Abstract
The correlations between the color of douchi and different protein hydrolysates from the post-fermentation period were analyzed. The results showed that peptides had the highest correlation with the color change, while free amino acids were second, which indicated that peptides and free amino acids were more involved in the Maillard reaction. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis revealed that the 7S component may be more involved in the Maillard reaction during the pre-fermentation of douchi, and that 11S subunits may be more involved in the formation of melanin in the initial stage of post-fermentation. The water-soluble extracts of two douchi samples in the most rapid formation stage of melanin were analyzed by reversed phase-high performance liquid chromatography. The results showed that the hydrophilicity of hydrolysates decreased depending on color. Meanwhile, the amino acid analyses demonstrated that the four amino acids with the maximum loss ratios were all hydrophilic in this stage, which indicated a loss in the hydrophilic components due to their involvement in the Maillard reaction.
INTRODUCTION
Douchi is a type of traditional fermented soy product that is widely consumed in Southern China. It is mainly used as a condiment because of its unique flavor and taste. There are three types of douchi based on the microorganisms used for fermentation, which include the Mucor-type, Bacteria-type, and Aspergillus-type douchi. The Aspergillus-type douchi,[Citation1–Citation4] and Bacteria-type douchi[Citation5–Citation7] have been widely researched on their physical, chemical, and functional properties; however, few reports on the Mucor-type douchi are available, and most of these have focused on the comparisons of various activities among the different types of douchi. Li et al.[Citation8] found that douchiqu fermented with Aspergillus oryzae, Mucor-wutungkiao, Bacillus subtilis natto, and Bacillus subtilis B1 had significantly different (p < 0.05) angiotensin-converting enzyme (ACE) inhibitor activities, with decreasing IC50 values of 0.4991, 0.3535, 0.2294, and 0.0901 mg/mL, respectively. Chen et al.[Citation3] used Aspergillus oryzae, Actinomucor elegans, and Rhizopus arrhizus to prepare douchi and investigated the anti-α-glucosidase activity of their aqueous douchi extracts. The results indicated that A. oryzae could utilize cooked black soybean to generate certainα-glucosidase inhibitors more effectively than A. elegans and R. arrhizus.
The Mucor-type Douchi produced in Southwestern China, such as that from the city of Chongqing or from the province of Sichuan, which produces high-quality products, should have a characteristic flavor, a palatably soft texture, and a bright brown color. The color, especially, is identified as the most important factor for judging the ripening extent and determining the consumer acceptability of Mucor-type douchi. Typically, the Mucor-type Douchi was produced by natural fermentation which caused the different Douchi colors from various batches by different climatic environments during the year. So, the production process should be improved to control the quality of Mucor-type Douchi.
The change in color is an important feature of the Maillard reaction (MR).[Citation9] The MR occurs between reducing sugars and amino compounds.[Citation10] Protein and its hydrolysates are sources that are involved in the MR along with the sugars that were hydrolyzed from carbohydrates during the ripening of the Mucor-type douchi.[Citation11] Hence, the ripened Mucor-type douchi has a bright brown color. Wang et al.[Citation11] investigated the effect of the MR on the ACE-inhibitory activity of douchi during fermentation and found that the MR consumed ACE-inhibitory peptides and decreased the ACE-inhibitory activity during the fermentation of Mucor-type douchi. Thus, the color of Mucor-type douchi should be associated with the degree of hydrolyzation of protein during fermentation. Research on the correlation between protein hydrolysates and color during fermentation of Mucor-type Douchi would provide a theoretical basis for the improvement of its production process. However, little is known about the correlation between the color and the protein hydrolysates during the fermentation of Mucor-type douchi.
This study used Actinomucor elegans, which is often used for research on Mucor-type douchi,[Citation3,Citation12] to prepare Mucor-type douchi and investigated the trends of color change and of protein hydrolysates of protein during fermentation. Then, their correlation was analyzed. The source and characteristics of the protein hydrolysates involved in the MR were also investigated.
MATERIALS AND METHODS
Materials
A. elegans was kindly provided by the microbiology teaching and research section from the College of Food Science of Southwest University. Soybeans were purchased from a local market in Beibei, Chongqing, China. Bovine serum albumin (BSA) and Folin-phenol reagents were purchased from Sigma Chemical Co., St. Louis, MO, USA. Sodium dodecyl sulfate (SDS), Tris, ammonium persulfate (APS), β-mercaptoethanol (2-ME), Coomassie Blue R-250, and N,N,N′,N′-tetramethylethylenediamine (TEMED) were procured from Bio-Rad Laboratories (Hercules, CA, USA). Glycine and bromophenol blue (BPB) were obtained from Sangon Biotech Co., Ltd. (Shanghai, China), and 30% acrylamide (the mass ratio of acrylamide to bisacrylamide was 29:1) was obtained from Beijing Solarbio Science and Technology Co., Ltd. (Beijing, China). All other chemicals were of analytical grade.
Preparation of Douchi
Mucor-type douchi was prepared from the soybeans. The steps and parameters for the preparation were as follows:
Soybeans were soaked in three times the volume of tap water for 4 h at 25°C. Once drained, they were steamed at 100°C for 2 h.
Pre-fermentation. The soybeans were inoculated with 1% (v/w)A. elegans inoculums (10ʹ spores/mL) as a pure-culture fermentation starter. The soybeans were cultured at 28°C and 90% humidity for 10 d in an incubator (LTI-601SD; Tokyo Rikakikai Co. Ltd, Tokyo, Japan). The product used as the douchi qu.
Post-fermentation. Douchi qu was washed with tap water and then combined with 10% salt, 5% fermented glutinous rice, and 5% distilled spirit (based on the dry weight of soybeans). This mixture was sealed in bottles and ripened at 28°C in an incubator for 150 d. The product is douchi.
At various fermentation phases, samples were lyophilized to a constant weight in a vacuum freeze-dryer (ALPAAI-4LSC; Christ, Osterode, Germany) and the pulverized into a powder using a mortar and pestle. The code and fermentation time of the samples are listed in .
TABLE 1 Sample codes and fermentation times of douchi
Determination of Color
The color of the samples were evaluated by the Hunter L, a, and b scale (with L representing the lightness on a scale of 0 (dark) to 100 (white), +a for redness, –a for greenness, +b for yellowness and –b for blueness) using a Hunter Lab colorimeter (Hunter Lab D65 Spectrocolorimeter Ultrascan PRO, Reston, VA, USA). The mashed samples were placed in a clear glass Petri dish (3 replicates), and color measurements were performed in triplicate.
The colorimeter was calibrated before each series of measurements using a white ceramic plate (L* = 96.82, a* = +0.65, and b* = +2.32) and the Hunter L, a, and b values were recorded. The total color difference (ΔE) was calculated to evaluate the extent of the MR according to the methods of Wang et al. (2013), which is a parameter that quantifies the overall color difference for a given sample compared to a reference samples (Pe0). The mean L, a, and b values were used to determine the ΔE among the samples. The ΔE was calculated using the following equation:[Citation13]
Determination of the Soluble Protein Content
Lyophilized douchi samples (1 g) were suspended in 30 mL of distilled water. The mixture was mixed on a magnetic stirrer at room temperature for 10 min, followed by sonication (KQ-100E, Ultrasonic Instrument Co., Ltd., Kunshan, China) for 30 min. The mixture was extracted in a shaking bath (SHZ-B, Jiangren Technical Co., Ltd., Shanghai, China) for 1 h at 30°C. The suspension was centrifuged at 12,000 × g for 30 min. The precipitates were extracted two times using 10 mL of distilled water following the procedure just described. The supernatants were combined and diluted with distilled water to 50 mL for the extraction of the douchi. The soluble protein was assayed by the Lowry method and the soluble protein content was calculated as follows:
Determination of the Total Free Amino Acid Content
The total free amino acids were analyzed by the ninhydrin method as described by Doi et al.[Citation14] All samples were assayed in triplicate and the content of free amino acids was calculated as follows:
The content of free amino acids (%) = (the weight of free amino acids [g]/the weight of the douchi sample [g]) × 100%
Determination of the Peptide Content
One milliliter of the extract solution of douchi was mixed with 1 mL of 20% TCA and then centrifuged at 10,000 ×g for 15 min. The soluble protein in the supernatant was assayed by the Lowry method to obtain the weight of the peptides in the douchi extraction. All samples were assayed in triplicate. The soluble protein was assayed by the Lowry method and the content of the soluble protein was calculated as follows:
Determination of the Macromolecular Soluble Protein Content
The content of macromolecular soluble protein was calculated as follows:
SDS-PAGE
For sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), 0.8 g of douchi powder was suspended in 10 mL of 0.03 M Tris-HCl buffer (pH 8.0). The mixture was mixed with a magnetic stirrer at room temperature for 10 min. Then, the mixture was extracted in a shaking bath for 2 h at 30 °C. The suspension was centrifuged at 12,000 × g for 30 min. SDS-PAGE analysis for supernatant was performed according to the method of Zhang et al.[Citation15] with some modifications. The sample solution was mixed at a 4:1 (v/v) ratio with a 5-fold-concentrated loading buffer containing β-mercaptoethanol. The mixed solution was heated in boiling water for 5 min and analyzed by SDS-PAGE using 5% stacking gels and 12% resolving gels in a miniature electrophoresis unit (Bio-Rad Laboratories, Hercules, CA) at a constant current of 15 mA/gel; the current was increased to 25 mA/gel when the BPB ran into the resolving gel. Fifteen μL samples were loaded into the gel together with 10 μLof the protein marker. After electrophoresis, the gel was stained with 0.1% (w/v) Coomassie Blue R-250 in 25% (v/v) isopropanol and 10% (v/v) acetic acid for 2 h and destained with 5% (v/v) alcohol and 10% (v/v) acetic acid until the zones on the blue background were clear. The PageRulerTM Unstained Protein Ladder (marker) was used to estimate the molecular weight distributions of the gelatins. The gel was scanned with a G:BOX EF gel-imaging system (Syngene, Cambridge, UK).
RP-HPLC Analysis of the Samples from the Period of Rapid Melanin Formation
The hydrophilicity change of the hydrolysates was analyzed using reversed phase-high performance liquid chromatography (RP-HPLC). The douchi powder 0.5 g of was suspended in 10 mL of distilled water, and the mixture was mixed on a vortex for 10 s, followed by sonication for 10 min. The mixture was extracted in a shaking bath for 2 h at 30°C. The suspension was centrifuged at 12,000 × g for 10 min. The supernatant was filtered through a cellulose acetate filter (0.45-µm pore size, Sartorius, Gottingen) and then analyzed by HPLC under the following conditions: Mobile phase: a mixture with 45% acetonitrile and 55% distilled water; Flow rate: 0.5 mL/min; Column: Agilent ZORBAX SB-C18 (4.6 mm i.d. × 250 mm); Injection volume: 10 µL; Detection wavelength: 270 nm.
Amino Acid Analysis of the Samples from the Period of Rapid Melanin Formation
Amino acid analysis was performed using a modified method described by Sanni et al.[Citation16] Five milliliters of 6 N HCl were added to a 100 mg sample in a sealed tube. The tube was placed in an oil bath at 110 ± 1 °C for 24 h. Then, the hydrolysate was filtered and an aliquot of 1 mL was dried with an EYELA concentrator (TVE-1000, Tokyo Rikakikai Co., Ltd., Tokyo, Japan). Ten milliliters of 0.02 M HCl was added to reconstitute the sample and the supernatant was filtered through a cellulose acetate filter (0.45-µm pore size). A Hitachi L-8800 amino acid auto analyzer (Hitachi Co. Ltd., Japan) was used for separating the amino acids.
Statistical Treatment of Data
The statistical analysis was performed using SPSS 13.0 for Windows (SPSS Inc, Chicago, IL).
RESULTS AND DISCUSSIONS
Correlation Between Color and Protein Hydrolysates in the Fermentation Process
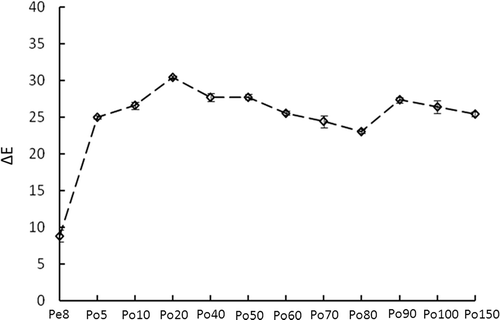
The color of douchi was evaluated by ΔE () which also has strong correlation with the MR.[Citation11] The color exhibited a trend of initially rapid increasing and then fluctuating in a range of 30.40 to 23.03. The greatest color increase occurred from 8th day pre-fermentation to 5th day post-fermentation and the ΔE value increases from 8.80 to 24.94. This trend is consistent with Wang et al.,[Citation11,Citation17] who used wutungkiao Mucor as the fermentative bacteria to prepare their douchi; the color of the douchi deepened quickly in the initial post-fermentation stage, and then slowly increased in the period from 7 to 28 d post-fermentation. After the 40th day post-fermentation, the ΔE value decreased slowly and reappeared as a small peak on the 90th day post-fermentation. This tendency likely occurred as a result of a change in the oxygen content in the environment causing a change in the microflora. At the beginning of the post-fermentation period, oxygen was consumed by A. elegans. After the 40th day post-fermentation, A. elegans growth is suppressed and various anaerobic microorganisms such as lactic acid bacteria gradually grew as oxygen was exhausted. These microorganisms could become the dominant flora[Citation12,Citation18] on the 90th day post-fermentation, and the hydrolysates of the metabolic products from them could be beneficial for the MR to make the ΔE value increase again.
The soluble protein content in the sample increased significantly (p < 0.05) in the pre-fermentation period. From the post-fermentation period, the content of soluble protein continued its upward trend and reached its maximum (12.11%) on the 10th day post-fermentation; the content then decreased slowly till the 80th day. The number of A. elegans reduced gradually after the 10th day post-fermentation, and some anaerobic microorganisms gradually grew because of the consumption of oxygen, which caused some soluble protein to be consumed as a nitrogen source with little protease was production. So, the soluble protein content showed downward trend in this stage. Similar with the color changing trend, the soluble protein content increased after the 80th day post-fermentation, likely due to the growth of anaerobic microorganisms that secrete proteases and increase hydrolysis of the insoluble protein. These hydrolysates could involve in the MR to result in color deepening of the douchi.
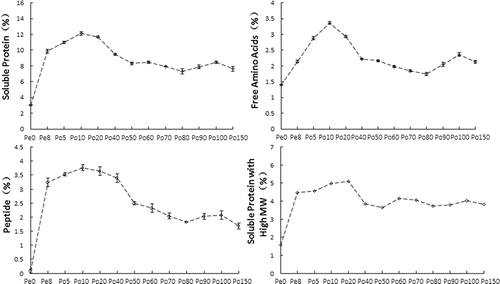
The soluble protein content increased at the initial stage of fermentation mainly due to proteolysis during which the insoluble macromolecular protein is degraded into soluble protein.[Citation19,Citation20] The composition of the soluble protein can be divided into free amino acids, peptides, and macromolecular soluble proteins. The changes in the concentrations of protein hydrolysates during the fermentation process of douchi are shown in .
Similarly to the soluble protein results, the free amino acid, peptide and macromolecular soluble protein contents increased significantly (p < 0.05) in the pre-fermentation period and the initial stage of post-fermentation; they kept fluctuating as microbial community changed. During the 80th–90th day post-fermentation, the content of peptides and free amino acids increased significantly (p < 0.05), while the macromolecular soluble protein content did not increase significantly (p > 0.05). These results demonstrate that the components of hydrolysates hydrolyzed by proteases secreted by the anaerobic microorganisms are mainly peptides and free amino acids at this stage, which could cause the color deepening of the douchi. Hence, it can also be implied that peptides and free amino acids are more involved the MR than macromolecular soluble protein at this stage.
Pearson’s correlation coefficients were calculated for a general assessment of the correlations between the contents of soluble protein and the color change values of the douchi. The strengths of the relationships between the variables were assessed using effect sizes as suggested by Cohen.[Citation21] He defined three levels of effect sizes: small (0.1–0.3), medium (0.3–0.5), and large (> 0.5), corresponding to absolute correlations of 0.1, 0.3, and 0.5, respectively. When the correlations between the contents of the soluble protein and the color change values are analyzed for all samples and the Pearson’s correlation coefficient is 0.049, no relevance is indicated. If the sample scope is limited to only post-fermentation, the Pearson’s correlation coefficient is 0.494, which indicates a moderate correlation (0.3–0.5). This correlation suggests that the MR occurs mainly in the post-fermentation phase, especially in the early post-fermentation stage, and a large portion of the hydrolysates associated with the MR were produced in this stage. On this basis, the correlations between the contents of free amino acids, peptides and macromolecular soluble protein and the color change were analyzed for the post-fermentation stage; the peptides were significantly correlated with the color change (> 0.5), while the free amino acids and macromolecular soluble protein had moderate correlations with the color changes (0.3–0.5), but the free amino acids were more strongly correlated than the macromolecular soluble protein (). These results are consistent with the results from the previously described analyses. Ogasawara et al.[Citation22] demonstrated that the characteristic flavor of ripened miso, which is also a fermented product of soybeans, is mainly based on the formation of the MR products involved with peptides with a MW from 1000 to 5000 Da, which is consistent with the conclusions above.
TABLE 2 Correlation analyses between protein hydrolysates and the color change during the post-fermentation stage of douchi
Sources Analysis of the Hydrolysates Involved in the MR During the Rapid Melanin Formation Stage
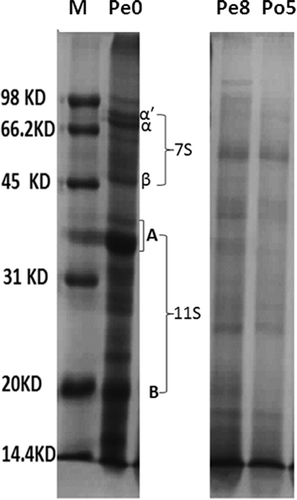
During the process of fermentation of douchi, the greatest extent of color deepening occurred from the 8th day pre-fermentation to the 5th day post-fermentation, which indicates the rapid generation of melanin during this stage. The two samples were analyzed by SDS-PAGE (). These results were used to determine the source of the hydrolysates that are involved in the MR.
After pre-fermentation, the 7S protein bands disappeared and the acid subunit and basic subunit of the 11S protein were still partially present, implying that the 7S components were more easily consumed than the 11S components during the pre-fermentation period. Usui et al.[Citation23] examined the masking of the allergen structure of soy protein by the attachment of chitosan through a Maillard-type protein-polysaccharide conjugation. From their SDS-PAGE results, the 7S components disappeared after a week of the MR; the 11S components were still present, but the bands were lighter in color after two weeks of the MR. Therefore, the 7S components could become involved in the MR quicker than the 11S components during the fermentation of Mucor-type douchi. That could be due to the difference of their hydrolysis extent and amino acids composition. The 7S components could be hydrolyzed more rapidly than the 11S components and more involved in the MR during pre-fermentation. Furthermore, the 11S components contained more Cys than the 7S components.[Citation24] Huang et al.[Citation13] proved that Cys could inhibit color formation of the MR products, which could cause less involvement of the 11S components in the MR during pre-fermentation.
After the stage of the most rapid melanin increase (Po5), the 11S protein bands disappeared completely. Thus, the hydrolysates of 11S may also become more involved in the MR during this stage, which rapidly deepened the colors of douchi. It is likely the hydrolysis of the 11S components produced many hydrolysates without Cys, which accelerate the MR. The results was similar with the report of Zhou et al.,[Citation25] who researched the MR by soy protein isolate and dextran. They found that the degree of protein glycosylation increased rapidly at the reaction time from 18 to 30 h, and the 11S components disappeared gradually at this stage while most of the 7S components disappeared before 18 h.
Hydrophilicity Analysis of Hydrolysates During the Rapid Melanin Formation Stage
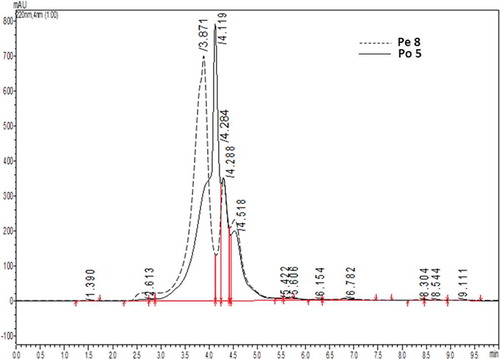
The characteristics of the water-soluble extracts from Pe8 and Po5 were analyzed using RP-HPLC (). Compared to the 8th day pre-fermentation, the retention times of the hydrolysates at the 5th day post-fermentation were delayed, indicating a change in polarity. The extracts from the Pe8 and Po5 stages consisted primarily of three components, and the latter two components had similar retention times of 14.288 and 14.518 min, respectively. In contrast, the retention time of the first component from Pe8 was 13.871 min, and this time was delayed to 14.119 min in Po5. These results indicate that some of the hydrophilic components were lost, and the hydrophobicity of the hydrolysates increased in the first 5 days post-fermentation. In view of the rapid generation of melanin during this stage, the hydrophilic hydrolysates may be more heavily involved in the MR. Lan et al.[Citation26] used soy protein hydrolysates and pentosan as the substrate to produce melanin and increase the browning intensity of the product. They found that hydrophobic amino acids are almost nothing to decrease after MR at 80°C, but more hydrophobic amino acids were reduced in the MR as the temperature rose, which was consistent with the results of this study because the fermented temperature of the douchi was lower than 80°C.
The total amino acid contents were analyzed for the Pe8 and Po5 stages (). From the 8th day pre-fermentation to the 5th day post-fermentation, the total amino acid content of the douchi decreased by 20.99%, and the contents of all of the amino acids decreased. These results are consistent with the results of Liu et al.[Citation27] and Zhang et al.[Citation2] The ratio between the hydrophilic amino acids and the hydrophobic amino acids decreased from 0.84 to 0.74, also demonstrating that, relative to the hydrophilic amino acids, the hydrophobic amino acids are more highly retained in the douchi sample. Hence, it is consistent with the results of RP-HPLC. The soybeans were soaked and steamed; then, were pre-fermented at 90% humidity for 10 days and washed with tap water before being sealed in a bottle. Thus, some water was contained in the post-fermentation system, which could be beneficial to hydrophilic peptides and amino acids to become involved in the MR.
TABLE 3 The changes in total amino acids of douchi from the 8th day of pre-fermentation to the 5th day of post-fermentation
The four amino acids with the highest rate of loss (Arg, His, Asp, and Lys) are all hydrophilic amino acids present in the early post-fermentation stage of the douchi, which is also consistent with above results. Liu et al.[Citation27] used soy protein hydrolysates and xylose as a substrate to build the MR model and to analyze the amino acid contents before and after the MR. These authors found that the three amino acids that decreased the most during the MR were Cys, Arg, and Lys. Niu et al.[Citation28] used wheat germ protein as a substrate and also found that Cys, Arg, and Lys decreased significantly during the MR process. Kwak et al.[Citation29] studied different amino acids involved in the MR and found that Arg and Lys are lost more easily after the MR. In addition, Tong et al. and Cui et al. studied the changes in fish protein hydrolysate before and after the MR and found that the contents of Cys, Arg, Lys/His, and Asp decreased significantly. Combining these findings, it is evident that the peptides containing Arg, Lys, His, and Asp in the post-fermentation process of the douchi may be more involved in the MR.
The Cys content decreased by 13.40% during the first 5 days of post-fermentation, which is the smallest decrease observed among the amino acids and does not agree with various reports of high Cys losses. The study of Huang et al.[Citation30] revealed that Cys inhibits melanogenesis in the MR, and that its major role in the Amadori product, which is formed by the condensation and rearrangement of amino compounds and reducing sugars interfering with peptides and sugar Amadori formation, is to effectively preventing Amadori compounds from further forming deoxy-reductones and cyclization, inhibiting the generation of color substances. During the first 5 days post-fermentation, melanin is generated rapidly and Cys may not be very involved in inhibiting the MR during this stage. As post-fermentation continues, the douchi lightens in color and Cys may be more involved in the reaction.
CONCLUSIONS
This study found that, during the fermentation process of douchi by A. Elegans, the color of douchi deepens quickly in the early post-fermentation stage, and the greatest extent of deepening in color occurred from the 8th day pre-fermentation to the 5th day post-fermentation. The changed trend of soluble proteins, including free amino acids, peptides and macromolecular soluble proteins, all followed similar trends to that of douchi color. The correlations between the contents of the three different types of hydrolysates and the douchi color change value during the post-fermentation period were analyzed. The results demonstrate that the peptide content was significantly correlated with the douchi color change, and the free amino acids and macromolecular soluble protein were moderately correlated with the douchi color change, though the correlation with free amino acids was higher than that with soluble protein macromolecules. It is reasoned that the peptides are more involved in the MR, while the free amino acids also contribute somewhat to the deepening in the douchi color during the post-fermentation period. The SDS-PAGE results revealed that the 7S protein subunits in douchi all disappeared, while the 11S subunits were still partially preserved after the 8th day of the pre-fermentation period. These results imply that the 7S component may be more involved in the MR during the pre-fermentation of douchi. During the first 5 days post-fermentation, the 11S subunits all disappeared. It is speculated that, during the rapid melanin formation stage, the hydrolysates from the 11S subunits may be more involved in the formation of melanin. The water-soluble extracts from the stage of the rapid melanin formation stage were analyzed by RP-HPLC. The results show that the hydrophilicity of the hydrolysates decreased after the 5th day post-fermentation, which indicates a loss in hydrophilic components because they are involved in the MR. Amino acid analyses revealed that during the first 5 days post-fermentation, the four amino acids that decreased the most are all hydrophilic, which is consistent with the conclusions from the HPLC analysis. Based on these findings, it is speculated that hydrophilic peptides and free amino acids may be more involved in the MR during rapid melanin formation stage.
ACKNOWLEDGMENT
The authors would like to thank Guohua Zhao at the College of Food Science, Southwest University, for his advice.
FUNDING
The authors gratefully acknowledge the support from the National Natural Science Foundation of China (Grant No: 31301425) and the Fundamental Research Funds for the Central Universities (Grant No: XDJK2011B001, 2362014xk11).
Additional information
Funding
REFERENCES
- Zhang, J.H.; Tatsumi, E.; Ding, C.H.; Li, L.T. Angiotensin I-Converting Enzyme Inhibitory Peptides in Douchi, A Chinese Traditional Fermented Soybean Product. Food Chemistry 2006, 98, 551–557.
- Zhang, J.H.; Tatsumi, E.; Fan, J.F.; Li, L.T. Chemical Components of Aspergillus-Type Douchi, a Chinese Traditional Fermented Soybean Product, Change During the Fermentation Process. International Journal of Food Science and Technology 2007, 42, 263–268.
- Chen, J.; Cheng, Y.Q.; Yamaki, K.; Li, L.T. Anti-alpha-Glucosidase Activity Of Chinese Traditionally Fermented Soybean (Douchi). Food Chemistry 2007, 103, 1091–1096.
- Wang, L.J.; Cheng, Y.Q.; Yin, L.J.; Bhandari, B.; Saito, M.; Li, L.T. Changes During Processing and Sodium Chloride Supplementation on the Physical and Chemical Properties Of Douchi. International Journal of Food Properties 2010, 13, 131–141.
- Fan, J.F.; Zhang, Y.Y.; Chang, X.J.; Saito, M.; Li, Z.G. Changes in the Radical Scavenging Activity of Bacterial-Type Douchi, A Traditional Fermented Soybean Product, During The Primary Fermentation Process. Bioscience Biotechnology and Biochemistry 2009, 73, 2749–2753.
- Luo, Y.C.; Li, B.; Ji, H.; Ji, B.P.; Ji, F.D.; Chen, G.; Tian, F. Effect of Soaking and Cooking on Selected Soybean Variety for Preparation of Fibrinolytic Douchi. Journal of Food Science and Technology-Mysore 2009, 46, 104–108.
- Luo, Y.C.; Li, B.; Ji, H.; Ji, B.P.; Ji, F.D.; Chen, G.; Tian, F. Effect of soybean varieties on the fibrinolytic activity and sensory characteristics of Douchi. Journal of Food Processing and Preservation 2010, 34, 457–469.
- Li, F.J.; Yin, L.J.; Cheng, Y.Q.; Yamaki, K.; Fan, J.F.; Li, L.T. Comparison of Angiotensin I-Converting Enzyme Inhibitor Activities of Pre-Fermented Douchi (A Chinese Traditional Fermented Soybean Food) Started with Various Cultures. International Journal of Food Engineering 2009, 5.
- Morales, F.J.; Romero, C.; JimenezPerez, S. Fluorescence Associated with Maillard Reaction in Milk and Milk-Resembling Systems. Food Chemistry 1996, 57, 423–428.
- Langner, E.; Rzeski, W. Biological Properties of Melanoidins: A Review. International Journal of Food Properties 2014, 17, 344–353.
- Wang, H.; Li, Y.Y.; Cheng, Y.Q.; Yin, L.J.; Li, L.T. Effect of the Maillard Reaction on Angiotensin I-Converting Enzyme (ACE)-Inhibitory Activity of Douchi During Fermentation. Food and Bioprocess Technology 2013, 6, 297–301.
- Li, F.J.; Yin, L.J.; Lu, X.; Li, L.T. Changes in Angiotensin I-Converting Enzyme Inhibitory Activities During the Ripening of Douchi (A Chinese Traditional Soybean Product) Fermented by Various Starter Cultures. International Journal of Food Properties 2010, 13, 512–524.
- Huang, M.G.; Liu, P.; Song, S.Q.; Zhang, X.M.; Hayat, K.; Xia, S.Q.; Jia, C.S.; Gu, F.L. Contribution of Sulfur-Containing Compounds to the Color-Inhibiting Effect and Improved Antioxidant Activity of Maillard Reaction Products of Soybean Protein Hydrolysates. Journal of the Science of Food and Agriculture 2011, 91, 710–720.
- Doi, E.; Shibata, D.; Matoba, T. Modified Colorimetric Ninhydrin Methods For Peptidase Assay. Analytical Biochemistry 1981, 118, 173–184.
- Zhang, Y.N.; Zhao, X.H. Study on the Functional Properties of Soybean Protein Isolate Cross-Linked with Gelatin by Microbial Transglutaminase. International Journal of Food Properties 2013, 16, 1257–1270.
- Sanni, A.I.; Onilude, A.A.; Ibidapo, O.T. Biochemical Composition of Infant Weaning Food Fabricated from Fermented Blends of Cereal and Soybean. Food Chemistry 1999, 65, 35–39.
- Wang, H.; Yin, L.J.; Cheng, Y.Q.; Li, L.T. Effect of Sodium Chloride on the Color, Texture, and Sensory Attributes of Douchi During Post-Fermentation. International Journal of Food Engineering 2012, 8.
- Chen, T.T.; Xiong, S.Q.; Jiang, S.Y.; Wang, M.J.; Wu, Q.L.; Wei, H. Molecular Identification of Microbial Community in Chinese Douchi During Post-Fermentation Process. Food Science and Biotechnology 2011, 20, 1633–1638.
- Xu, C.L.; Hao, K.; Peng, C.F.; Cai, W.H.; Jin, Z.Y. Effects of Fermentation Temperature and Time on the Physicochemical and Sensory Characteristics of Douchi. Food Technology and Biotechnology 2005, 43, 307–311.
- Chen, Y.F.; Lee, S.L.; Chou, C.C. Fermentation with Aspergillus Awamori Enhanced Contents of Amino Nitrogen and Total Phenolics as Well as the Low-Density Lipoprotein Oxidation Inhibitory Activity of Black Soybeans. Journal of Agricultural and Food Chemistry 2011, 59, 3974–3979.
- Cohen, J. The Cost of Dichotomization. Applied Psychological Measurement 1983, 7(3), 249–253.
- Ogasawara, M.; Yamada, Y.; Egi, M. Taste Enhancer from the Long-Term Ripening of Miso (Soybean Paste). Food Chemistry 2006, 99, 736–741.
- Usui, M.; Tamura, H.; Nakamura, K.; Ogawa, T.; Muroshita, M.; Azakami, H.; Kanuma, S.; Kato, A. Enhanced Bactericidal Action and Masking of Allergen Structure of Soy Protein by Attachment of Chitosan Through Maillard-Type Protein-Polysaccharide Conjugation. Nahrung-Food 2004, 48, 69–72.
- Zhao, X.Y.; Chen, J.; Lu, Z.F.; Ling, X.Q.; Deng, P.; Zhu, Q.J.; Du, F.L. Analysis of the Amino Acids of Soy Globulins by AOT Reverse Micelles and Aqueous Buffer. Applied Biochemistry and Biotechnology 2011, 165(3–4), 802–813.
- Zhou, X.Y.; Qi, J.R.; Yin, S.W.; Yang, X.Q.; Zhu, J.H.; Huang, L.X. Formation of Soy Protein Isolate-Dextran Conjugates by Moderate Maillard Reaction in Macromolecular Crowding Conditions. Journal of the Science of Food and Agriculture 2013, 93, 316–323.
- Lan, X.H.; Liu, P.; Xia, S.Q.; Jia, C.S.; Mukunzi, D.; Zhang, X.M.; Xia, W.S.; Tian, H.X.; Xiao, Z.B. Temperature Effect on the Non-Volatile Compounds of Maillard Reaction Products Derived from Xylose-Soybean Peptide System: Further Insights into Thermal Degradation and Cross-Linking. Food Chemistry 2010, 120, 967–972.
- Liu, P.; Huang, M.G.; Song, S.Q.; Hayat, K.; Zhang, X.M.; Xia, S.Q.; Jia, C.S. Sensory Characteristics and Antioxidant Activities of Maillard Reaction Products from Soy Protein Hydrolysates with Different Molecular Weight Distribution. Food and Bioprocess Technology 2012, 5, 1775–1789.
- Niu, L.Y.; Jiang, S.T.; Pan, L.J.; Zhai, Y.S. Characteristics and Functional Properties of Wheat Germ Protein Glycated with Saccharides Through Maillard Reaction. International Journal of Food Science and Technology 2011, 46, 2197–2203.
- Kwak, E.J.; Lim, S.I. The effect of Sugar, Amino Acid, Metal Ion, and NaCl on Model Maillard Reaction under pH Control. Amino Acids 2004, 27, 85–90.
- Huang, M.G.; Zhang, X.M.; Eric, K.; Abbas, S.; Hayat, K.; Liu, P.; Xia, S.Q.; Jia, C.S. Inhibiting the Color Formation by Gradient Temperature-Elevating Maillard Reaction of Soybean Peptide-Xylose System Based on Interaction of L-Cysteine and Amadori Compounds. Journal of Peptide Science 2012, 18, 342–349.