Abstract
In the current study, the effect of air exposure to the gelatin solution on improvement of gel structure was investigated in terms of the steady and dynamic shear rheological properties. Prepared gelation solution (5% w/v) was covered to prevent air incorporation and it was subjected to 5 h gelatin and rheological analyses were carried out for the comparison of non-covered ones. It was observed that the preventing of air into the gelatin solution affected the rheological parameters. Apparent viscosity and complex viscosity values of samples increased during gelation and these values were measured to be tremendously high (1.894 and 8.346 Pa s, respectively) in non-covered gelatin solution while they were 0.474 and 1.611 Pa s in covered samples after 5 h gelation, respectively. Similarly, storage modulus (G′) of samples increased with the increase in gelation time and it was recorded to be 52.203 Pa in gelatin solution exposed to air while it was 9.848 Pa in gelatin solution covered to prevent air incorporation. These results showed importance of air in gelatin solution to the food industry using gelatin in food formulation for the structure of processed foods.
Keywords:
Introduction
Gelatin is a stabilizer derived from the ubiquitous collagen protein by acid or alkaline hydrolysis. A number of studies have been devoted to the processing of gelatin from bone, sinew, skin, or cartilage of animals.[Citation1–Citation3] It is a collagen based protein and collagen molecule, composed of three α-chains intertwined in the so-called collagen triple-helix, is approximately 300 nm in length, and has average molecular weight of 105 kDa.[Citation4] During the collagen denaturation, this triple-helix conformation is lost and depending on hydrogen bonds, destruction of polymers transforms coiled form.[Citation4] A progressive increase occurs in gel strength of gelatin gels on cooling to below 30°C[Citation5] and gelatin starts to melt because of the dissociation of triple helices as the temperature is increased above 35°C[Citation6] Due to its unique rheological properties, gelatin is widely used in the food, photographic, cosmetic, and pharmaceutical industries as a stabilizer, emulsifier, texturizer, and gelling agent.[Citation7] The worldwide production of gelatin derived from pig-skin (46%), bovine (29.4%), bones (23.1%) and other sources (1.5%) is about 326,000 tons per year and it gradually raises year by year.[Citation8] In the food industry, gelatin is used as texturizer and chewiness provider for confections, emulsifier and stabilizer for baked foods, hydrocolloid, and viscosity improver for meat and dairy products.[Citation2] The quality of food-grade gelatin mostly depends on its gelling ability, affected by concentration, pH, presence of interacting compounds, gel maturation time, and temperature.[Citation9] The gelling properties of gelatin are also influenced from the content of certain amino acids present in the structure. Proline and hydroxyproline are major amino acids that are responsible for melting and gelling temperatures of different sources of gelatin. A decrease in proline and hydroxyproline level in the composition of gelatin causes lower melting and gelling temperatures.[Citation10] According to Burjanadze,[Citation11] total Gly-Pro-Hyp sequence is the main factor for the thermal stability of collagen. The ability of gelation and creation of strong gel structure of gelatin provide unique usage area for it. For that reason, gelling performance of the gelatin is very important criteria in the food formulations. During the experiments with gelatin solution performed by our research group, it was observed that the gel strength of gelatin was significantly affected from the air incorporation into the solution. Because of these surprising differences, this study was conducted to see the effect of air. The main aim of the current study was to investigate the effect of air exposure to the gelatin solution on the gel strength in terms of steady and dynamic shear rheological characterization. This is most probably the first report regarding the air and gelatin interaction in the literature.
Material and Methods
Physicochemical Analysis
Beef gelatin was used in the study. pH value, moisture, protein, and ash contents of the gelatin sample were determined as outlined in AOAC.[Citation12] The pH value of the sample was determined with a pH meter (WTW 315i set model, Weilheim, Germany). Protein content of the gelatin was determined using an automatic nitrogen analyzer (FP 528 LECO, ABD) based on the Dumas method. aw value of the sample was determined using an aw-meter (AquaLab, 2.0, Decagon, USA). The color values of the sample were measured by a colorimeter (Lovibond RT Series Reflectance Tintometer, England). The L (brightness), a (± red–green) and b (± yellow–blue) color coordinates were recorded according to the CIELab color space system.
Steady Shear Rheological Analysis
Steady shear rheological characteristics of gelatin solution (5% w/v) were determined using a controlled stress rheometer (THERMO-HAAKE, RheoStress 1, Karlsruhe Germany) equipped with a temperature control unit (Haake, Karlsruhe K15 Germany) and a plate-plate configuration (plate diameter 35 mm and gap size 0.500 mm). Measurements were carried out in the shear rate range of 5–100 s–1 at constant temperature (25ºC). Sample was placed between the plate—plate geometry and the measurement was started immediately. Total 25 data points were recorded at 10 s intervals during the shearing. Each measurement was replicated four times on four different samples from the same gelatin solution with two repetitions. The apparent viscosity was measured as a function of shear rate. Recorded data were fitted to Oswald de Waele model using RheoWin Data Manager (RheoWin Pro V. 4.0, HAAKE, Karlsruhe, Germany) and consistency coefficient (K) and flow behavior index (n) values were calculated according to the model used to describe shear-induced behavior of the gelatin solution as follows:
Dynamic Shear Rheological Analysis
Dynamic shear rheological characteristics of gelatin solutions were determined using a strain/stress controlled rheometer (Thermo-HAAKE, Rheostress 1, Karlsruhe, Germany) equipped with a temperature-control unit (Thermo HAAKE, Karlsruhe K15 Germany). The measurements were carried out using a plate-plate configuration with a plate diameter of 35 mm and a gap of 0.500 mm. Sample was placed between the plate-plate geometry and the measurement was started immediately. Before starting to the frequency sweep tests, stress sweep test was applied to see the linear viscoelastic region (LVR) of gelatin solution (5% w/v). Then, frequency sweep test was conducted for all samples using a dynamic oscillatory shear rheometer. Dynamic shear measurements were performed in the frequency range of 0.1–10 Hz at a constant shear stress (0.2 Pa) in the LVR and constant temperature (25°C). Dynamic shear values recorded at the frequency values ranging between 0.1–10 Hz were used to evaluate the dynamic mechanical spectra of the samples. Each measurement was replicated three times on three different samples from the same solution with two repetitions.
The dynamic mechanical spectra parameters of G′ (elastic or storage modulus) and G′′ (viscous or loss modulus) were calculated using the following equations:[Citation13]
Loss tangent which is a dimensionless number giving a clear indication of whether the material behavior is solid-like or liquid-like, was determined using the following equation.[Citation14]
Equations of complex modulus G* and complex viscosity η* as following were used to characterize the overall response of the sample against to the sinusoidal strain:
Statistical Analysis
All statistical calculations were carried out using the Statistical Analysis System (SAS) Software.[Citation15] One-way analysis of variance (ANOVA) was applied using the general linear model procedure. Duncan multiple range test was used to show the differences among mean values with the significance level of 0.05.
Results and Discussion
Physicochemical Properties of Gelatin
Physicochemical analysis showed that gelatin had 0.710% ash, 8.328% moisture, and 88.203% protein. pH value of gelatin solution (1% w/v) was determined to be 5.383 and aw value of the sample was recorded to be 0.408. L, a, and b values of the gelatin powder were determined to be 73.362, 2.965, and 29,114, respectively. Binsi et al.[Citation16] reported that the moisture and protein contents of fish skin gelatin were 4.2 and 94.6%, respectively. They also informed that the pH value of the gelatin solution (1% w/v) was 6.4. In another study, moisture and protein content of gelatin were reported to be 4.52 and 92.31%, respectively.[Citation17] Badii and Howell[Citation18] conducted a research on structure and gelling properties of fish gelatin and they reported the moisture level and pH value to be 12.1% and 6.0, respectively.
Steady Shear Rheological Properties of Gelatin Solutions
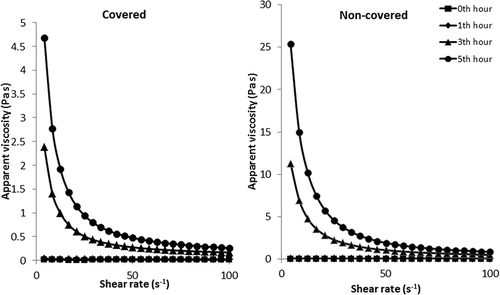
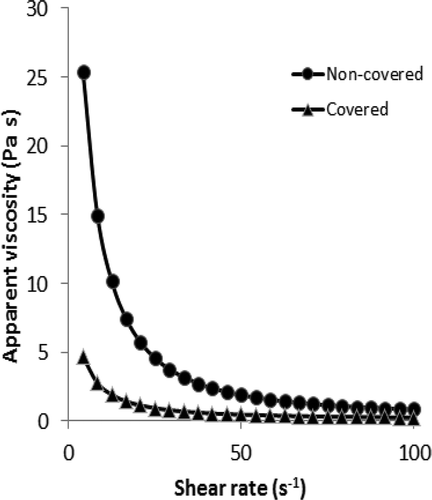
shows the steady shear rheological parameters of gelatin solution which is covered or non-covered during gelation. It can be clearly seen from the table that the gelation of gelatin needs certain time. Steady shear rheological parameters of samples increased with gelation time. Surprisingly, covering of solution to prevent the exposure of air into the solution significantly affected the rheological properties of the final solution. Apparent viscosity at 50 s–1 values of gelatin solution which is covered with a stretch wrap (5% w/v) was determined to be 0.008 Pa s at the beginning, 0th hour. After 1 h, apparent viscosity at 50 s–1 started to increase rapidly and it was recorded as 0.025 and 0.275 Pa s after 1 and 3 h, respectively, for covered gelatin solution. Apparent viscosity values at 50 s–1 were determined to be tremendously high in non-covered gelatin solution. As can be clearly seen from , apparent viscosity value of non-covered gelatin solution at 50 s–1 (5% w/v) was measured to be 0.049 after 1 h gelation. Approximately, two fold difference was observed between the apparent viscosity values of covered and non-covered gelatin solution after 1 h gelation. Similar to the covered sample, apparent viscosity at 50 s–1 increased during the gelation in non-covered sample, but the increase in viscosity was quite higher than that of the covered sample. Apparent viscosity of non-covered sample at 50 s–1 reached to 1.894 Pa s after 5 h gelation while the apparent viscosity value of covered gelatin solution was 0.474 Pa s. The steady shear rheological analysis showed that the preventing of air to come contact with gelatin solution sample results a significant decrement in the improvement of the gelation in gelatin solution. The gelatin solution which is gaped during the stored time gelled rapidly compared to non-gaped gelatin sample. Marcotte et al.[Citation19] reported that the viscosity of gelatin at 4% (w/v) concentration was measured to be 0.017 Pa s at 20ºC and 0.0050 Pa s at 60ºC. Oswald de Waele model was used to describe the effect of shear rate on apparent viscosity and the model parameters were tabulated in . Similar to the apparent viscosity, consistency coefficient increased tremendously during the gelation. And also, preventing the air exposure into the solution affected the Oswald de Waele parameters. Consistency coefficient was determined to be 0.006 Pa sn at the beginning of the gelation and it increased rapidly during the gelation. After 5 h gelation, it reached to 14.34 and 81.24 Pa sn for covered and non-covered samples, respectively. The increase in consistency coefficient caused a decrease in the flow behavior index values. It was recorded to be higher than unity but the increase in the gelation time decreased the flow behavior index values of sample due to the decrement in the fluidity and increment in the elasticity of gelatin solution sample. shows the rheological behavior of gelation solution. As is seen from the figure, the flow behavior of gelatin solution was quite close to Newtonian at the beginning of the gelling, but after that, with the increase in duration, the solutions started to behave like non-Newtonian. The flow behavior index values decreased tremendously with the increase in gel strength. Marcotte et al.[Citation19] reported that the gelatin solution (2% w/v) showed Newtonian behavior. However, Binsi et al.[Citation16] investigated rheological and functional properties of gelatin from the skin of the Bigeye snapper and they reported the flow behavior of gelatin as non-Newtonian. So, it could be said that the rheological behavior of gelatin depends on the gelling property. Apparent viscosity of samples decreased with the increase in shear rate. illustrates the difference between the flow behavior of covered and non-covered gelatin solution. It is clear from the figure that the covering of sample decreased the apparent viscosity values during shearing of the sample in the range of 5-100 s–1. Oswald de Waele model was determined to be adequate to describe the effect of shear rate on apparent viscosity of gelatin solution. Coefficient of determination was found to be high (). Only, due to the improvement of the gelation in the non-covered sample, Oswald de Waele model could not describe the behavior of gelatin solution after 5 h gelation due to the increase in elasticity of the sample. Binsi et al.[Citation16] reported that Casson and Herschel–Bulkley models were suitable in predicting the flow behavior of gelatin solutions at various concentrations and temperatures and because they calculated a yield stress value for the gelation solution at various concentration. They calculated yield stress as 1.16 Pa for 30 mg/mL at 10°C and they observed that after this temperature, the viscosity as well as yield stress values became markedly time dependent owing to the degree of crosslinks formed during gelling process. Higher yield stress values at lower temperature levels indicated appearance of gel characteristics of gelatin.
TABLE 1 Steady shear rheological parameters of covered and non-covered gelatin solution during gelation+
Dynamic Mechanical Spectra of Gelatin Solutions
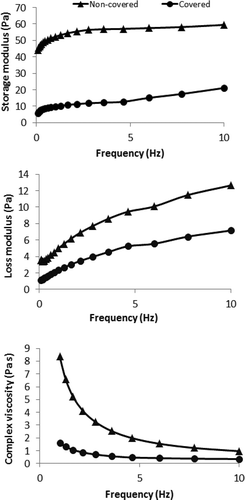
Due to the gelation of sample, it is thought that the determination of dynamic mechanical spectra of the gelatin solution during the gelation is required for the well-established characterization. illustrates the dynamic mechanical parameters of gelatin solutions. Storage modulus (G′) and loss modulus (G′′) of covered and non-covered samples generally increased with the increasing frequency. Similar to the steady shear rheological parameters, dynamic mechanical shear parameters were significantly affected from the air exposure. G′ and G′′ values of samples increased significantly because of the air during the gelation. The dynamic shear rheological parameters recorded at 1 Hz were tabulated in . As can be seen from , covering the sample to prevent the air exposure caused a significant change in the dynamic mechanical spectra of sample. Covering the sample decreased the improvement of gel structure significantly. Storage modulus was recorded to be 0.018 Pa at the beginning of the gelation. During the gelation, storage modulus increased significantly and it was recorded to be 4.890 and 9.848 Pa, for the gelation time of 3 and 5 h, respectively, in the covered gelatin solution. In the non-covered samples, storage modulus was determined to be significantly higher than that of covered samples. It was recorded to be 29.67 and 52.203 Pa in the non-covered sample after 3 and 5 h gelation time, respectively. Air provided a significant improvement in the gelation of gelatin. Similarly, loss modulus of samples increased during gelation of covered and non-covered samples and the high increase was recorded in the non-covered samples. After 5 h later, loss modulus of covered sample was determined to be 2.354 Pa while that of non-covered sample was 4.996 Pa. At the beginning of the gelation, loss modulus of sample was higher than storage modulus due to the liquid character of the sample, but after the duration for gelling, storage modulus was recorded to be significantly higher than loss modulus. Bulcke et al.[Citation20] reported that the gelation solution showed higher storage modulus than loss modulus and they stated that the mechanical spectra of the gelatin-based hydrogels are characteristic for a well-developed network. It was also reported that the sol state of gelatin can be represented by a rheological parameter function G′′ > G′, whereas the gel state is represented by G′ > G′′ due to an increased elasticity. So, the gelling point can be defined as a point of tan δ =1 (G′ = G′′).[Citation21,Citation22] According to this definition, gelling point of the gelatin sample was determined during 1th hour of gelation for non-covered sample. Badii and Howell[Citation18] reported that the storage modulus of gelatin solution at different concentrations was higher than loss modulus showing that the solid like behavior for the gelatin gel. Complex viscosity values of sample also increased during the gelation for both covered and non-covered samples. shows the change in complex viscosity values of samples in the frequency range of 1–10 Hz. As can be clearly seen from the figure, the complex viscosity decreased with the increase in frequency for all samples. For covered and non-covered gelatin solution samples, the increase in gelation time increased the complex viscosity of samples significantly (p < 0.05). The increase of parameters in covered samples was found to be significantly lower compared to that of non-covered samples (p < 0.05). The improvement on the gel structure was determined to be high in non-covered gelatin solution samples. Complex viscosity value was recorded as 0.018 Pa s at the beginning of the gelation. It is measured to be 0.039, 0.821, and 1.611 Pa s for the gelation durations of 1, 3, and 5 h in the covered gelatin solution, respectively. It was determined to be 0.364, 4.752, and 8.346 Pa s in non-covered sample. shows the effect of covering to prevent the air exposure to the gelatin in terms of the dynamic mechanical spectra of gelatin solution. Storage modulus, loss modulus, and complex viscosity values were determined to be tremendously higher after 5 h gelation in non-covered gelatin solution compared to covered one. Complex modulus which is a function of storage modulus and loss modulus was also measured to be significantly higher in non-covered sample (p < 0.05). It was recorded to be 52.443 Pa in non-covered samples after 5 h gelation while it was 10.127 Pa in covered sample. Loss tangent decreased significantly during the gelation of sample in both covered and non-covered sample but the lowest value was determined for non-covered samples during the gelation ().
TABLE 2 Dynamic mechanical spectra values of covered and non-covered gelatin solution during gelation+
FIGURE 3 Change in storage modulus, loss modulus, and complex viscosity values versus frequency of covered and non-covered gelatin solutions during gelation.
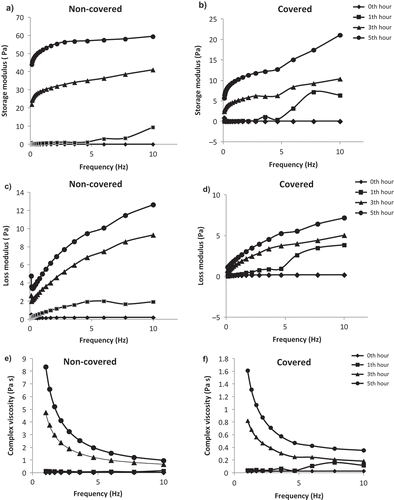
FIGURE 4 Differences between the storage modulus, loss modulus and complex viscosity values of covered and non-covered aqueous gelatin solution after 5 h gelation.
As a conclusion, gelatin is widely used in many food formulations in the food industry. It has significant functions in the food formulations namely absorbing the water, enhancing the stability and viscosity and creating a gel structure. This study reports the gelling ability of gelatin depending on the air exposure during the gelation. It can be clearly claimed that the gelation of gelatin accelerated due to the air incorporation. Steady shear and dynamic shear rheological parameters showed that the gelatin solution which is covered to prevent the air contact gelled slowly compared to sample which is non-covered. Air incorporation into the gelatin sample increased the gelation and apparent viscosity, complex viscosity and especially storage modulus of non-covered samples increased rapidly and tremendously. These results will have techno-functional and important effects in food formulations especially for the one stabilized with gelatin in the food industry.
References
- Gómez-Guillén, M.C.; Pérez-Mateos, M.; Gómez-Estaca, J.; López-Caballero, E.; Giménez, B.; Montero, P. Fish Gelatin: A Renewable Material for the Development of Active Biodegradable Films. Trends in Food Science and Technology 2009, 20, 3e16.
- Karim, A.A.; Bhat, R. (2009). Fish Gelatin: Properties, Challenges, and Prospects as an Alternative to Mammalian Gelatins. Food Hydrocolloids 2009, 23(3), 563e576.
- Gilsenan, P.M.; Ross-Murphy, S.B. Rheological Characterizations of Gelatins from Mammalian snd Marine Sources. Food Hydrocolloids 2000, 14,191e195.
- Papon, P.; Leblon, J.; Meijer, P.H.E. Gelation and Transitions in Biopolymers. In The Physics of Phase Transitions; Papon, P.; Leblond, J.; Meijer, P.H.E.; Ed.; Springer: Berlin, Heidelberg, 2007; 189–213.
- Sarbon, N.M.; Badii, F.; Howell, N.K. The Effect of Chicken Skin Gelatin and Whey Protein Interactions on Rheological and Thermal Properties. Food Hydrocolloids 2015, 45, 83–92.
- Fitzsimons, S.M.; Mulvihill, D.M.; Morris, E.R. Segregative Interactions Between Gelatin and Polymerized Whey Protein. Food Hydrocolloids 2008, 22, 485–491.
- Surh, J.; Decker, E.A.; McClements, D.J. Properties and Stability of Oil-In-Water Emulsions Stabilized by fish Gelatin. Food Hydrocolloids 2006, 20, 596–606.
- GME. (2008). Gelatin Manufacturers of Europe. Retrieved from http://www.gelatine.org/en/gelatine/ overview/127.htm ( accessed March 15, 2008).
- Johnston-Banks, F.A. Gelatin. In Food Gels; Harris, P.; Ed.; Elsevier Applied Science Publishers: London, 1990; 233–289.
- Haug, I.J.; Draget, I.K.; Smidsrod, O. Physical and Rheological Properties of fish Gelatin Compared to Mammalian Gelatin. Food Hydrocolloids 2004, 18, 203–213.
- Burjanadze, T.V. New Analysis of the Phylogenetic Change of Collagen Thermostability. Biopolymers 2000, 53, 523–528.
- AOAC International. Official Methods of Analysis, 17th Ed. AOAC, International, Arlington, VA, 2000.
- Steffe, J.F. Rheological Methods in Food Process Engineering. Freeman Press, East Lansing, USA, 1996.
- Gunasekaran, S.; Ak, M.M. Dynamic Oscillatory Shear Testing of Foods—Selected Applications. Trends in Food Science and Technology 2000, 11, 115–127.
- SAS. 1988. SAS/STAT User’s Guide ( 6.03), SAS Institute Inc., Cary, NC.
- Binsi, P.K.; Shamasundar, B.A.; Dileep, A.O.; Badii, F.; Howell, N.K. Rheological and Functional Properties of Gelatin from the Skin of Bigeye Snapper (Priacanthus Hamrur) Fish: Influence of Gelatin on the Gel-Forming Ability of Fish Mince. Food Hydrocolloids 2009, 23, 132–145.
- Cho, S.H.; Jahncke, M.L.; Chin, K.B.; Eun, J.B. The Effect of Processing Conditions on the Properties of Gelatin from Skate (Raja Kenojei) Skins. Food Hydrocolloids 2006, 20, 810–816.
- Badii, F.; Howell, N.K. Fish Gelatin: Structure, Gelling Properties and Interaction with Egg Albumen Proteins. Food Hydrocolloids 2006, 20, 630–640.
- Marcotte, M.; Hoshahili, A.R.T.; Ramaswamy, H.S. Rheological Properties of Selected Hydrocolloids as a Function of Concentration and Temperature. Food Research International 2001, 34, 695–703.
- Van Den Bulcke, A.I.; Bogdanov, B.; De Rooze, N.; Schacht, E.H.; Cornelissen, M.; Berghmans, H.Structural and Rheological Properties of Methacrylamide Modified Gelatin Hydrogels. Biomacromolecules 2000, 1, 31–38.
- Yoshimura, K.; Terashima, M.; Hozan, D.; Ebato, T.; Nomura, Y.; Ishii, Y.; Shirai, K. Physical Properties of Shark Gelatin Compared with Pig Gelatin. Journal of Agriculture and Food Chemistry 2000, 48, 2023–2027.
- Winter, H.H.; Chambon, F. Analysis of Linear Viscoelasticity of a Cross-Linking Polymer at the Gel Point. Journal of Rheology 1986, 30, 367–382.