Abstract
The aim of the present study was to determine impacts of different additives on fatty acid composition and total tocopherol content of cottonseed oil during deep-frying process using a chemometric approach. A quarter-fractional factorial design with two levels and five factors was used for preparing the frying cottonseed oil blends. In the experimental design, additives were such as ascorbic palmitate, mixed tocopherols, dimethylpolysiloxane, lecithin, and sesame oil were used as factors. The effect of additives on fatty acid composition and tocopherol content were evaluated with normal distribution (ND) graphs during ten hours frying at 170 ± 5°C. The data were statistically assessed by one-way analysis of variance at a significance level of p < 0.05. The analysis of variance test results were in good agreement with ND graphs and data indicated that the interaction between ascorbic palmitate and dimethylpolysiloxane (A × C factor) showed a significant and protective effect on fatty acids. Meanwhile, the sesame oil (E factor) and A × C factor were showed a significant effect on level of tocopherols. The results of study indicated that present approach could be used to assess and improve the frying stability of any oil with respect to fatty acid composition and tocopherols.
INTRODUCTION
Deep-fat frying is the most common quick food preparation technique, which is widely used in homes, restaurants, and food manufacturing industries. It converts the food materials into something more attractive and delicious. During the frying process, material is dipped in oil and heated at high temperature usually in between 150–190°C. Due to high temperature, the physicochemical and sensory properties of oil are changed due to the formation of various undesirable harmful compounds. Therefore, quality and stability of frying oil is decreased with the increase of frying time.[Citation1,Citation2]
During frying, vegetable oils undergo various processes such as oxidation, hydrolysis, and polymerization which lead to deterioration of frying oil with the formation of new products such as free fatty acids, hydroperoxide, and plymers and changes occurred in fatty acid composition (FAC), cis to trans fatty acid (TFA) ratio and level of bioactive compounds.[Citation3] As a result, these parameters have an inverse effect on the shelf life and quality parameters of oils.[Citation4]
Several studies reported that FAC and tocopherols are important features of different frying vegetable oils which have great impacts on flavor and stability of fried foods.[Citation5–Citation7] The previous studies show that oxidation occurs more quickly in highly unsaturated oils than the less unsaturated one. Therefore, saturated and hydrogenated oils are frequently used for frying applications, despite their health negative effects.[Citation8–Citation10] Tocopherols are natural occurring potent antioxidants that help to prevent the oxidation of unsaturated fatty acids (USFAs). Tocopherols including α-, β-, γ-, and δ- homologous are considered to be high anti-oxidants.[Citation11–Citation13] Tocopherols play an anti-oxidative role during frying process by preventing oxidation process.[Citation14] But tocopherol species are lost along with extended frying time and the oxidation rate of USFAs is increased.
During the frying application it is important to monitor the losses of tocopherols for assessing the deterioration in vegetable oils. It has been reported that at frying temperatures, the losses of tocopherols and decrease of unsaturation occur with increase of frying cycles.[Citation6] Among most of the common vegetable oils, cottonseed oil (CSO) is considered to be more stable oil, due to unique FAC and significant level of tocopherols (~1000 mg/kg oil). However, refining process reduces the level of tocopherols. CSO is included in the preferred oils for frying applications, and has a 2:1 ratio of polyunsaturated fatty acids (PUFA) to saturated fatty acids (SFAs). Its FA profile generally consists of 70% USFAs (18% oleic and 52% linoleic) and 26% SFAs. The SFAs provides a degree of stability to the oil that makes it suitable for frying applications. CSO is also rich in natural tocopherols and antioxidants. Therefore, these natural antioxidants are retained at high levels in fried products and responsible for longer shelf life of products.[Citation15–Citation19]
In order to improve stability of frying oils; emulsifiers, natural and synthetic antioxidants, anti-polymerizing, anti-spattering, and anti-foaming agents have been used to protect them from oxidation. Commercial rapeseed, sunflower, corn, palm, and hydrogenated vegetable oils are chosen for frying applications. Regarding these circumstances, excellent frying performance has been noted for those vegetable oils which contain the maximum number of antioxidants.[Citation20,Citation21] Due to the nutritional and health related issues, many efforts have also been made to develop stable frying oil formulations using several additives. The main purpose of the present study was to develop new formulations for frying oil by preparing blends with CSO and different types of additives using quarter-fractional factorial design (Q-FFD) chemometric approaches. This study was focused on the stability of refined CSO and its blends—with various additives and impacts of additives on FAC and tocopherol contents during the frying process.
EXPERIMENTAL PROCEDURES
Materials
Samples
A refined, bleached, and deodorized CSO sample was obtained from Helvacizade edible oil refinery company (Konya, Turkey). The frozen pre-fried French fries (Superfresh, Turkey) were obtained from the local market of Konya, Turkey. Additives used for preparing the frying CSO blends; ascorbic palmitate, mix tocopherols (containing equal amounts of alpha, beta, delta, and gamma tocopherols), dimethylpolysiloxane (DMPS), lecithin, and cold-pressed sesame oil were acquired from different companies.
Chemicals and standards
All chemicals, reagents, and solvents used in the study were of gas chromatography (GC) and high performance liquid chromatography (HPLC) grade. Hexane, acetone, acetonitrile, methanol, phosphoric acid, and potassium hydroxide (KOH) were purchased from Merck (Darmstadt, Germany) and Sigma Aldrich (Buchs, Switzerland). Pure standards of tocopherols (DL-α-tocopherol, (+)-β-tocopherol, (+)-δ-tocopherol, and (+)-γ-tocopherol; 99%, HPLC purity) and FA standards mixtures (G003886 SUPELCO), were obtained from Sigma Chemical Co. (St. Louis, MO).
Experimental Design Study for Preparing the Frying CSO Blends
Food additives; ascorbic palmitate (A), mixed tocopherols (B), DMPS (C), lecithin (D), and cold-pressed sesame oil (E), were added into the refined CSO immediately prior to frying operations according to experimental design study. A Q-FFD with two levels and five factors was used to estimate the impact of additives on tocopherols and FAC changes during the deep-frying process. Five variables would give 25 = 32 training data set; however, a two-level and five factors Q-FFD corresponds to 25/4 = 8 training data set (frying CSO blends). The experimental design of the present study and level of food additives are shown in . In experimental design table, five factors; ascorbic palmitate (A), mix tocopherol (B), DMPS (C), lecitin (D), and cold-pressed sesame oil (E) were varied at two levels, a high (+1) and low (–1) level, and the levels were assigned according to European unity statute and Turk nutrient codex.
TABLE 1 Q-FFD factorial design and concentration levels of the additives for the CSO blends
Frying Procedure
The frying experiments were conducted using 2 L capacity of Tefal Oleoclean fryer (Rumilly, France). Frying procedures were carried out at 170 ± 5°C for 10 h continuously in a day. During the frying period, no additional fresh CSO was added. When the required temperature was reached, 8% frozen French fries/oil were introduced into hot oil and fried for 8 min. During the frying process, the lid of the fryer was closed while the basket was immersed into the hot oil. After 8 min, fried French fries were removed and the frying operation was continued for a new potato sample. With eight different frying CSO blends and one refined CSO, a total of nine frying experiments were carried out for 10 h without interruption. In order to determine the frying performance of CSO blends, 50 mL of oil was taken after each hour and cooled at room temperature. The frying procedure was performed according to the previous study.[Citation19] The samples were stored in a refrigerator in brown glass-stoppered flasks to avoid further chemical changes and filtered with 0.45 µm pore size filter before chromatographic analyses.
Analytical Methods
Determination of FAs by gas chromatography-flame ionization detector (GC-FID)
Analysis of FAC was carried out by Agilent 6890 series GC system (Agilent Technologies, USA) fitted with a capillary column packed with 100% cyanopropyl methyl polysiloxane (Supelco SP-2380 model, 60 m × 250 µm × 0.2 µm i.d.; Bellefonte, PA, USA) and equipped with a flame ionization detector. Before FAC analyses, oil samples were converted to fatty acid methyl esters (FAMEs). In brief, 0.1 g of the frying oil sample was weighed in a sample tube and dissolved in 10 mL hexane. Then 1 mL of 2 N potassium hydroxide in methanol was added and shaken for one minute before the centrifugation procedure. After centrifugation, the clear supernatant was transferred to a GC auto-sampler vials.
One μL FAMEs were injected into the GC-FID system using an auto-sampler with a split ratio of 100:1. The oven initial temperature was set at 50°C for 2 min and then increased at a rate of 4°C/min up to 240°C, where it was held for 10 min. Both the injector and the detector temperatures were set at 250°C. The flow rate of carrier gas (hydrogen) and make-up gas (nitrogen) was set at 1 mL/min–1. The data were recorded using the Agilent ChemStation data processor. FAMEs peaks were identified by comparison with retention times of known standards (Sigma Chemical Co.) and quantification was determined as the percent area of each peak relative to the sum of all peak areas. All analyses were conducted in duplicate and results are provided as average values.
Determination of tocopherol by high performance liquid chromatography-fluorescence detector (HPLC-FLD)
The contents of tocopherol isomers (α-, β-, γ-, and δ-tocopherols) in oil samples were determined directly by reversed-phase HPLC technique. Tocopherols were analyzed by Agilent 1200 Series HPLC system (Agilent Technologies, USA) equipped with AGT-G1354A model quaternary pump with degasser, AGT-G1321A model fluorescence detector (FLD) with a xenon discharge lamp (λEx: 295 nm, λEm: 330 nm), AGT-G1316B model thermostatted column compartment and AGT-G1328B model manual injector systems.
By this procedure, the 1 g of frying oil samples and 10 mL of acetone (HPLC grade) were added into the test tube and shaken for 30 s. After shaking, the samples were filtered with 0.45 µm pore size, using a 20 µL manual loop system. Finally, the samples were injected into the HPLC-FLD system directly. Chromatographic separation of tocopherol was achieved by Hypersil ODS 5 μm, 250 mm × 4.6 mm i.d., (Hewlett-Packard, USA) column. A mobile phase (48% MeCN-48% methanol-4% water) containing 0.2 mL phosphoric acid was used at the rate of 1.5 mL/min–1. All data were collected and recorded by using of Agilent ChemStation 2001–2008 data processor. Tocopherols were identified by comparing their retention times with those of pure standards of α-, β-, γ-, and δ-tocopherols, and were quantified on the basis of the peak area of the standards using calibration curves and results were expressed in parts per million (ppm). The analysis was performed in triplicate for each sample and the average values were reported.
Statistical Analysis
The results were expressed as the means of three determinations and the standard deviations. The statistical significant differences were obtained at a p < 0.05. The data were collected and recorded by using Agilent ChemStation 2001 and 2008 data processors and the statistical evaluation of the results was carried out using Microsoft Excel, 2007. Significant differences were identified between the additives using analysis of variance (ANOVA) and F-tests for multiple comparisons at 95% confidence level.
RESULTS AND DISCUSSION
Changes in FAC of the Frying CSO Blends
In order to evaluate the quality of frying fats and oils, oxidative stability is an important parameter. The oxidative stability of frying oils is also related to their FA profiles and the degradation of USFAs take place at higher temperature and longer frying time.[Citation4] In this study, different CSO blends were prepared with different concentration of ascorbic palmitate (A), tocopherols (B), DMPS (C), lecithin (D), and cold-pressed sesame oil (E) to check the stability of frying CSO. The FAC of CSO is appropriate in terms of health benefits when used for frying applications CSO has significant level of PUFAs (54.01%) including linoleic (18:2Δ9c,12c, omega-6) and linolenic (18:3Δ9c, 12c, 15c, omega-3) acids. The sum of monounsaturated and SFAs were found to be 19.92 and 23.75%, respectively. Monounsaturated includes oleic acid (18:1Δ9c), while SFAs include myristic (C14:0), palmitic acid (16:0), and stearic acid (C18:0) as shown in . FAC of deodorized CSO obtained in the present study lies within the official as specified in the Turkish Standard Codex TS 887; ICS 67.200.10.[Citation22] Olive oil could be used for frying, as it is more stable at frying temperatures, but the high cost restricts for frying applications.[Citation23] In recent years, to improve oxidative stability and healthier properties, many efforts have been made in European countries to develop new frying oil formulations using several additives.[Citation4]
TABLE 2 Fatty acid composition analyses results for frying CSO blend oils during frying process (%w/w)
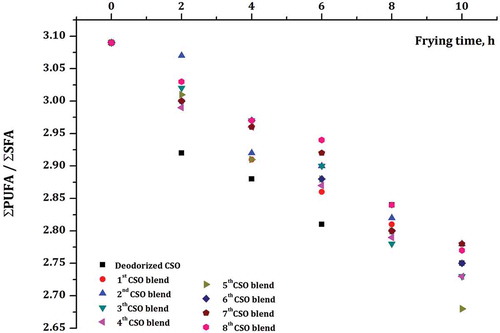
The changes in FAC of the developed new frying CSO blends were monitored during deep-frying process and data shown in . During the frying applications, a significant decrease was observed for the content of linoleic and linolenic acids, and also a distinct increase was observed for the content of TFAs. The total SFAs (myristic, palmitic, stearic, and arachidonic acids) were increased from 23.57 to 26.10% from fresh (deodorized CSO) to 10th h (8th blended) oils. Whereas, PUFAs were decreased from 54.27% (fresh oil from deodorized CSO) to 50.77% (10 h from 8th blended) during the continuous frying processes. Therefore, ratio of total PUFAs to SFAs (∑PUFA/∑SFA) was decreased from 3.09 to 2.77 from fresh (deodorized CSO) to 10th h (8th blended) frying period as shown in and . shows the calculated oxidizability (Cox) values of the frying CSO blends and this was computed by the percentage of unsaturated C18 FAs by applying the Eq. (1) as proposed by Fatemi and Hammond,[Citation24] and for all CSO blends. A linear decrease of Cox values was observed with the frying time.
shows the oxidation of FAs increases with the decrease of unsaturation in the frying CSO samples. The ratio of linoleic/linolenic (PUFA) to palmitic acid (SFA) is measured to be applicable indicators of the level of deterioration.[Citation25,Citation26] In , results show a significant decrease of ∑PUFA/∑SFA ratio at 5th and minimum at 2nd and 7th frying oil blends from 3.09 to 2.68% and 3.09 to 2.78%, respectively, during frying at 170°C. Cox values (the index of the oil oxidation stability) showed good correlation with ∑PUFA/∑SFA ratios. Among the CSO blends, the 2nd and 5th frying oil blend showed the highest and lowest Cox value from 5.80 to 5.49% and 5.80 to 5.35%, respectively, at the end of the 10 h frying process. During repetition of the frying process and high temperature, the amount of TFAs was increased. In frying oil blends, the TFAs as linoelaidic acid (18:2Δ9c, 12t, and 18:2Δ9t, 12c) were identified and increased from 0.26 (fresh deodorized CSO) to 0.57% (10th hour from 8th blended) oils. The results of the reported studies shows that prolonged frying process at high temperature may increase the TFAs concentration[Citation20,Citation21] and supported the outcome of present work. clearly shows that increased the amounts of TFAs and decreased the values of total tocopherols were observed during frying process. Several researchers have investigated the relationship between TFA intake and coronary heart disease risks. Therefore, the maximum allowable limit (MLA) of TFAs in European countries is <1%.[Citation27]
In the present study, the correlation between the changes in FAC and tocopherol content, concentration and kind of additives in frying oil blended samples by chemometric approach,[Citation28] was established by using Q-FFD. The analyzed data of the results (chemometric approaches, study of the regression models, etc.) was performed using Excel (Microsoft, 2007) database. Regression analysis was performed, based on the experimental data, and was fitted into an empirical second order polynomial model as shown below in Eq. (2);
TABLE 3 Probability and effect values of the additives for fatty acid composition and tocopherol analyses
FIGURE 2 Normal distribution curve of the CSO blends for (a) fatty acid composition and (b) tocopherol analysis with the effects of different additives; ascorbic palmitate (A), mixed tocopherols (B), dimethylpolysiloxane (DMPS) (C), lecithin (D), cold-pressed sesame oil (E).
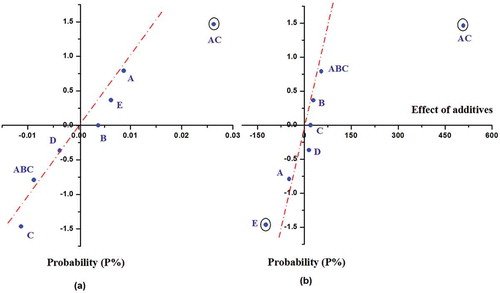
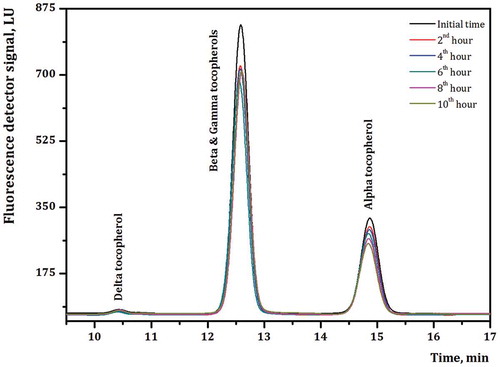
FIGURE 3 Tocopherol composition chromatograms obtained from HPLC-FLD system for frying deodorized CSO analyses.
In order to evaluate the impacts of the additives on the frying performance of CSO blends, the ∑PUFA/∑SFA data, obtained from the beginning and end of the frying process were utilized as response factors. Regression coefficient matrix was composed for each CSO blends using response factor data. The results of the empirical second-order polynomial model (theoretical predicted values) were given for FAC analysis by following Eq. (3) and the quality of the fitted model’s statistical significance was checked by an ANOVA test.
To evaluate the impacts of additives, ND curves were used. The curves for FAC and tocopherol analyses were plotted () using probability values (P) calculated by Eq. (4), where q is the ranking order of the effect and n is the number of factors, and data of regression coefficient matrix given in .
The ND graph for FAC analyses (), showing the impacts of additives as a point form, the spots directly away from the linear line are thought to be more effective on the changes of FA profile in terms of ∑PUFA/∑SFA values during the frying process. The more points directly away from the linear line, and showed the effects of these changes. In present study, the impacts of C (DMPS), AC (Ascorbic palmitate × DMPS) and ABC (Ascorbic palmitate × Mix Tocopherol × DMPS) factors, points directly away from the linear line, were evaluated to be compatible with the polynomial model Eq. (3), for the changes in the ∑PUFA/∑SFA values. The effect of the “AC” factor was +0.026 × 2 = +0.052 unit and this positive effect represents that “AC” factor has protective effect according to the average decrease (–2.749 × 2 = –5.498 unit) of the ∑PUFA/∑SFA value. In order to verify of ND results, ANOVA test was applied to all data obtained from FAC analyses at 95% confidence level as given in supplemental . As can be seen from supplemental , the ANOVA test was in agreement with the ND graph taking consideration of the Fcritical value for the impact of the A × C factors. The ANOVA test results of the FAC also indicated the impact of AC (Ascorbic palmitate × DMPS) factor is significant.
Changes in Tocopherol Profile of the Frying CSO Blends
Indeed, the FAC alone may not adequately explain the oxidative stability and quality of frying oils. In other words, oxidative stability of the frying oils depends on their FAC and the content of unsaponifiable minor components, especially tocopherol content.[Citation29] Tocopherols are significant minor constituents in vegetable oils, acting as natural antioxidants and considered to be responsible to control the rate of oxidative degradation. Antioxidant activity of tocopherols is related with number of mechanisms, including scavenging active oxygen species and free radicals, and through action as efficient chain terminators in auto-oxidation reactions.[Citation30]
Several researchers reported that the addition of the food additives especially antioxidants significantly improved the oxidative stability of the frying oil and extended the usage life of the frying oils.[Citation9,Citation29,Citation31] The changes in tocopherol content of the frying CSO blends, including additives in different concentrations, during deep-frying process are presented in , the level of ∑ tocopherols per 1000 g oils studied. For all frying CSO blends, a linear increase of the loss in total tocopherol contents with frying time was observed. After 10 h frying process, it could be noticed () that there were significant losses of tocopherols. It has been already reported that oils heated at frying temperatures degrade more quickly in the presence of more oxygen and water and in absence or low level of tocopherols.[Citation32] At the end of the frying processes, the total tocopherol contents of the frying CSO blends are shown in . The highest tocopherol loss was detected in the 6th CSO blend (66.53%) the lowest was in 7th CSO blend (14.21%). When the importance of tocopherols as antioxidants in higher levels was considered, the 6th CSO blend was expected to exhibit greater oxidative stability compared to the other CSO blends. Slow degradation of tocopherols can improve the storage stability of fried food products and present better nutritional values.
TABLE 4 Tocopherol analyses results for frying CSO blend oils (∑ tocopherol, mg/kg oil)
To establish the impacts of the additives on the frying performance of CSO blends, the tocopherol analyses results, HPLC-FLD system data, obtained from the beginning and end of the frying process were utilized as response factors, ∑ tocopherol values. Regression coefficient matrix was composed for each CSO blends using response factor data. The results of the polynomial model were presented for tocopherol analysis by following Eq. (5) and to evaluate the impacts of additives, ND curves were used. The curves for tocopherol analyses were plotted ().
The ND graph for tocopherol analyses (), the spots directly away from the linear line are thought to be more effective on the changes of tocopherol content during the frying processes. In the present research, the impacts of A (Ascorbic palmitate), E (mix Tocopherol × DMPS) and A × C (Ascorbic palmitate × DMPS) factors were evaluated and found to in good agreement with the polynomial model Eq. (5) for the changes in the ∑ tocopherol value. The effect of “E” factor was –122.710 × 2 = –245.420 unit and this negative effect represents that “E” factor has a precipitating effect for the average decrease (–1380.670 x 2 = –2761.34 unit) of ∑ tocopherol content. Whereas, the effect of “A × C” factor was +508.363 × 2 = +1016.726 unit and this positive effect represents that “A × C” factor has a protective effect according to the average decrease.
At the same time, the quality of the fitted model’s statistical significance was checked by an ANOVA test in supplemental . ANOVA test was in good agreement with the ND graph, taking consideration of the Fcritical values, for the impact of the E and A × C factors. The ANOVA test results of the tocopherol analyses also indicated that, the impact of E and A × C factors is significant with 13.7810 and 236.5199 Fcritical values, respectively.
CONCLUSION
The present study determines the real impacts of additives (ascorbic palmitate, mix tocopherol, DMPS, lecithin, and cold-pressed sesame oil) on FAC and total tocopherol content of eight CSO blends using a chemometric design. The results of present study indicated that stability of commercial frying CSO could be enhanced by adding ascorbic palmitate with DMPS (A × C) additive by Q-FFD approaches. The ANOVA tests are in good agreement with the ND graphs and the data indicated that A × C factor showed a protective effect on FAs. Meanwhile, the impacts of sesame oil (E) and A × C factor were found to be contained precipitating and protective effects to the average decrease of total tocopherol content. Consequentially, the advancement of knowledge could be applied to prepare a more stable blend of frying oil with respect to FAC and tocopherols with the addition of additives. Reliable data could be developed for the help of processors, policy makers, and consumers.
NOMENCLATURE
| CSO: | = | cottonseed oil |
| Q-FFD: | = | quarter-fractional factorial design |
| DMPS: | = | dimethylpolysiloxane |
| ND: | = | normal distribution |
| ANOVA: | = | one way-analysis of variance |
| FAs: | = | fatty acids |
| FAC: | = | fatty acid composition |
| SFAs: | = | saturated fatty acids |
| USFAs: | = | unsaturated fatty acids |
| MUFA: | = | monounsaturated fatty acids |
| PUFAs: | = | polyunsaturated fatty acids |
| α-, β-, γ-, and δ-Toc: | = | alpha, beta, gamma, and delta tocopherols |
| FAMEs: | = | fatty acid methyl esters |
| GC-FID: | = | gas chromatography-flame ionization detector |
| HPLC-FLD: | = | high performance liquid chromatography fluorescence detector |
| ∑PUFA/∑SFA | = | total polyunsaturated to saturated fatty acids |
ACKNOWLEDGMENTS
The authors acknowledge the Industry and Trade Ministry of Research and Development Center, Selcuk University Coordinators of Scientific Research, Helvacizade Edible Oil Company, and TUBITAK fellowship programs, 2221 and 2216, for foreign citizens. The authors are also thankful to Dr. Huseyin Kara, who provided facilities and good environment for the research work at chemistry department, Selcuk University, Konya, Turkey.
FUNDING
The present study is a part of master thesis entitled Increasing Usage Efficiencies of Cottonseed Oils Produced in Our Country by Improving Their Refinery Process was supported by Republic of Turkey Industry and Trade Ministry of Research and Development Centre with San-Tez Projects-150.STZ.2007-2 project number and Selcuk University Coordinators of Scientific Research with SU-08201044 and SU-08401007 project numbers.
Additional information
Funding
REFERENCES
- Çaglar, A.; Duman, E.; Ozcan, M.M. Effects on edibility of reused frying oils in the catering industry. International Journal of Food Properties 2012, 15, 69–80.
- Nasirullah; Rangaswamy, B.L. Oxidative stability of healthful frying oil medium and uptake of inherent nutraceuticals during deep frying. Journal of the American Oil Chemists’ Society 2005, 82, 753−757.
- Andrikopoulos, N.K.; Dedoussis, G.V.Z.; Falirea, A.; Kalogeropoulos, N.; Hatzinikola, H.S. Deterioration of natural antioxidant species of vegetable edible oils during the domestic deep-frying and pan-frying of potatoes. International Journal of Food Sciences and Nutrition 2002, 53, 351–363.
- Choe, E.; Min, D.B. Mechanisms and factors for edible oil oxidation. Comprehensive Reviews in Food Science and Food Safety 2006, 5, 169–186.
- Che Man, Y.B.; Jaswir, I. Effect of rosemary and sage extracts on frying performance of refined, bleached, and deodorized (RBD) palm olein during deep-fat frying. Food Chemistry 2000, 69, 301−307.
- Barrera-Arellano, D.; Ruiz-Me´ndez, V.; Marquez Ruiz, G.; Dobarganes, C. Loss of tocopherols and formation of degradation compounds in triacylglycerol model systems heated at high temperature. Journal of the Science of Food and Agriculture 1999, 79, 1923−1928.
- Jaswira, I.; Che Man, Y.B.; Kitts, D.D. Optimization of physicochemical changes of palm olein with phytochemical antioxidants during deep-fat frying. Journal of the American Oil Chemists’ Society 2000, 77, 1161−1168.
- Verleyen, T.; Kamal-Eldin, A.; Mozuraityte, R.; Verhé, R.; Dewettinck, K.; Huyghebaert, A.; Greyt, W.D. Oxidation at elevated temperatures: Competition between α-tocopherol and unsaturated triacylglycerols. European Journal of Lipid Science and Technology 2002, 104, 228–233.
- Aladedunye, F.A.; Przybylski, R. Protecting oil during frying: A comparative study. European Journal of Lipid Science and Technology 2009, 111, 893–901.
- Matthäus, B. Utilization of high-oleic rapeseed oil for deep-fat frying of French fries compared to other commonly used edible oils. European Journal of Lipid Science and Technology 2006, 108, 200–211.
- Steel, C.J.; Dobarganes, M.C.; Barrera-Arellano, D. The influence of natural tocopherols during thermal oxidation of refined and partially hydrogenated soybean oils. Grasas Aceites 2005, 56, 46−52.
- Holownia, K.I.; Erickson, M.C.; Chinnan, M.S.; Eitenmiller, R.R. Tocopherol losses in peanut oil during pressure frying of marinated chicken strips coated with edible films. Food Research International 2001, 34, 77–80.
- Lukonge, E.; Labuschagne, M.T.; Hugo, A. The evaluation of oil and fatty acid composition in seed of cotton accessions from various countries. Journal of the Science of Food and Agriculture 2007, 87, 340–347.
- Kamal-Eldin, A. Effect of fatty acids and tocopherols on the oxidative stability of vegetable oils. European Journal of Lipid Science and Technology 2006, 108, 1051–1061.
- Gunstone F.D., Vegetable Oils in Food Technology; Blackwell Press: Oxford, UK, 2002; p. 1−337.
- Lee, J.H.; Pangloli, P. Volatile compounds and storage stability of potato chips fried in mid-oleic sunflower oil. International Journal of Food Properties 2013, 16, 563–573.
- Daniel, D.R.; Thompson, L.D.; Shriver, B.J.; Wu, C.K.; Hoover, L.C. Non hydrogenated cottonseed oil can be used as a deep fat frying medium to reduce trans-fatty acid content in french fries. Journal of the American Dietetic Association 2005, 105, 1927−1932.
- Houhoula, D.P.; Oreopoulou, V.; Tzia, C. The effect of process time and temperature on the accumulation of polar compounds in cottonseed oil during deep-fat frying. Journal of the Science of Food and Agriculture 2003, 83, 314−319.
- Arslan, F.N.; Kara, H.; Ayyildiz, H.F.; Topkafa, M.; Tarhan, I.; Kenar, A. A chemometric approach to assess the frying stability of cottonseed oil blends during deep-frying process: I. Polar and Polymeric Compound Analyses. Journal of the American Oil Chemists’ Society 2013, 90, 1179–1193.
- Gertz, C. Chemical and physical parameters as quality indicators of used frying fats. European Journal of Lipid Science and Technology 2000, 102, 566–572.
- Uluata, S.; Ozdemir, N. Antioxidant activities and oxidative stabilities of some unconventional oilseeds. Journal of the American Oil Chemists’ Society 2012, 89, 551–559.
- Edible cottonseed oils specified in the Turkish Standard Codex TS 887; ICS 67.200.10.
- Ramadan, M.F.; Wahdan, K.M.M. Blending of corn oil with black cumin (Nigella sativa) and coriander (Coriandrum sativum) seed oils: Impact on functionality, stability, and radical scavenging activity. Food Chemistry 2012, 132, 873–879.
- Fatemi, S.H.; Hammond, E.G. Analysis of oleate, linoleate, and linolenate hydroperoxides in oxidized ester mixtures. Lipids 1980, 15, 379−385.
- Houhoula, D.P.; Oreopoulou, V.; Tzia, C. A kinetic study of oil deterioration during frying and a comparison with heating. Journal of the American Oil Chemists’ Society 2002, 79, 133–137.
- Onal, B.; Ergin, G. Antioxidative effects of alpha-tocopherol and ascorbyl palmitate on thermal oxidation of canola oil. Nahrung 2002, 46, 420–426.
- Medina-Juárez, L.A.; Gámez-Meza, N.; Ortega-García, J.; Noriega-Rodriguez, J.A.; Angulo-Guerrero, O. Trans fatty acid composition and tocopherol content in vegetable oils produced in Mexico. Journal of the American Oil Chemists’ Society 2000, 77, 721–724.
- Lundstedt, T.; Seifert, E.; Abramo, L.; Thelin, B.; Nyström, Å.; Pettersen, J.; Bergman, R. Experimental design and optimization. Chemometrics and Intelligent Laboratory Systems 1998, 42, 3−40.
- Abdalla, A.E.M. Antioxidative effect of olive oil deodorizer distillate on frying oil and quality of potato chips. Lipid/Fett 1999, 101, 57–63.
- Kamal-Eldin, A.; Andersson, R. A multivariate study of the correlation between tocopherol content and fatty acid composition in vegetable oils. Journal of the American Oil Chemists’ Society 1997, 74, 375−380.
- Gertz, C. Optimising the baking and frying process using oil-improving agents. European Journal of Lipid Science and Technology 2004, 106, 736–745.
- Juáreza, M.D.; Osawa, C.C.; Acuña, M.E.; Sammán, N.; Gonçalves, L.A.G. Degradation in soybean oil, sunflower oil, and partially hydrogenated fats after food frying, monitored by conventional and unconventional methods. Food Control 2011, 22, 1920–1927.