Abstract
Controlled freezing-point storage means that the storage temperature is in a non-freezing temperature zone between the freezing point of water and food. It has been demonstrated that controlled freezing-point storage can prolong the storage-life of fresh food. Green beans (Phaseolus vulgaris L.) have been planted extensively in the northeast region of China and harvested seasonally. Degradation of cell wall polysaccharides has greatly affected the texture properties of the green bean cell walls. During controlled freezing-point storage, activities of cellulase, pectin methyl esterase, and polygalacturonase were inhibited effectively. It resulted in a slow degradation of cellulose and pectin, less expansion of the cell wall, and less dissolution of pectin layers. As a result, the web structure of the cell wall was preserved. At controlled freezing temperature, the hardness of green beans decreased slightly. Homogalacturonan of chelate pectin was degraded into galacturonic acid, and the side chains of the soluble alkali pectin were degraded into arabinose and galactose. At the later stage of controlled freezing-point storage, the web structure of the cell wall polysaccharides was destroyed. However, the pectin degradation in the green beans was inhibited significantly during controlled freezing-point storage.
INTRODUCTION
The plant cell wall is synthesized during cell expansion at the first stages of development and is composed of cellulose, hemicelluloses, pectins, enzymes, and structural proteins. During fruit ripening, cell wall weakening and solubilization of the middle lamella lead to a decrease in cell cohesion.[Citation1] Furthermore, there are major changes in the pectic polymers in the cell walls.[Citation2] Neutral sugars (mainly galactose and arabinose) are major components of the cell wall neutral pectins. During this softening, there is a loss of neutral sugars and acidic pectin (rhamnogalacturonan) of all cell walls.[Citation3] The major enzymes implicated in the softening of fruits are pectinesterase, polygalacturonase cellulase, and β-galactosidase.
The degradative changes associated with ripening involve multiple structural alterations within the cell wall that affect pectins and the cellulose-xyloglucan framework.[Citation4] These structural alterations lead to an increased polyuronide and hemicellulose solubilization. The degradation of side chains in pectic polysaccharides and cellulose-hemicelluloses network under the activity of enzymes was considered one of the most significant factors of fruit softening through modifying the network of cell wall polysaccharides.[Citation5,Citation6] During storage at 20°C, both fruit quality and polysaccharide contents decrease quickly.[Citation7] Particularly, browning reactions that occur during storage could result in an unacceptable product.[Citation8] Nevertheless, low-temperature storage was more effective to maintain postharvest fruit and vegetable quality, in particular the polysaccharide contents as health beneficial foods.[Citation9]
Due to its high nutritional quality and sensory properties, green beans (Phaseolus vulgaris L.) are grown extensively in all major continental areas. The quality and shelf life of green beans are known to vary with such factors as the cultivars, maturity, processing, and storage conditions. Seasonality and perishability of these vegetables explain the necessity of applying preservation technologies, such as freezing, in order to extend their shelf life. Controlled storage is one of the most important methods of storage and preservation of these vegetables for a long time and it allows for the consumption of the green beans far from the production areas. Industrial processing of green beans, as well as the development of green bean pods, is characterized by cell wall dissolution that leads to changes in cell wall polysaccharides.[Citation10,Citation11]
In fact, little is known about changes of cell wall material (CWM) and cell wall polysaccharide fractions of green beans during storage. In particular, our study aims to assess the degraded changes and structural alterations within the cell wall in green bean pods during storage at different temperatures (25°C, 8°C, and controlled freezing [CF] temperature). What’s more, this work aims to evaluate the activity of CWM degrading enzymes in relation to green bean pod texture at different conditions. The understanding of degradative changes and structural alterations within the cell wall during storage could be of great importance for improving the quality and shelf life of green beans.
MATERIALS AND METHODS
Plant Material and Chemicals
Mature green beans of a major cultivar (Phaseolus vulgaris L.) were obtained from a local farm (Heilongjiang Academy of Agricultural Science, Harbin, China). The green bean samples were picked randomly from 200 plants, and a total of 20 kg were packed in plastic boxes and transported to the laboratory within two hours on the day of harvest. Samples, collected from fresh green beans and three storage conditions (25°C, 8°C, and CF temperature), were immersed in liquid nitrogen, packaged in polyethylene bags, and stored in an ultra low-temperature freezer (Thermo Fisher Scientific Inc., SAN JOSE, CA) at –70°C. The seeds were manually removed from the frozen pods every 5 days for further analyses. Fresh samples were analyzed as a control and an assessment of the firmness measurements. Monosaccharide standards were purchased from Sigma Chemical Co. (St. Louis, MO). All the other chemicals used were of analytical grade. All measurements are represented as the average of triplicate; three samples were taken from one trial.
Storage Conditions
Three storage conditions were used: room temperature storage, cold storage, and controlled freezing-point storage (CF storage). For room storage, the room temperature was set at 25 ± 2°C and controlled by an air-conditioner (MSHJ11UV, Mitsubishi, Japan). The green bean samples were placed on a table in the room. For cold storage, the green beans were placed on the glass board in the refrigerator (KK28E76, Siemens, Germany). The temperature was set at 8 ± 2°C. CF storage is the non-freezing temperature zone between the freezing point of water and that of an individual material. The freezing zone of green beans ranges from 0 to –0.66°C. In this investigation, the CF temperature was set at –0.2°C. A special arrangement was made for CF storage as previously reported.[Citation12] Two plastic buckets were used, the green beans were placed in an inner bucket, which was then placed inside an outer bucket. The ice-water mixture was filled between the walls of the two buckets, so that the temperature in the inner bucket was precisely controlled at –0.2°C. A digital thermometer (ZDR-11, Zheda Electric Equipment Co., China) was used to monitor the temperature. In this study, all the samples were stored in low air flow (air rate <0.15 m/s), and the relative humidity of storage was 80–90% at 8 and –0.2°C and 30–40% at 25°C. The tests were performed on beans from different storage conditions at different end time since being in room temperature and in 8°C storage the beans had deteriorated to such an extent that the tests could not be performed. For firmness measurement green bean samples were taken at the fresh stage, then from 25°C (after 4 and 8 days of storage), 8°C and CF temperature (after 4, 8, 12, 16, and 20 days). When investigating cell wall polysaccharides and sugar composition, samples were taken at the fresh stage, then from 25°C (5 days of storage), 8°C (after 5, 10, and 15 days), and CF storage (after 5, 10, 15, and 20 days).
Purification and Fractionation of CWM
Purification of CWM
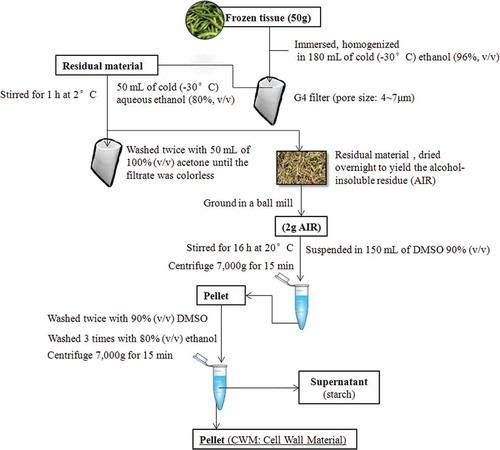
Frozen tissue (50 g) was immersed in 180 mL of cold (–30°C) ethanol (96%, v/v), homogenized (HR1724, Philips, Netherlands) by four bursts of 45 s, and collected on a G4 filter (pore size: 4˜7μm). Different steps of purification process of CWM are shown in . The material was suspended in 50 mL of cold aqueous ethanol (80%, v/v) stored beforehand at –30°C in lab freezers. The material was stirred for 1 h at 2°C then filtered again, washed twice with 50 mL of 100% (v/v) acetone until the filtrate was colorless, and dried overnight to yield the alcohol-insoluble residue (AIR). The AIR was subsequently ground in a ball mill (model MM2, Retsch, Ochten, Netherlands). To remove starch, the AIR (2 g) was suspended in 150 mL of 90% (v/v) dimethyl sulfoxide (DMSO) and stirred for 16 h at 20°C. The suspension was centrifuged (7000 g for 15 min) and the pellet was washed twice with 90% (v/v) DMSO and three times with 80% (v/v) ethanol. The supernatant, which contained predominantly starch, was discarded.[Citation11] Pectic polymers were extracted from the resulting CWM using the method of Selvendran et al.[Citation13] with minor modifications.
Fractionation of CWM
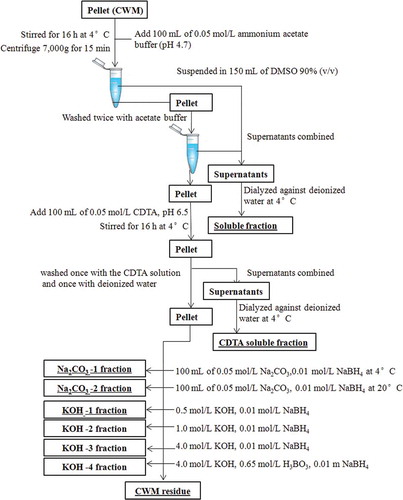
Different steps of fractionation of CWM are shown in . To the pellet obtained from purification process, 100 mL of 0.05 mol/L ammonium acetate buffer (pH 4.7) was added and the suspension was stirred for 16 h at 4°C. The suspension was centrifuged and the pellet was washed twice with acetate buffer. The supernatants were then combined and this “buffer” soluble fraction was dialyzed exhaustively against deionized water at 4°C. Then, 100 mL of 0.05 mol/L cyclohexylenedinitrilotetraacetate (CDTA, pH 6.5) was added to the pellet, and the suspension was stirred for 16 h at 4°C. The suspension was centrifuged and the pellet was washed once with the CDTA solution and once with deionized water. The supernatants were combined and fill dialyzed at 4°C against deionized water (CDTA soluble fraction). The pellet was subsequently extracted with 100 mL (O2-free) of 0.05 mol/L Na2CO3 containing 0.01 mol/L NaBH4 at 4 and 20°C. Next, hemicelluloses and residual pectins were extracted with four KOH fractions. Respectively, 0.5, 1.0, and 4.0 mol/L KOH contained 0.01 mol/L NaBH4 and 4.0 mol/L KOH contained 0.65 mol/L H3BO3 and 0.01 m NaBH4. All extractions were performed with constant stirring under N2 for 16 h at 20°C to leave a residue consisting mainly of cellulose. All Na2CO3 and KOH supernatants were filtered, adjusted to pH 5.0 with acetic acid, dialyzed exhaustively against deionized water, and lyophilized. During neutralization of the 0.5 and 1.0 mol/L KOH supernatants, a precipitate was formed, which was isolated and analyzed separately.[Citation11,Citation14]
Monosaccharide Composition
The analysis of monosaccharide compositions was carried out by the method of Yang et al.[Citation15] with a slight modification. The polysaccharide was hydrolyzed for 3 h with 10 mL of 2 mol/L trifluoroacetic acid (TFA) at 105°C. Derivatization of the released monosaccharides was then carried out using the alditol acetate reagent according to the method of Kang et al.[Citation16] The alditol acetate derivatives were loaded onto a HP-5 capillary column and determined by a flame ionization detector. The following program was adopted for gas chromatography GC/MS (7890A-5975C Agilent) analysis: injection temperature: 210°C; detector temperature: 250°C; column temperature programmed from 110 to 190°C at 3°C/min, holding for 4 min at 190°C, then increasing to 210°C at 3°C/min and finally holding for 20 min at 210°C. Helium was used as the carrier gas and maintained at 1.0 mL/min. Inositol was used as the internal standard.
Firmness measurements
The firmness was measured after 0, 4, 8, 12, 16, and 20 days at 8°C and CF temperature using a texture analyzer (TA.XT2, Stable Micro Systems Texture Technologies, Scarsdale, NY) fitted with a 3 mm diameter flat probe. The analyzer was linked to computer that recorded the data via a software program called Texture Expert 32 Version 3.0 (Stable Micro Systems Software). The ice stored green beans were equilibrated to 20–25°C at room temperature. A 26-mm P/36R probe was used to shear a bean, with pre-test speed of 2.0 mm/s, test speed of 0.5 mm/s, and post-test speed of 2.0 mm/s, compression of 50%, probe force of 20 g. The maximum force developed during the test was recorded. All textural analyses were replicated ten times and results were presented as mean values. Each disc was measured in the internal and external zone.[Citation17] The external zone is the surface of green been pod and the internal zone is the pulp of green bean pods. For 25°C storage, firmness was measured after 0, 4, and 8 days, once in room temperature, the beans had deteriorated to such an extent that the tests could not be performed.
Transmission electron microscopy (TEM) analysis
For electron microscopy analysis, green beans were cut into 1 mm × 1 mm, fixed with 3.5% glutaraldehyde, stored at 4°C for 12 h, washed three times with 0.1 M phosphate buffer (pH 6.8), and then fixed with 1% osmium tetroxide for 1 h at 4°C. After that, green bean slices were washed again three times with 0.1 M phosphate buffer (pH 6.8), dehydrated through a graded ethanol series of 30 to 100%. The samples were embedded with Epon 812 and polymerized at 35°C for 15 h, at 15°C for 18 h, and at 60°C for 15 h. The epoxy resin was polymerized for 20 h in an oven set at 60°C. Around 90-nm thin sections were prepared on ultramicrotome, collected, and transferred to 100-mesh copper EM grids. The sections were post-stained with uranyl acetate and lead citrate and examined in a TEM-1200 transmission electron microscope operating at an accelerating voltage of 100 KV.
Statistical analyses
Statistical analyses were performed using XLSTAT 2009 (http://www.xlstat.com/). Data were compared on the basis of standard deviation of the mean values. Differences between mean values were assessed using a one-way analysis of variance with a post hoc determination using Duncan’s multiple range tests. The level of significance was set at P < 0.05.
RESULTS AND DISCUSSIONS
Cell Wall Composition of Green Beans
Green beans were subjected to sequential extraction with different extractants (). The CDTA fraction contents showed a sharp increase at 25°C within 5 days of storage (from 18 to 76 mg/g DW of pod). However, this increase was delayed at 8°C and significantly inhibited with CF storage. The same variation was observed within the residual CWM. In a same manner, the pectin fractions (Na2CO3-1, Na2CO3-2) and hemicellulosic polysaccharides (KOH-3 and KOH-4) decreased rapidly at 25°C but gradually at 8°C and CF storage. The degradation of protopectin is inhibited by lower temperature (CF). The changes of pectic polysaccharides were inhibited by CF storage, and then the senescence was delayed. CF storage controlled the solubilization and depolymerization of pectin and inhibited enzymatic hydrolysis.
TABLE 1 Changes in cell wall polysaccharide contents of green bean during storage
The KOH-1 and KOH-2 fraction contents decreased significantly at 25 and 8°C storages but remained unchanged at CF temperature. In fact, the degradation of cellulose is inhibited because the cellulase (Cel) activity is effectively inhibited at CF storage.
Sugar Composition of Soluble Pectin Fractions
From different storage conditions, sugar composition of soluble fractions shows a slight change (). In particular, we distinguish a decrease of arabinose and a slight increase of galactose. On the other hand, xylose and glucose increased rapidly at 25°C compared to other storage conditions which show no change. Low temperature storage showed an inhibitory effect on these changes. Sugar degradation was more rapid during warm storage.
TABLE 2 Changes in sugar composition of soluble fractions of pods during storage
Sugar Composition of CDTA Pectin Fractions
As shown in , in the CDTA fraction, rhamnose, xylose, mannose glucose increased after 5 days of warm storage, but they show a significant decrease at 8°C and CF storage. The arabinose and galactose decrease in different storage conditions. On the other hand galacturonic acid shows a significant increase in all storage conditions. The loss of galactose and arabinose from the cell wall during storage may favor a firmer texture by promoting cross-linking of pectin chains or pectin-extensin associations.[Citation18]
TABLE 3 Changes in sugar composition of CDTA fractions of pods during storage
Sugar Composition of Na2CO3 Pectin Fractions
The Na2CO3-soluble fractions of green beans were extracted during storage at different temperatures (). The rhamnose content remained unchanged in all storage conditions. The arabinose and galactose residues decreased rapidly in warm storage however this degradation was delayed at 8°C and CF storage. The highest mole percentage of arabinose residue was obtained in Na2CO3 fraction and the ratio of arabinosyl to rhamnosyl residue decreased during storage. The change was not evident in early stages of CF temperature, but is obvious in the later stage of warm storage. This ratio decreased more rapidly compared to cool storage. Galacturonosyl to rhamnosyl residue ratio and galactosyl to rhamnosyl residue ratio were all lower at warm storage stages compared to cool storage.
TABLE 4 Changes in sugar composition of Na2CO3 fractions of pods during storage
Sugar Composition of Hemicellulosic Fractions
In the present study, the primary sugars in hemicellulosic polysaccharide fractions were arabinose, galactose, and glucose residues (). The presence of galacturonic acid, galactose, and arabinose in cellulosic fractions was indicative of pectin co-extraction. It was possible to demonstrate the association between these kinds of polymers. There was a loss of galactose and arabinose during cool and warm storage. The galactose content decreased more rapidly during warm storage compared to cool storage.
TABLE 5 Changes in sugar composition of hemicellulosic fractions of pods during storage
Green Bean Firmness
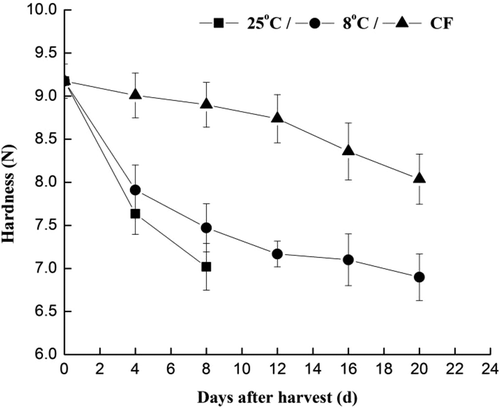
The firmness of green beans decreased significantly in all treatments over time. In fact, firmness of beans during CF storage showed little changes compared to warm storage. Indeed, at low temperatures the enzyme activities were lowered leading to an attenuated decrease of firmness. Similar patterns of firmness were observed in peach fruit stored at 5°C.[Citation19] Similar results reported with grape fruits; compared to fruit stored at high oxygen (O2) atmosphere, grape fruits stored in air had the fastest softening rate losing 27% of their firmness in about 15 days.[Citation20]
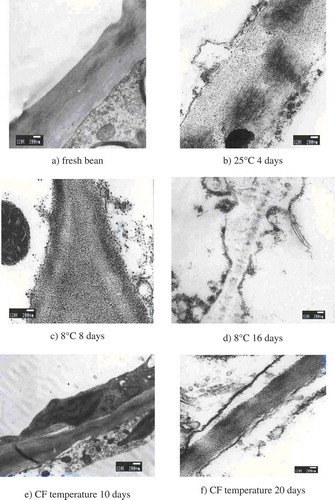
During storage at 8 and 25°C, the firmness decreased rapidly after 4 days and then it decreased slowly (). The firmness of stored green beans was negatively correlated with content of starch and protopectin significantly. According to TEM micrographs (), the degradative changes associated with different storage conditions show clearly that structural alteration was related to warm storage. During low temperature storage softening and cell wall degradation was delayed.
For fresh green beans, the cell walls were smooth, and its microfibril presented intact lamellae structure. The cell wall presented as a regular polygon. The vacuolization was in the cell. The cytoplasm and its inclusions were attached to the cell wall which was present in a thick and dense state. Starch particles were mainly distributed in the cytoplasm. The cell wall was divided up into clear-dim-clear sectors. The middle lamella was a thin and dim layer having higher electronic density (). When green beans were stored at 25°C for 4 days, the microfibril structure of cell walls became disordered. Outer layer of cell walls had significant floccus and a part of the cell-wall degradation matters were spread into the cell. The most common changes in the cell wall structure during ripening were related to pectins, which are the major components of primary cell wall and middle lamella.[Citation21] The fiber became soft and the structure of cell wall changed to incompact state. Softening of the fruit observed during ripening is due to the hydrolytic change of protopectin to pectin. Also, the middle collide layer of cell wall was broken up resulting in irregular cell shape (). When green beans were stored at 8°C for 8 days, the microfibril of cell walls began to decompose, and a part of decomposed cell wall spread into the cell. The middle collide layer of cell wall was broken up, but the cell walls were still kept an intact structure (). When green beans were stored at 8°C for 16 days, the cell wall was decomposed. The decomposed cell wall became flocculent and spread into the cell. Electronic density in middle lamella decreased markedly. Although the middle collide layer disappears and cell inclusion was completely degraded, the cell wall can be recognized by TEM ().
When green beans were stored at CF temperature for 10 days, the cell wall was smooth, and microfibril of the cell wall was intact and in regular arrangement. The cell contained a plenty of inclusions. Also, the middle collide layer and cell wall structure stayed intact (). However, when green beans were stored at CF temperature for 20 days, part of microfibril of cell walls was degraded, and a part of degraded cell wall was spread into the cell. The middle collide layer of cell wall was decomposed. The cytoplasm and cell walls were separated. The structure of cell wall still remained intact ().
DISCUSSION
The ripening-related solubilization of cell wall polysaccharides, which made up the major structural network of the cellular wall, appears common to most species.[Citation14] The pectins are mainly consisted of two co-extensive residues homogalacturonans (HGAs) and rhamnogalacturonans which form the so-called “hairy regions” of pectins due to their high number of branched side chains (arabinans, galactans, and arabinogalactans). Based on this structure, the changes of the galacturonosyl to rhamnosyl residue ratio are often used to reflect the modifications of pectic main chains. The changes of galactosyl to rhamnosyl residue ratio and those of arabinosyl to rhamnosyl residue ratio are used for reflecting the modifications of pectic side chains.
In this study, the rhamnose residue content in CDTA fraction increased more rapidly during 25 and 8°C storages than during CF storage, which probably resulted from the increase in the low molecular pectin content and contributed to the increase in CDTA fraction content. Galacturonic acid, which made up the main chain of pectins, is rapidly degraded in the four pectic fractions during 25 and 8°C storages compared to CF storage. This suggests that the rupture of main pectic chains contributes significantly to green bean softening.
Depolymerization of pod hemicellulosic polysaccharides during softening was observed in this study. Other changes in cell wall components, which often occur during vegetable storage, are the loss of neutral sugar side chains from numerous polysaccharides. Green bean softening involved the solubilization and the loss of neutral sugars in hemicellulosic polysaccharides as well as the depolymerization of hemicelluloses. It is the neutral sugars that are often solubilized and then lost to a considerable extent during green bean softening. The modifications of pectins during softening have been reported in many other vegetables and fruits, and have been proposed to be a significant factor causing softening.[Citation22]
In the present study, the inhibition of the loss of arabinose and galactose in pectins during CF temperature storage supported their participation in softening of green beans. This loss might be attributed to the degradation of pectic side chains riching in arabinans, galactans, and arabinogalactans. The loss of arabinose and galactose were detected in CWM-residue, suggesting that the loss of neutral sugars contributed significantly to the softening.
These biochemical changes that contribute to wall swelling, wall stiffness, intercellular adhesion, and the alterations in different physical properties of cellular walls and tissue texture are not well known. However, it is increasingly apparent that softening and textural changes are brought about by the actions of a multitude of cell-wall-localized enzymes acting on specific, potentially highly localized substrates in a prescribed manner.[Citation22]
The present results showed that different sugars had different rates of loss, but the degradation of arabinosyl residue appeared mainly during the second 4 days of storage in the four pectin fractions and the CWM-residue. The large decline in arabinosyl residue from the insoluble residue matches up with the increase in arabinosyl residue in the more easily extractable CDTA and sodium carbonate extracts, suggesting that the release of arabinosyl residue from tightly bound polymers to a less firmly attached form is an important mechanism of softening. This is consistent with previous findings, and might be attributed to the increase in activity of arabinofuranosidases.[Citation23] The separation of galactose and the loss of galacturonic acid might be attributed to the activities of α-D-galactosidase[Citation24] and polygalacturonase (PG), respectively. However, these enzymes probably differ in the ways of functioning and the chronology of changes in activity, which contribute to the difference of degradation of corresponding sugars, as it is shown in the present investigation.
CONCLUSION
In the current research, flesh firmness of green beans decreased rapidly within first four days of room storage, and then the decrease became slower. Low temperature delayed the softening of green beans. The activity of CWM degrading enzymes was significantly delayed at CF storage which consequently inhibited the degradative changes and structural alterations within the cell wall. Therefore, the green beans stored at CF temperature should be recommended for prolonging the shelf-life of the product compared to 25 and 8°C storages. Combining with hot air treatment and permeability film package, green beans can be preserved up to 28 days. Research on the application of the CF storage technique should be extended to cover more fruit and vegetable varieties.
ACKNOWLEDGMENTS
The authors would like to thank anonymous reviewers and the Editor for comments on the earlier version of this article. The authors are also very grateful to Miss S. Bedoui for providing language assistance.
FUNDING
The present work was supported by the Ministry of Science and Technology of the People’s Republic of China (MOST) through China postdoctoral program awarded to W.E. under the China-Africa Science and Technology Partnership program (CASTEP).
REFERENCES
- Brummell, D.A. Cell wall disassembly in ripening fruit. Functional Plant Biology 2006, 33, 103–119.
- Tucker, G.; Grierson, D. Fruit ripening. In: The Biochemistry of Plants. A Comprehensive Treatise; Davies, D.D.; Ed.; Academic Press: New York, NY, 1987; Vol. 12.
- Thomson, A.K. Fruit and Vegetables: Harvesting, Handling, and Storage, 2nd Ed; Blackwell Publishing Ltd.: Oxford, 2003; 480.
- Redgwell, R.J.; MacRae, E.; Hallett, I.; Fischer, M.; Perry, J.; Harker, R. In vivo and in vitro swelling of cell walls during fruit ripening. Planta 1997, 203, 162–173.
- Perez, S.; Rodriguez-Carvajal, M.A.; Doco, T. A complex plant cell wall polysaccharide: Rhamnogalacturonan II. A structure in quest of a function. Biochimie 2003, 85, 109–121.
- Yashoda, H.M.; Prabha, T.N.; Tharanathan, R.N. Mango ripening-chemical and structural characterization of pectic and hemicellulosic polysaccharides. Carbohydrate Research 2005, 340, 1335–1342.
- Mizuno, M. Anti-tumor polysaccharides from mushrooms during storage. Biofactors 2000, 12, 275–281.
- Vaios, T.K.; Serafim, B.; Athanassia, K.; Panayiotis, S.R. Color degradation of beans during storage. International Journal of Food Properties 2006, 9 (1), 61–71.
- Wiboonsirikul, J.; Masayuki, M.; Pramote, K.; Shuji, A. Properties of extract from okara by its subcritical water treatment. International Journal of Food Properties 2013, 16 (5), 974–982.
- Stolle-Smits, T.; Beekhuizen, J.G.; Vandijk, C.; Voragen, A.G.J.; Recourt, K. Cell-wall dissolution during industrial processing of green beans (Phaseolus-Vulgaris L). Journal of Agricultural and Food Chemistry 1995, 43, 2480–2486.
- Stolle-Smits, T.; Beekhuizen, J.G.; Kok, M.T.C.; Pijnenburg, M.; Recourt, K.; Derksen, J.; Voragen, A.G.J. Changes in cell wall polysaccharides of green bean pods during development. Plant Physiology 1999, 121, 363–372.
- Guo, L.; Ma, Y.; Sun, D.W.; Wang, P. Effects of controlled freezing-point storage at 0°C on quality of green bean as compared with cold and room-temperature storages. Journal of Food Engineering 2008, 86, 25–29.
- Selvendran, R.R.; Stevens, B.J.H.; ÓNeill, M.A. Developments in the isolation and analysis of cell walls from edible plants. Society of Experimental Biologie Seminar Series 1985, 28, 39–78.
- Jin, C.H.; Suo, B.; Kan, J.; Wang, H.M.; Wang, Z.J. Changes in cell wall polysaccharide of harvested peach fruit during storage. Journal of Plant Physiology and Molecular Biology 2006, 32, 657–664 (in Chinese).
- Yang, B.; Wang, J.S.; Zhao, M.M.; Liu, Y.; Wang, W.; Jiang, Y.M. Identification of polysaccharides from pericarp tissues of litchi (Litchi chinensis Sonn.) fruit in relation to their antioxidant activities (Vol. 341, 634 pages, 2006). Carbohydrate Research 2006, 341, 1975–1975.
- Kang, X.J.; Qu, J.S.; Gu, Z.Z. Analysis of Angelica dahurica polysaccharide. Chinese Journal of Analytical Chemistry 2006, 34, 533–535.
- Vicente, A.R.; Costa, M.L.; Martinez, G.A.; Chaves, A.R.; Civello, P.M. Effect of heat treatments on cell wall degradation and softening in strawberry fruit. Postharvest Biology and Technology 2005, 38, 213–222.
- Galindo, F.G.; Brathen, E.; Knutsen, S.H.; Sommarin, M.; Gekas, V.; Sjoholm, I. Changes in the carrot (Daucus carota L. cv. Nerac) cell wall during storage. Food Research International 2004, 37, 225–232.
- Jin, C.H.; Shuiye, Y.S.; Kang, J.; Suo, B.; Wang, Z.J. Degradation of cell wall polysaccharides during postharvest fruit ripening and softening of different apple varieties. Physiology and Molecular Biology of Plants 2006, 32, 617–626 (in Chinese).
- Deng, Y.; Wu, Y.; Li, Y.F. Changes in firmness, cell wall composition, and cell wall hydrolases of grapes stored in high oxygen atmospheres. Food Research International 2005, 38, 769–776.
- Kannika, R.; Sanguansri, C. Effect of cultivar and ripening stage on quality and microstructure of frozen mangoes (Mangifera indica Linn.). International Journal of Food Properties 2014, 17 (5), 1093–1108.
- Brummell, D.A.; Harpster, M.H. Cell wall metabolism in fruit softening and quality and its manipulation in transgenic plants. Plant Molecular Biology 2001, 47, 311–340.
- Brummell, D.A.; Dal Cin, V.; Crisosto, C.H.; Labavitch, J.M. Cell wall metabolism during maturation, ripening, and senescence of peach fruit. Journal of Experimental Botany 2004, 55, 2029–2039.
- Jin, C.H.; Mizuno, M.S.; Wang, Z.J.; Lu, Z.X.; Terai, H.F.; Tsuchida, H.N. Study on the degradation specificity of cell wall polysaccharides by β-galactosidase in apples. Journal of Yangzhou Univinersity. (Agric Life Sci Edn) 2002, 23, 71–74 (in Chinese).