Abstract
Modification of gelatine with a novel natural polyphenol, Galla chinensis extract, was investigated. The gel strength, elastic and viscous moduli, swelling property, thermal stability, X-ray diffraction, ultrastructure, and Fourier transform infrared spectra were determined to evaluate the properties of the modified gelatine. Gel strength and thermal stability tests indicated that Galla chinensis extract increased the stability of gelatine within a certain concentration range. On the other hand, the swelling of Galla chinensis extract-containing gelatine decreased with increasing Galla chinensis extract content because of the formation of a denser structure. The elastic and viscous moduli were enhanced by Galla chinensis extract, but the melting and gelling points did not show any significant improvement. Scanning electron microscopy studies suggested that Galla chinensis extract played an important role in modifying the gelatine microstructure. X-ray diffraction revealed that Galla chinensis extract could intercalate the gelatine chains and strengthen the intermolecular interactions. These results suggested that Galla chinensis extract may be used as a novel natural crosslinker to stabilize gelatine, and gels treated with Galla chinensis extract could be found with different properties.
INTRODUCTION
Gelatin is one of the most popular biodegradable polymers. It can be obtained by the physical and chemical degradation of collagen or by thermal denaturation.[Citation1] These processes involve breaking the triple-helix structure into random coils to obtain gelatin. Currently, gelatin is widely used in pharmaceutical, food, photographic, and cosmetic applications because of its plasticity, adhesiveness, and low antigenicity. And some of the common applications of gelatin in the biomedical field include drug delivery, wound dressing, tissue engineering, and gene therapy.[Citation2] In the food industry, gelatin is considered one of the most promising biopolymers for packaging applications and food ingredient.[Citation3] Near 40°C, aqueous solutions of gelatin are in the sol state and form physical thermos reversible gels upon cooling.[Citation4] Above this temperature, gelatine chains behave as random coils, which provided that it is particularly significant in food ingredient.[Citation5]
However, the poor mechanical properties and thermal stability of gelatin during processing, limit its potential applications. Therefore, improving the functional properties of gelatin gels from by-products of seafood processing and animal skin or bone could widen its potential utility. Toward that end, the thermal and mechanical stability of gelatin can be improved through crosslinking,[Citation6] as confirmed by scanning electron microscopy (SEM) and X-ray diffraction measurements. The modification of gelatin by physical and chemical methods has been extensively investigated for a number of biomaterials applications.[Citation7] However, many of these methods suffer from various drawbacks. For example, ultra-violet (UV) irradiation only modifies the surface of gelatin. In addition, the use of chemical crosslinkers such as aldehyde-containing compounds is not recommended because of toxicity concerns.[Citation8] Although genipin has been previously investigated as a natural crosslinking agent with less toxicity for biomaterial applications,[Citation9] it has many inherent disadvantages such as difficulties in extraction, limited sources, and high cost. Studies regarding protein crosslinking using oxidized dialdehyde polysaccharides have been carried out previously.[Citation3] However, such polysaccharides were oxidized and/or denatured with reagents such as xanthan gum, which is not approved for use in food or medicine.[Citation9] Hence, the development of novel, inexpensive, non-toxic, and readily available crosslinking agents is desired.[Citation10] For example, it has been reported that procyanidin, a phenolic compound derived from vegetables and fruits, stabilizes collagen as a crosslinker, without destructing its triple helical structure.[Citation11]
Numerous studies have been conducted on the application of plant extracts in various medicines and foods.[Citation12,Citation13] Galla chinensis, an extract obtained from the abnormal growth of Rhus leaf tissue in response to the secretion of parasitic aphids (family Pemphigidae), has been shown to contain a large amount (in more than 60% of its weight) of gallotannin, a polyphenolic compound.[Citation14] Additionally, Galla chinensis was reported to display strong antioxidant activities and provide benefits against coronary heart disease, cancer, high blood pressure, and diabetes because of its flavone glycosides that can scavenge free radicals.[Citation15,Citation16] As such, Galla chinensis extract (GCE), which shows excellent antioxidant activity and can crosslink, could be an ideal additive for gelatin hydrogels for improving their physiochemical properties. However, not many studies have reported the effect of GCE on the physicochemical properties and stability of gelatin. This work aimed to develop a novel natural crosslinking agent for gelatin by using GCE and evaluates certain properties such as gel strength, thermal stability, and swelling ratio. Dynamic viscoelastic analysis of the samples was also carried out. Furthermore, the surface properties of the dried gel were observed via SEM, Fourier transform infrared (FTIR), and X-ray diffraction.
MATERIALS AND METHODS
Materials and Chemicals
Gelatin (type B, bovine bone) was purchased from Gelatin Co., Ltd., Qingdao, China. NaHCO3, HCl, and 2,4,6-trinitrobenzenesulfonic (TNBS) acid were obtained from Sigma (St. Louis, MO, USA) or Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Galla chinensis (origin: Zhejiang province, China) was purchased from China Jinan Ronghetang Group Co., Ltd (Jinan, China). All other chemicals used in this study were of analytical grade.
Extraction of Natural Functional Materials
Galla chinensis (50 g) was dried, ground into a powder, and passed through a sieve (60-mesh).[Citation17] Next, the powder was mixed with 500 mL of deionized water, boiled for 1 h, and then filtered. Then, the extract was dissolved in 250 mL of ethanol (100%). The crude extracts were centrifuged at 900 × g for 10 min, and the supernatant was utilised throughout the study. The crude extracts were evaporated and dried under vacuum (below 40ºC) to form a powder. The crude extracts separated from Galla chinensis were obtained in 10% yield and the dry matter was referred to as GCE.[Citation9]
Hydrogel Sample Preparation
First, gelatin was mixed with distilled water (6% w/v) for 15 min and then heated at 50°C under mechanical stirring until it completely dissolved to obtain the hydrogel forming solution (HFS). Then, the GCE solution was incorporated into the HFS to prepare the hydrogel at concentrations of 0.03, 0.06, 0.09, and 0.12 g/100 mL. The corresponding gelatin composites were named G-GCE1, G-GCE2, G-GCE3, and G-GCE4, respectively. The HFS without extract was prepared in the same way as the control. All mixtures were stirred at 50ºC for 30 min to obtain homogeneous solutions. The air bubbles in HFS were removed by a rotary evaporator (Heidolph Laborta-4002). The resulting mixture was cooled to room temperature, conditioned at 4°C for 24 h to form hydrogels, and then lyophilized using a vacuum dryer. The resulting freeze-dried samples were used in the subsequent tests.
Gel Strength Measurements
The strength of the hydrogels was measured according to a procedure described in a previous report[Citation18] with some modifications. The strength of the hydrogels was determined using a TMS-PRO food texture analyser (Food Technology Co., Virginia, USA) with a flat-faced cylindrical plunger (1.27 mm diameter) at a testing speed of 60 mm· min−1. The maximum force (in grams) was obtained as the plunger penetrated the hydrogels. The break force was calculated using Eq. (1):
where, σ, F, and r are the break force, maximum force, and radius of the cylindrical plunger, respectively. Each sample was tested in triplicate.
Viscoelastic Properties
The dynamic viscoelastic analysis of the HFS was carried out on a physical MCR 101 rheometer rotary viscometer (Anton Paar Physica, Graz, Austria) using a cone-plate geometry (cone angle = 4, gap = 1.0 mm). Temperature ramps from 0 to 50°C and back to 0°C took place at a scan rate of 5°C/min, a frequency of 1 Hz, a target strain of 2%, and an applied stress of 3.0 Pa. In addition, all experiments were performed within the linear viscoelastic region. The elastic modulus (G′), viscous modulus (G″), and phase angle were recorded as a function of temperature. The error of the considered parameters in different tests of a single sample was less than 6% in all cases.[Citation19]
Differential Scanning Calorimetry (DSC)
Calorimetric measurements were performed using a Netzsch DSC 200PC equipment (Netzsch, Bavaria, Germany) with an attached refrigerated cooling system (RCS). Nitrogen was used as the purge gas at a flow rate of 40 mL·min–1 through the DSC cell and at 100 mL·min–1 through the RCS unit. The temperature and enthalpy calibrations were performed using high-purity standards (n-decane, benzene, and indium). Hermetic aluminium pans were utilized. Approximately 10–12 mg of dried sample was hermetically sealed in an aluminium pan, while an empty sealed pan was used as the reference. Freshly conditioned dried samples were scanned between 10 and 150°C at a heating rate of 5 K min–1.[Citation20] The peak value of the spectrum is generally referred to as the denaturation temperature (Td).
Thermogravimetric Analysis (TGA)
Dried gel samples were scanned using a thermogravimetric analyser (Netzsch TG 200PC, Germany) from 20 to 800°C at a rate of 10°K/min under a nitrogen atmosphere to avoid thermo-oxidation reactions.[Citation21] Nitrogen was used as the purge gas at a flow rate of 20 mL/min. For this analysis, 5 mg of each sample was used.
Swelling Behavior of Dry Gelatin during Rehydration
The swelling ratio was determined according to the method described by Charulatha and Rajaram.[Citation22] The dried gel was cut into small pieces (2 × 2 cm) and weighed in an air-dried condition (temperature: 10°C, relative humidity: 70%), and then placed into distilled water at 4°C for 90 min. The dried gels were gently shaken for 90 min and blotted with filter paper with a pore size ≤30 μm to remove the surface water prior to weighing every 15 min. The swelling ratio was determined using Eq. (2):
where, Wt and Wd are the hydrated and dried weights of the xerogel (freeze-dried gel), respectively.
Determination of the Degree of Crosslinking
A solution of TNBS acid was used to measure the percentage of uncrosslinked ε-amino groups remaining in the samples after crosslinking by a UV assay.[Citation23] For each group, samples (10 mg) were immersed in 1.0 mL of freshly prepared 0.5% (v/v) TNBS and 1.0 mL of 4% (w/v) sodium bicarbonate. The crosslinked gel samples were incubated for 4 h at 40°C in the dark. Subsequently, 3.0 mL of 6 M hydrochloric acid was added, and the mixture was heated at 60°C for 30 min. The final solution was diluted two-fold with ultrapure water. The absorbance of the solution was measured at 346 nm with a spectrophotometer (UNIC 7200, UNIC apparatus Co. Ltd, Shanghai, China) against a blank. All samples were tested in triplicate. The control was assumed to contain 100% of the available free amine groups and the value was used to determine the percentage of remaining free amine groups after the addition of GCE. Finally, the relationship between the absorbance and the ε-amino groups was calculated using Eq. (3):
where, 1.46 × 104 L/mole·cm is the molar absorptivity of THP-lys, b is the cell path length in cm, and is the sample weight in grams.
X-Ray Diffraction Studies
The X-ray diffraction patterns of the freeze-dried samples were obtained using an 18-kW rotating anode X-ray diffractometer (Bruker, D8 Advance, Germany) using Ni-filtered Cu radiation generated at 40 kV and 40 mA as the X-ray source. The 2θ range was 5–40° at a scanning speed of 4°/min and a step size of 0.1° (2θ). The crystallinity (Xc) of the samples was calculated using Eq. (4):
where, Fc is the diffraction peak area relevant to the crystal state structure, and Fa is that relevant to the non-crystal structure calculated from the diffraction pattern.
FTIR Spectral Analysis
FTIR spectra of gelatin with and without GCE were collected on a Nicolet 200SXV equipment at 25°C.[Citation24] The scans were carried out in the spectral range 500–4000 cm–1 at a resolution of 4 cm–1 with automatic signal gain, and 64 scans were collected. The spectral data was analysed using the Omnic v8.0 data collection software program.
Field Emission Scanning Electron Microscopy (FE-SEM) Analysis
The surface and cross-section morphologies of the samples were examined using a field emission SEM (HITACHI S 4300, Japan) under standard high-vacuum conditions with an accelerating voltage of 15 kV. All samples were fractured in liquid nitrogen and coated with a fine layer of gold prior to obtaining the micrographs.[Citation25]
RESULTS AND DISCUSSION
Gelatin Gel Strength
Gel strength is a vital functional property of hydrogels. The specific applications of a gel are often determined by the gel strength.[Citation26] Additionally, the size of gelatin chains, concentration, molecular weight distribution of gelatin, and hydrogen bonds between water molecules and free hydroxyl groups of amino acids play an important role in the gel strength.[Citation27,Citation28]
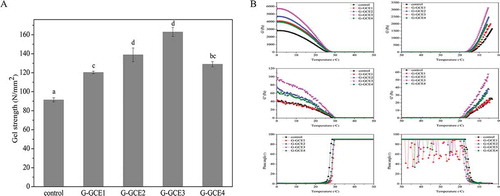
depicts the changes observed in the gel strength of gelatin with different amounts of GCE. The results indicated that the incorporation of GCE in gelatin caused a significant increase in the gel strength as compared to that of the control. Specifically, the addition of GCE caused the gel strength to increase to 46.9 N/mm2. The results suggested that hydrogen bond formation between water and the free hydroxyl groups of amino or polyphenol groups led to the crosslinking between gelatin and GCE. Moreover, further increase in the GCE (GCE4) content caused a decrease in the gel strength, likely because the components of the gelatin or collagen precipitated owing to excess rotary evaporator (Heidolph Laborta-4002) polyphenols and rotary evaporator (Heidolph Laborta-4002) incomplete reactivity of GCE in gelatin, as reported previously.[Citation28] In addition, when the GCE content was further increased, the gelatin changed to granular precipitate that is difficult to form gel (data not shown). This phenomenon could be explained by a previous report that indicated that proteins could be precipitated by polyphenols when the polyphenol content exceeded a certain concentration.[Citation29] It can be assumed that higher ratios of GCE to gelatin in the solution accompany higher degrees of binding of the polyphenols to the gelatin and the higher the degree of binding the greater the tendency to form precipitates.[Citation30] Thus, the addition of GCE of low concentration to gelatin resulted in a stronger gel, and a positive correlation was observed between the GCE content and gel strength, similar to a previous report concerning gelatin gels crosslinked by rutin.[Citation18]
Viscoelastic Properties
Viscoelastic properties reflect the changes of viscoelastic modulus in sol-gel conversion. shows the temperature-dependent viscoelastic properties of the gels with or without the addition of GCE, and the viscoelastic properties of the samples with different GCE contents are plotted as a function of temperature. In accord with previous reports, the elastic modulus (G′), viscous modulus (G″), and phase angle showed sigmoidal curves during heating and cooling. GCE-containing gelatin exhibited a large increase in G′ and G″, and G-GCE3 showed the largest increase compared to the control. In general, this increase resulted from changes in the conformation of gelatin during heating.[Citation31] Previous reports[Citation32] also showed that an increase in the G′ of gelatin during gelation is related to an increase in the triple helical content, the basic unit of collagen. Thus, gelatin molecules partly revert to the triple helical structure during gel formation. Furthermore, some studies Chiou et al.[Citation31] and Montero et al.[Citation32] suggested that a high concentration of helical structures in gelatin resulted in larger elastic modulus values. G-GCE3 showed the largest G′ and G″ during gel formation (); i.e., when the number of chemical junctions promoting the formation of triple helical structure increase, more bonds result, leading to more crosslinking and a larger increase in the modulus.[Citation19] Thus, the GCE-containing hydrogels had a greater crosslinking density than the control; similar results were found in subsequent tests. However, the GCE content had no obvious effect on the transition temperature, as seen in the phase angle.
DSC Measurements
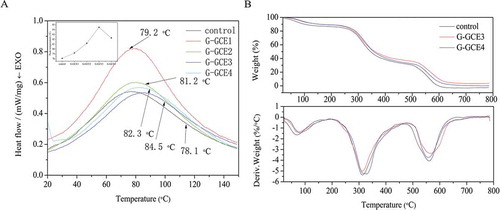
It is vital to investigate the thermal stability of GCE-containing gelatin for applications in biomaterials and the food industry, as the hydrogels may be heated during their processing and consumption.[Citation18] To examine the effect of the GCE content on the thermal stability of the dried gels, the peak values of the samples were determined by DSC (). It is generally accepted that the endothermic peak is associated with the transformation of gelatin chains from the triple helical structure to the random coil structure.[Citation11] The peak value is generally referred to as the denaturation temperature (Td). In this work, as compared to the control, the Td values of GCE-containing gelatin shifted toward higher temperatures. Specifically, the Td value was 78.1°C for the control, 84.5°C for G-GCE3, and 82.3°C for G-GCE4. This trend was similar to the gel strength result, i.e., an increase and subsequent decrease with increasing GCE content. This might be due to the greater number of crosslinking covalent bonds which were broken exothermically.[Citation33] However, it is of great interest that when the GCE content reached a critical value, Td decreased. This was likely because high GCE contents led to incomplete reactivity. Moreover, increased thermal stability helped to maintain the triple helical structure of gelatin, which is beneficial for the mechanical properties and biocompatibility of gelatin-based materials.[Citation11]
TGA
TGA was carried out to evaluate the weight loss and derivative thermogravimetry (DTG) for gelatin with different GCE contents. The weight loss of gelatin and gelatin-GCE are shown in as a function of temperature. All samples displayed similar behaviour with four main thermal degradation steps in the temperature range 20–800°C. The first stage of thermal degradation occurred between 20 and 170°C, and was related to the loss of low-molecular-weight compounds and water.[Citation34] The second stage in the range 180–460°C was associated with the decomposition of the gelatin chains.[Citation35] The weight loss event above 500°C was related to the degradation of thermally stable compounds.[Citation36]
The temperature of 50% mass, residue at 500ºC, maximum rate of weight loss, and temperature corresponding to the maximum rate of weight loss (Tm) of the control and different gelatin-GCE are given in . The data suggest that GCE-containing gelatin was more thermally stable than the control. Specifically, the interaction between the polymers may have stabilized the gelatin chains. Nevertheless, the thermal stability of G-GCE4 was lower than that of G-GCE3, and it may be hypothesized that small fragments derived from precipitates because of higher ratios of GCE to gelatin, which may have a higher heat sensitivity, are more susceptible to thermal degradation.[Citation37]
TABLE 1 The temperatures of 50% mass (T50), the residue at 500°C, the temperature corresponding to the maximum rate of mass loss (Tm), the maximum rate of mass loss of the control and different gelatin-GCE
Swelling Properties
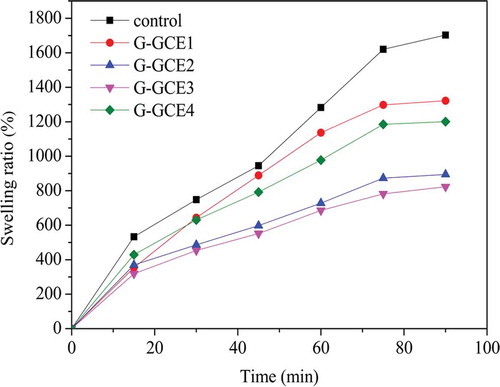
It is known that gelatin is soluble in aqueous solutions, and soaking in water for a few minutes is sufficient to induce the swelling of dried gelatin. The influence of varying GCE contents on the swelling capacity of the dried gel are shown in . The swelling of the control or the treated gelatin samples increased quickly until 75 min, and the maximum was measured at 90 min. The maximum swelling ratio was defined as the MSR. The MSR of the control, G-GCE1, G-GCE2, G-GCE3, and G-GCE4 were 1703.23, 1321.89, 894.30, 823.12, and 1200.90%, respectively. The MSR of all the samples treated with GCE were lower than that of the control, and the amount of GCE affected the swelling ability. Specifically, crosslinking could significantly decrease the swelling even at low GCE contents. In contrast, the decrease in the swelling ratio brought by G-GCE4 was much lower than other higher GCE contents, which was similar to the results obtained from the gel strength and thermal stability experiments. This is probably because high GCE contents in gelatin may lead to aggregation and result in a decreased compatibility between the gelatin chains and GCE.[Citation15] In the same manner, other authors also reported that the addition of crosslinking agents to gelatin may reduce the swelling ratio. Specifically, Johnson[Citation38] found that the capacity to swell of gel markedly reduced as the concentration of gallic acid increased. Yan et al.[Citation18] also used rutin to crosslink gelatin and found that the degree of swelling significantly reduced as the concentration of rutin increased.
Crosslinking Density of Gelatin Samples
The crosslinking degree of gelatin samples has been calculated from the moles of free amino groups per gram of gelatin.[Citation6] The number of uncrosslinked amino groups in the gelatin samples is determined by the reaction of TNBS with amino groups. shows the degree of crosslinking as a function of GCE content. G-GCE3 yielded the maximum percentage of crosslinks, while G-GCE4 had fewer crosslinks than G-GCE3. The percentage of crosslinks was still lower than 1 even at higher GCE contents because some free ε-amino groups were wrapped owing to the increased GCE content, resulting in the aggregation of gelatin and gelatin nanoparticles through inter- and intra-molecular interactions.[Citation39] Higher GCE contents (lower than the critical value) facilitated crosslinking because more availability of hydroxyl groups could crosslink with amino groups in the gelatin chains. The ability of GCE to interact with gelatin chains can be attributed to interactions between the hydroxyl groups and gelatin, which increases the mechanical and thermal stability.[Citation40] The crosslinking of gelatin with GCE can be adjusted by varying the concentration of the crosslinking agent.
TABLE 2 Degree of crosslinking, expressed as the percent of free ε-amino groups lost after crosslinking
X-Ray Diffraction Studies
It is well known that the X-ray diffraction patterns of gelatin show two characteristic peaks; the first peak indicates the distance between the molecular chains and the second peak is an extensive broadening peak in the 2θ range 15–25°.[Citation41] shows the X-ray diffraction patterns of the samples with varying GCE contents. When gelatin and GCE had very low compatibility, significant peaks of each polymer were seen in the curves;[Citation18] therefore, the curves were expressed simply as the mixed patterns of GCE and gelatin with different concentrations and showed no peaks of other polymers. It is evident that the intensity of the peak gradually decreased with increasing GCE content, indicating the gradual loss of crystallinity. The crystallinity values of the samples are shown in . The decrease in the crystal peak intensity might be due to the intermolecular interactions between the hydroxyl groups in GCE and NH2 side-chain groups in gelatin, which limits the molecular movements and thus prevents crystallization. These findings are supported by the literature.[Citation42]
TABLE 3 Crystallinity (Xc) of the samples with different GCE contents
FIGURE 4 (a) X-ray diffraction diagrams of gelatin with and without GCE; (b) FT-IR spectra of gelatin with and without GCE (control and G-GCE3).
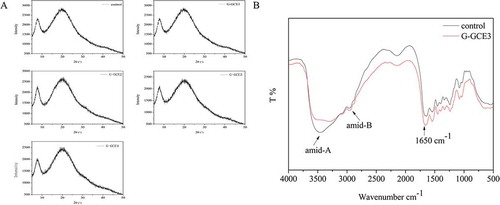
Higher GCE contents led to lower degrees of crystallinity. These results could be associated with the observed increase in the Td values with increasing GCE content. When G-GCE complex forms, amino groups in gelatin form hydrogen bonding with hydroxyl groups in GCE. This formation breaks the hydrogen bonding between amino groups and hydroxyl groups in gelatin and then results in an amorphous structure of the complex. The increase in the Td values with increasing GCE content might be due to crosslinking covalent bonds which were broken exothermically. When GCE was added, the number of crosslinking covalent bonds increased, so the degrees of crystallinity decreased and Td values increased. The decreased crystallinity of the samples further implied that the hydrogen bonding between gelatin and GCE could lead to good compatibility. Moreover, the reduced crystallinity would play a role in the composites’ degradability, mechanical properties, and swelling ratio, as confirmed by the previously mentioned results.
FTIR Spectral Analysis
FTIR spectra of gelatin samples incorporated with GCE at select concentrations are depicted in . It is generally accepted that conformational changes and functional groups of gelatin samples at the molecular level can be determined by FTIR through detailed spectral analysis.[Citation15] The results, reported in , showed that the peak corresponding to the stretching vibration of the N-H bands between 3300 and 3600 cm–1 became sharper and wider when gelatin was incorporated with GCE; this observation supported that polyphenols in GCE have enough C=O and O-H bonds to form intra- and inter-molecular hydrogen bonds.[Citation43] In addition, an amide-A band, resulting from the stretching vibration of N-H group, appeared at 3445.94 and 3299.89 cm–1 for the control and G-GCE4, respectively. It is generally accepted that the position of this band shifts to a lower frequency when the N-H group of a peptide is involved in hydrogen bonding.[Citation44] Hence, the results reported here are in line with that of Hoque et al.,[Citation37] who found that the N-H group of gelatin combined with the OH group of polyphenols via hydrogen bond formation. The absorbance of amide-B appeared at 2937.85 and 2934.28 cm–1 for the control and G-GCE4, respectively, arising from the asymmetric stretch vibration of –NH3 as well as =CH of the short chains of gelatin.[Citation15] The amide-B region of GCE-containing gelatin was shifted to a lower wavenumber, indicating the interaction of –NH3 groups between the gelatin chains.
In addition, the characteristic region (1500–1675 cm–1) corresponding to the stretching and bending vibration of N-H and C=O bands in amino groups were much sharper and stronger in G-GCE4. This was mainly due to the formation of intermolecular hydrogen bonds because of the N-H moieties and the C=O groups with O-H species in polyphenols.[Citation43] Hence, both the O-H and C=O groups bands in GCE directly strengthen the stretching vibration of the corresponding characteristic bands.
The FTIR analysis suggested that the incorporation of GCE could strengthen the intermolecular interactions in the gelatin samples and form hydrogen bonds with the corresponding functional group resulting from the reduction of the free hydrogen, which formed more compact networks between the gelatin chains. This might explain the lower swelling ratio, higher thermal stability, and viscoelastic modulus in GCE-containing gelatin.
SEM Analysis
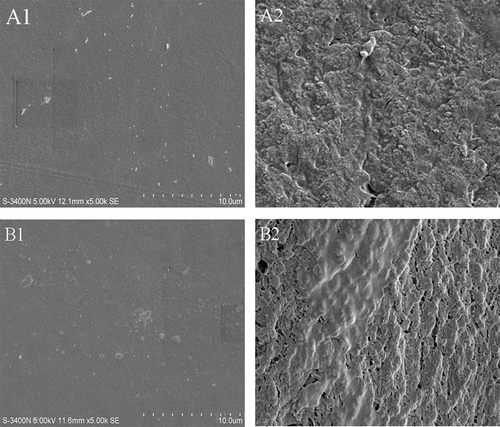
SEM can provide a better understanding of the relationship between the GCE content and the films’ structural characteristics. The SEM micrographs of the outer surface and cross-section of gelatin films at different GCE contents are illustrated in . The surfaces of the samples showed a compact and homogenous structure without any pores, while the cross-section of the samples exhibited a rougher surface, similar to previous reports.[Citation45] It is remarkable that the cross-section of the GCE-containing gelatin was rougher and had some discontinuous zones. A similar phenomenon was also observed at other GCE contents mixture (data not shown). Additionally, as the GCE content increased, the phenomenon was more pronounced because of the crosslinking of the phenolic compounds. The increased roughness might be governed by the greater number of bonds between phenolic compounds and gelatin strands via covalent and non-covalent interactions, which led to aggregation in the three-dimensional network.[Citation46] In addition, the discontinuous zones could be related to the formation of channels during the drying process.
CONCLUSIONS
In summary, the feasibility of GCE as a new natural crosslinker in the modification of gelatin was demonstrated. The physicochemical properties of gelatin before and after the incorporation of GCE were described, and structural information was gleaned. The results indicated that the introduction of GCE could enhance the gel strength and thermal stability, and significantly decrease the swelling of gelatin, up to a certain critical concentration (0.12 g/100 mL) of GCE. Additionally, GCE did not induce any significant changes on the melting and gelling points of the hydrogels. The crosslinking mechanism, as determined by FTIR, indicated that GCE mainly induced H-bonds, which reinforced the interactions between the gelatin chains. GCE can be used as a natural gelatin crosslinker and can modify the properties of gelatin. These results are significant to the development of novel crosslinking agents and applications as synthetic materials. Especially, the appropriate crosslinking by natural crosslinking agents could be a promising process to improve gel property of gelatin as food ingredient.
NOMENCLATURE
| SEM | = | scanning electron microscopy |
| HFS | = | hydrogel forming solution |
| G’ | = | elastic modulus |
| G” | = | viscous modulus |
| RCS | = | refrigerated cooling system |
| DSC | = | differential scanning calorimetry |
| Td | = | denaturation temperature |
| MSR | = | maximum swelling ratio |
| TGA | = | thermogravimetric analysis |
FUNDING
This study was supported by National Natural Science Funds (31371791), the most important open fund projects in Zhejiang province (xkzsc06), special funds for scientific research on public causes (201305013), and the Program for Changjiang scholars and Innovative Team Research in University.
Additional information
Funding
REFERENCES
- Mandl, I. The Macromolecular Chemistry of Gelatin, Vol. 5; Academic Press, New York, NY, 1964; 433 pp.
- Malafaya, P.B.; Silva, G.A.; Reis, R.L. Natural–Origin Polymers As Carriers and Scaffolds for Biomolecules and Cell Delivery in Tissue Engineering Applications. Advanced Drug Delivery Reviews 2007, 59, 207–233.
- Guo, J.; Ge, L.; Li, X.; Mu, C.; Li, D. Periodate Oxidation of Xanthan Gum and Its Crosslinking Effects on Gelatin-Based Edible Films. Food Hydrocolloids 2014, 39, 243–250.
- Djabourov, M. Architecture of Gelatin Gels. Contemporary Physics 1988, 29, 273–297.
- Achet, D.; He, X.W. Determination of the Renaturation Level in Gelatin Films. Polymer 1995, 36, 787–791.
- Bigi, A.; Cojazzi, G.; Panzavolta, S.; Roveri, N.; Rubini, K. Stabilization of Gelatin Films by Crosslinking with Genipin. Biomaterials 2002, 23, 4827–4832.
- Cao, N.; Fu, Y.; He, J. Mechanical Properties of Gelatin Films Cross-Linked, Respectively, by Ferulic Acid and Tannin Acid. Food Hydrocolloids 2007, 21, 575–584.
- Kosaraju, S.L.; Puvanenthiran, A.; Lillford, P. Naturally Crosslinked Gelatin Gels with Modified Material Properties. Food Research International 2010, 43, 2385–2389.
- Cui, L.; Jia, J.; Guo, Y.; Liu, Y.; Zhu, P. Preparation and Characterization of IPN Hydrogels Composed of Chitosan and Gelatin Cross-Linked by Genipin. Carbohydrate Polymers 2014, 99, 31–38.
- Liu, H.; Jiao, Z.; Guo, S. Effect of Epigallocatechin Gallate on the Properties of Gelatin. International Journal of Food Properties 2014, 17, 2119–2130.
- He, L.; Mu, C.; Shi, J.; Zhang, Q.; Shi, B.; Lin, W. Modification of Collagen with a Natural Cross-Linker, Procyanidin. International Journal of Biological Macromolecules 2011, 48, 354–359.
- Negi, P.S. Plant Extracts for the Control of Bacterial Growth: Efficacy, Stability, and Safety Issues for Food Application. International Journal of Food Microbiology 2012, 156, 7–17.
- Siripatrawan, U.; Noipha, S. Active Film from Chitosan Incorporating Green Tea Extract for Shelf Life Extension of Pork Sausages. Food Hydrocolloids 2012, 27, 102–108.
- Tian, F.; Li, B.; Ji, B.; Yang, J.; Zhang, G.; Chen, Y.; Luo, Y. Antioxidant and Antimicrobial Activities of Consecutive Extracts from Galla Chinensis:The Polarity Affects the Bioactivities. Food Chemistry 2009, 113, 173–179.
- Wu, J.; Chen, S.; Ge, S.; Miao, J.; Li, J.; Zhang, Q. Preparation, Properties and Antioxidant Activity of An Active Film from Silver Carp (Hypophthalmichthys Molitrix) Skin Gelatin Incorporated with Green Tea Extract. Food Hydrocolloids 2013, 32, 42–51.
- Jin, X.; Ning, Y. Antioxidant and Antitumor Activities of the Polysaccharide from Seed Cake of Camellia Oleifera Abel. International Journal of Biological Macromolecules 2012, 51, 364–368.
- Silva-Weiss, A.; Bifani, V.; Ihl, M.; Sobral, P.; Gómez-Guillén, M. Polyphenol-Rich Extract from Murta Leaves on Rheological Properties of Film-Forming Solutions Based on Different Hydrocolloid Blends. Journal of Food Engineering 2014, 140, 28–38.
- Yan, M.; Li, B.; Zhao, X.; Yi, J. Physicochemical Properties of Gelatin Gels from Walleye Pollock (< I> Theragra Chalcogramma</I>) Skin Cross-Linked by Gallic Acid and Rutin. Food Hydrocolloids 2011, 25, 907–914.
- Zhang, Y.-N.; Zhao, X.-H. Study on the Functional Properties of Soybean Protein Isolate Cross-Linked with Gelatin by Microbial Transglutaminase. International Journal of Food Properties 2013, 16, 1257–1270.
- Yan, M.; Li, B.; Zhao, X.; Yi, J. Physicochemical Properties of Gelatin Gels from Walleye Pollock (Theragra Chalcogramma) Skin Cross-Linked by Gallic Acid and Rutin. Food Hydrocolloids 2011, 25, 907–914.
- Nuthong, P.; Benjakul, S.; Prodpran, T. Characterization of Porcine Plasma Protein-Based Films As Affected by Pretreatment and Cross-Linking Agents. International Journal of Biological Macromolecules 2009, 44, 143–148.
- Charulatha, V.; Rajaram, A. Influence of Different Crosslinking Treatments on the Physical Properties of Collagen Membranes. Biomaterials 2003, 24, 759–767.
- Alfredo Uquillas, J.; Kishore, V.; Akkus, O. Genipin Crosslinking Elevates the Strength of Electrochemically Aligned Collagen to the Level of Tendons. Journal of the Mechanical Behavior of Biomedical Materials 2012, 15, 176–189.
- Ahmad, M.; Benjakul, S.; Prodpran, T.; Agustini, T.W. Physico-Mechanical and Antimicrobial Properties of Gelatin Film from the Skin of Unicorn Leatherjacket Incorporated with Essential Oils. Food Hydrocolloids 2012, 28, 189–199.
- Wu, J.; Ge, S.; Liu, H.; Wang, S.; Chen, S.; Wang, J.; Li, J.; Zhang, Q. Properties and Antimicrobial Activity of Silver Carp (Hypophthalmichthys Molitrix) Skin Gelatin-Chitosan Films Incorporated with Oregano Essential Oil for Fish Preservation. Food Packaging and Shelf Life 2014, 2, 7–16.
- Cho, S.M.; Gu, Y.S.; Kim, S.B. Extracting Optimization and Physical Properties of Yellowfin Tuna (Thunnus Albacares) Skin Gelatin Compared to Mammalian Gelatins. Food Hydrocolloids 2005, 19, 221–229.
- Sinthusamran, S.; Benjakul, S.; Kishimura, H. Characteristics and Gel Properties of Gelatin from Skin of Seabass (Lates Calcarifer) As Influenced by Extraction Conditions. Food Chemistry 2014, 152, 276–284.
- Naczk, M.; Grant, S.; Zadernowski, R.; Barre, E. Protein Precipitating Capacity of Phenolics of Wild Blueberry Leaves and Fruits. Food Chemistry 2006, 96, 640–647.
- Wang, Y.; Zhang, H.; Liang, H.; Yuan, Q. Purification, Antioxidant Activity and Protein-Precipitating Capacity of Punicalin from Pomegranate Husk. Food Chemistry 2013, 138, 437–443.
- Van Buren, J.P.; Robinson, W.B. Formation of Complexes Between Protein and Tannic Acid. Journal of Agricultural and Food Chemistry 1969, 17, 772–777.
- Chiou, B.-S.; Avena-Bustillos, R.J.; Shey, J.; Yee, E.; Bechtel, P.J.; Imam, S.H.; Glenn, G.M.; Orts, W.J. Rheological and Mechanical Properties of Cross-Linked Fish Gelatins. Polymer 2006, 47, 6379–6386.
- Montero, P.; Fernández-Dı́az, M.D.; Gómez-Guillén, M.C. Characterization of Gelatin Gels Induced by High Pressure. Food Hydrocolloids 2002, 16, 197–205.
- Bigi, A.; Panzavolta, S.; Rubini, K. Relationship Between Triple-Helix Content and Mechanical Properties of Gelatin Films. Biomaterials 2004, 25, 5675–5680.
- Guo, J.; Ge, L.; Li, X.; Mu, C.; Li, D. Periodate Oxidation of Xanthan Gum and Its Crosslinking Effects on Gelatin-Based Edible Films. Food Hydrocolloids 2014, 39, 243–250.
- Mu, C.; Guo, J.; Li, X.; Lin, W.; Li, D. Preparation and Properties of Dialdehyde Carboxymethyl Cellulose Crosslinked Gelatin Edible Films. Food Hydrocolloids 2012, 27, 22–29.
- Subject Index. Methods in Enzymology, Vol. 75: Academic Press, New York, NY, 1982; 1–824 pp.
- Hoque, M.S.; Benjakul, S.; Prodpran, T. Effects of Partial Hydrolysis and Plasticizer Content on the Properties of Film From Cuttlefish (Sepia Pharaonis) Skin Gelatin. Food Hydrocolloids 2011, 25, 82–90.
- Copyright. In: Advances in Physiology, Biochemistry, and Function; Johnson, A.D.; Gomes,W.R.; Eds.; Academic Press. New York, NY. 1977; p. iv.
- Dupont-Gillain, C.C.; Jacquemart, I.; Rouxhet, P.G. Influence of the Aggregation State in Solution on the Supramolecular Organization of Adsorbed Type I Collagen Layers. Colloids and Surfaces B: Biointerfaces 2005, 43, 179–186.
- Jayakumar, G.C.; Kanth, S.V.; Sai, K.P.; Chandrasekaran, B.; Rao, J.R.; Nair, B.U. Scleraldehyde As a Stabilizing Agent for Collagen Scaffold Preparation. Carbohydrate Polymers 2012, 87, 1482–1489.
- Wang, J.; Wan, Y.Z.; Luo, H.L.; Gao, C.; Huang, Y. Immobilization of Gelatin on Bacterial Cellulose Nanofibers Surface Via Crosslinking Technique. Materials Science and Engineering: C 2012, 32, 536–541.
- Peña, C.; de la Caba, K.; Eceiza, A.; Ruseckaite, R.; Mondragon, I. Enhancing Water Repellence and Mechanical Properties of Gelatin Films by Tannin Addition. Bioresource Technology 2010, 101, 6836–6842.
- Li, J.-H.; Miao, J.; Wu, J.-L.; Chen, S.-F.; Zhang, Q.-Q. Preparation and characterization of Active Gelatin-Based Films Incorporated with Natural Antioxidants. Food Hydrocolloids 2014, 37, 166–173.
- Doyle, B.B.; Bendit, E.; Blout, E.R. Infrared Spectroscopy of Collagen and Collagen-Like Polypeptides. Biopolymers 1975, 14, 937–957.
- Rattaya, S.; Benjakul, S.; Prodpran, T. Properties of Fish Skin Gelatin Film Incorporated with Seaweed Extract. Journal of Food Engineering 2009, 95, 151–157.
- Jridi, M.; Hajji, S.; Ayed, H.B.; Lassoued, I.; Mbarek, A.; Kammoun, M.; Souissi, N.; Nasri, M. Physical, Structural, Antioxidant and Antimicrobial Properties of Gelatin-Chitosan Composite Edible Films. International Journal of Biological Macromolecules 2014, 67, 373–379.