rolled, and put into tin pans. The final fermentation lasted 30 min at 28–30°C. The loaves were then baked at 180°C for 35–40 min. The bread loaves were prepared 1 day ahead of sensory evaluation. Based on a preliminary study, the experimental cookies and bread were prepared by replacing 10 and 2.5% of the wheat flour with oxidized rice bran, and were compared with controls made with 100% wheat flour.
Sensory Evaluation
The sensory characteristics of the cookies and bread were judged by a laboratory acceptance panel. The evaluation was performed 1 day after baking by 30 trained sensory panelists with an average age of 40 years. The bread loaves used for the sensory analysis were sliced to a thickness of approximately 1.2 cm by a slicing machine, and each slice was divided into two pieces. The serving sizes for the cookies and bread were 5.5 and 25.0 g, respectively. Samples were presented one at a time on plastic plates coded by the letters of alphabets with water to clean the palate between evaluations and evaluated in separated booths. Several attributes were selected for evaluation; 12 attributes for cookie and 11 attributes for bread. One serving of each cookie or bread, was provided to each panelist, who was asked to provide a score from 1 (dislike extremely) to 9 (like extremely) for each attribute. SensoTools (Sensometrics, Incheon, Korea) was used for the statistical analysis of the results. The significance of difference was tested by paired comparisons (p < 0.05).
RESULTS AND DISCUSSION
Analysis of the Central Composite Experiment
The DO values from the experimental runs are presented in , along with the coded and decoded values of the independent variables (TEMPO concentration, NaOCl concentration, and reaction mixture pH) for each experiment. Using statistical analysis software, a second-order polynomial was obtained (Eq. [Citation4]) to describe the effects of TEMPO concentration, NaOCl concentration, and reaction mixture pH on the chemoselective oxidation of rice bran:
Optimization of Chemoselective Oxidation Conditions and Verification of the Model
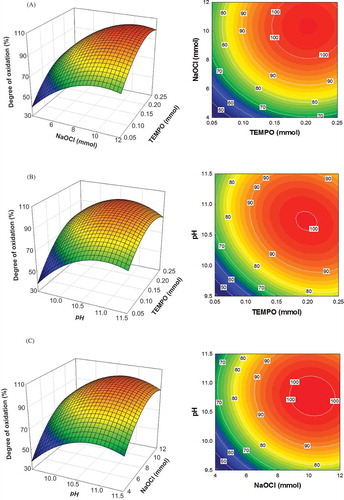
shows the effects of the independent variables on DO. In general, exploring the response surface indicated a complex interaction between the variables. shows the effect of TEMPO and NaOCl concentrations on DO at a pH of 10.5. DO increased with increasing TEMPO and/or NaOCl concentrations as shown in the contour plot in . shows the effect of TEMPO concentration and reaction mixture pH on the DO at a NaOCl concentration of 8.0 mM. At a high TEMPO concentration, a change in reaction mixture pH had little effect on the DO, whereas at a low TEMPO concentration, the DO increased when the pH of the reaction was increased. The effects of NaOCl concentration and reaction mixture pH on the DO at a TEMPO concentration of 0.15 mM are shown in . An increase in both the NaOCl concentration and reaction mixture pH increased the DO, indicating that both of these variables had quadratic effects on the DO. All three contour plots in exhibited similar behavior, in that the calculated values of DO increased with increasing values of the three independent variables (TEMPO concentration, NaOCl concentration, and reaction mixture pH). These results are in good agreement with the strong linear and quadratic effects of the three independent variables on the DO.
FIGURE 2 Response surface and contour plots showing the effects of variables on the degree of oxidation. (a) Effect of TEMPO and NaOCl on the DO at a reactant pH of 10.5; (b) effect of TEMPO and pH on the DO at a NaOCl concentration of 8.0 mmol; (c) effect of NaOCl and pH on the DO at a TEMPO concentration of 0.15 mmol.
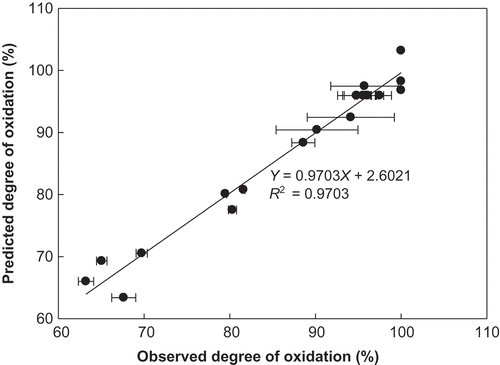
From these analyses, the optimum values for TEMPO concentration, NaOCl concentration, and reaction mixture pH were determined to be 0.16, 8.52, and 10.85 mM, respectively. An experiment was then conducted using these optimal conditions to examine the adequacy of the model. The actual experimental DO value was 98.77 ± 1.23, which did not differ significantly from the predicted value of 100%. This agreement between experimental and predicted values under conditions that were calculated to be optimal verified the validity of the model.
Effect of Chemoselective Oxidation on Water Solubility
The water solubilities (%) of native and chemoselectively oxidized rice brans were determined. After chemoselective oxidation, the water solubility of the oxidized rice bran increased to 84.65%, whereas that of native rice bran was 30.65%. This increase in water solubility after chemoselective oxidation could be due to the introduction of carboxyl groups, which are hydrophilic and also charged.[Citation20,Citation21]
Structural Analysis of Chemoselectively Oxidized Polysaccharide
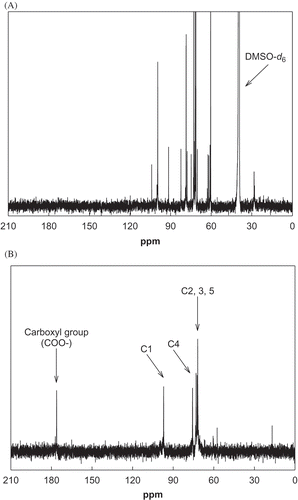
To confirm the chemoselective oxidation of primary hydroxyl groups in the polysaccharides of rice bran, a structural analysis was carried out using 13C-NMR and FT-IR. shows the 13C-NMR spectrum of rice bran before and after chemoselective oxidation. The signals of carbons C1, C2, C3, C4, and C5 were observed at 100.1, 73.4, 72.4, 78.8, and 71.6 ppm, respectively, in native rice bran () and at 97.28, 73.28, 72.95, 76.03, and 72.38 ppm, respectively, in oxidized rice bran ().[Citation22] A newly detected resonance at 177.3 ppm in the oxidized rice bran may represent a carboxyl group converted from the C6 primary hydroxyl group of the polysaccharides. Anelli et al. reported that secondary alcohols are oxidized to ketones during the oxidation of alcohols by oxoammonium.[Citation23] The absence of resonance in the 195–205 ppm region of the 13C-NMR spectrum shows that no ketone group was present in the oxidized rice bran, indicating that the secondary hydroxyl groups were not oxidized by TEMPO/NaOCl-mediated chemoselective oxidation.
TABLE 3 Sensory characteristics (maximum score of 9) of oxidized rice bran (ORB) incorporated cookies (the upper column) and bread (the lower column)
FIGURE 3 13C-NMR spectra of the native and selectively oxidized rice bran by TEMPO/NaOCl-mediated oxidation. (a) Native rice bran; (b) selectively oxidized rice bran.
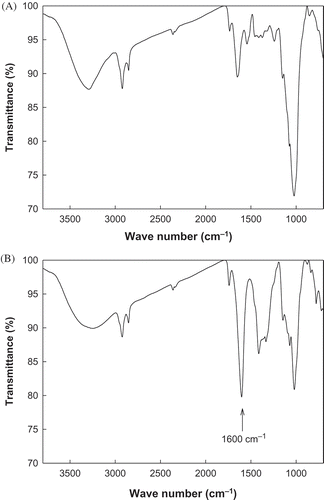
The FT-IR spectra of native and oxidized rice brans are shown in . FT-IR spectra of the oxidized rice bran revealed a sharp peak at 1,600 cm−1 relative to the native rice bran, indicating the presence of a C=O bond in the oxidized rice bran. A broad peak in the 3000–3500 cm−1 range in the spectrum of the native rice bran indicated the presence of an OH group, and this peak was also observed in the oxidized rice bran spectrum. This result suggests that secondary hydroxyl groups were not oxidized during the reaction; if they were, the peak in the range of 3000–3500 cm−1 would have disappeared or decreased in intensity. Therefore, the FT-IR spectra provided confirmation that the primary hydroxyl groups were selectively oxidized in the rice bran. This result agrees with that of a previous report.[Citation24]
Effect of Oxidized Rice Bran on the Sensory Properties of Cookies and Breads
The incorporation of oxidized rice bran into cookies had no significant effect on color, saltiness, sweetness, or egg flavor but did significantly increase the characteristics of toasted flavor, denseness, and hardness (). Although the scores for these characteristics were higher when oxidized rice bran was incorporated into the cookies, the mean scores for overall acceptability in the control versus oxidized rice bran-containing cookies were 5.73 and 6.17, respectively. The substitution of oxidized rice bran for some of the wheat flour in bread had no significant influence on any sensory parameter, except for color. The incorporation of oxidized rice bran resulted in an increased score for crust color (from 5.23 to 5.73). Compared with the control bread, the crust of the oxidized rice bran-containing bread was somewhat darker. The overall acceptability scores did not differ significantly between control and oxidized rice bran-containing breads, indicating that crust color did not affect the sensory quality of the bread. In summary, the partial replacement of wheat flour with oxidized rice bran enriched the dietary fiber content of cookies and bread without significantly affecting their sensory qualities.
CONCLUSIONS
The optimum conditions for the chemoselective oxidation of rice bran were identified as 0.16 mM TEMPO, 8.52 mM NaOCl, and a pH of 10.85 for the reaction mixture. The coefficient of determination (R2) for the response in the predictive model was 0.9703. The actual DO was 98.77%, which, within the margin of error, agreed perfectly with the predicted value of 100%. The water solubility of oxidized rice bran increased dramatically to 84.65%, compared with that of the native rice bran (30.65%). The 13C-NMR spectrum of the oxidized rice bran revealed a new chemical shift at 177.3 ppm, indicating the introduction of a carboxyl group at C6 by chemoselective oxidation of the primary hydroxyl group. Furthermore, the FT-IR spectrum of oxidized rice bran yielded a peak at 1600 cm−1, indicative of the C=O bond in the carboxylate. Because of the high water solubility of rice bran after chemoselective oxidation, oxidized rice bran could be a suitable source of water-soluble dietary fiber for the beverage and food industry, enabling the development and production of dietary fiber-enriched foods.
FUNDING
This research was financially supported by a grant (10162KFDA995) from the Korea Food & Drug Administration in 2013.
SUPPLEMENTAL MATERIAL
Supplemental data for this article can be accessed on the publisher’s website.
LJFP_A_926370_supplemental.docx
Download MS Word (14.8 KB)Additional information
Funding
REFERENCES
- Bardhan, J.; Chakraborty R.; Raychaudhuri U. Quality enhancement of mustard oil by tocotrienol rich fraction from rice bran oil. International Journal of Food Properties 2014, 17 (10), 2312–2321.
- Abdul-Hamid, A.; Luan, Y.S. Functional properties of dietary fiber prepared from defatted rice bran. Food Chemistry 2000, 68, 15–19.
- Chandi, G.K.; Sogi, D.S. Functional properties of rice bran protein concentrate. Journal of Food Engineering 2007, 79, 592–597.
- Parrado, J.; Miramontes, E.; Jover, M.; Gutierrez, J.F.; de Terán, L.C.; Bautista, J. Preparation of a rice bran ezymatic extract with potential use as functional food. Food Chemistry 2006, 98, 742–748.
- Chen, M.-H.; Bergman, C.J. A rapid procedure for analyzing rice bran tocophenol, tocotrienol, and γ-oryzanol contents. Journal of Food Composition and Analysis 2005, 18, 139–151.
- Lai, P.; Li, K.Y.; Lu, S.; Chen, H.H. Phytochemicals and antioxidant properties of solvent extracts from Japonica rice bran. Food Chemistry 2009, 117, 538–544.
- Brown, L.; Rosner, B.; Willett, W.W.; Sacks, F.M. Cholestrol-lowering effects of dietary fiber: A meta-analysis. American Journal of Clinical Nutrition 1999, 69, 30–42.
- Elleuch, M.; Bedigian, D.; Roiseux, O.; Besbes, S.; Blecker, C.; Attia, H. Dietary fibre and fibre-rich by-products of food processing: Characterisation, technological functionality, and commerical applications: A review. Food Chemistry 2011, 124, 411–421.
- Bragd, P.L.; van Bekkum, H.; Besemer, A.C. TEMPO-mediated oxidation of polysaccharides: Survey of methods and applications. Topics in Catalysis 2004, 27, 49–66.
- Rasenack, N.; Müller, B.W. Micron-size drug particles: Common and novel micronization techniques. Pharmaceutical Development and Technology 2004, 9, 1–13.
- Lee, J.E.; Jun, J.Y.; Kang, W.-S.; Lim, J.D.; Kim, D.E.; Lee, K.Y.; Ko, S. Effect of particle size on the solubility and dispersibility of endosperm, bran, and husk powders of rice. Food Science and Biotechnology 2008, 17, 833–838.
- Hogekamp, S.; Schubert, H. Rehydration of food powders. Food Science and Technology International 2003, 9, 223–235.
- Jillavenkatesa, A.; Kelly, J. Nanopowder characterization: Challenges and future directions. Journal of Nanoparticle Research 2002, 4, 463–468.
- Yalpani, M. A survey of recent advances in selective chemical and enzymic polysaccharide modifications. Tetrahedron 1985, 41, 2957–3020.
- Choi, K.O.; Yang, S.C.; Kim, D.E.; Kang, W.-S.; Shin, M.; Choi, Y.-H.; Ko, S. Improving solubility through carboxymethylation of different-sized endosperm, bran, and husk rice powders. Food Science and Biotechnology 2009, 18, 1439–1446.
- Singh, H.; Sodhi, N.S.; Singh, N. Structure and functional properties of acetylated sorghum starch. International Journal of Food Properties 2012, 15, 312–325.
- de Nooy, A.E.J.; Besemer, A.C.; van Bekkum, H. Highly selective nitroxyl radical-mediated oxidation of primary alcohol groups in water-soluble glucans. Carbohydrate Research 1995, 269, 89–98.
- Chang, P.-S.; Robyt, J.F. Oxidation of primary alcohol groups of naturally occurring polysaccharide with 2,2,6,6-tetramethyl-1-piperidine oxoammonium ion. Journal of Carbohydrate Chemistry 1996, 15, 819–827.
- Bragd, P.L.; Besemer, A.C.; van Bekkum, H. Bromide-free TEMPO-mediated oxidation of primary alcohol groups in starch and methyl α-D-glucopyranoside. Carbohydrate Research 2000, 328, 355–363.
- Chang, P.-S.; Seo, H.-M.; Kwon, O.-T.; Lee, H.-G.; Kim, Y.-S. Selective oxidation of primary alcohol group in 1-monostearoyl glycerol mediated by 2,2,6,6-tetramethyl-1-piperidine oxoammonium ion. Food Science and Biotechnology 2004, 13, 225–259.
- Ahn, S.M.; Lee, H.J.; Kim, S.W.; Lee, J.; Chang, P.-S. Physicochemical properties of selectively oxidized 1-monolaurin from 2,2,6,6-tetramethyl-1-piperidinyl oxoammonium ion/sodium hypochlorite-mediated reaction. Journal of Agricultural and Food Chemistry 2009, 57, 2920–2924.
- Singh, V.; Zakiuddin, S.; Divakar, S. 13C CP/MAS NMR spectroscopy of native and acid modified starches. Starch/Stärke 1993, 45, 59–62.
- Anelli, P.L.; Biffi, C.; Montanari, F.; Quici, S. Fast and selective oxidation of primary alcohols to aldehydes or to carboxylic acids and of secondary alcohols to ketones mediated by oxoammonium salts under two-phase conditions. Journal of Organic Chemistry 1987, 52, 2559–2562.
- Ding, B.; Ye, Y.Q.; Cheng, J.; Wang, K.; Luo, J.; Jiang, B. TEMPO-mediated selective oxidation of substituted polysaccharides—an efficient approach for the determination of the degree of substitution at C-6. Carbohydrate Research 2008, 343, 3112–3116.