Abstract
Maize is a main botanical source used for extraction of starch in the world market. New maize cultivars with different amylose contents and special starch metabolism characteristics have been generated. Three types of maize cultivars, namely, normal maize, waxy maize (wxwx homozygous mutant), and super-sweet maize (sh2sh2 homozygous mutant), were investigated to determine differences in endosperm structures, morphologies, and physicochemical properties of starches. Maize kernels exhibited significantly different contents of total starch, soluble sugar, and amylose. Normal maize kernels contained the largest proportion of floury endosperm, followed by waxy maize and then super-sweet maize. Normal maize starch and waxy maize starch were larger in size than super-sweet maize starch. Normal maize starch and waxy maize starch were spherical and polygonal in floury and vitreous endosperms, respectively. Super-sweet maize starch was spherical both in floury and vitreous endosperms. Waxy maize starch showed the strongest birefringence patterns, the highest crystallinity and the largest proportion of ordered structure in external region of granules, and the largest proportion of double helix components, followed by normal maize starch and then super-sweet maize starch. Waxy maize starch showed the highest peak viscosity, trough viscosity, breakdown viscosity, gelatinization temperatures (i.e., gelatinization conclusion temperature, gelatinization onset temperature, gelatinization peak temperature, and gelatinization enthalpy). By contrast, super-sweet maize starch showed the lowest corresponding values for these parameters.
INTRODUCTION
Maize starch accounts for 80% of all starches in the world and is the major component of many staple foods.[Citation1] Different maize classes and cultivars are grown in different countries. Maize starch physiochemical properties change with the starch structure and composition, which vary among genotypes and cultural practices.[Citation2] Maize is usually divided into four categories: (1) waxy maize, which contains nearly 0% amylose; (2) normal maize, which contains approximately 25% amylose; (3) high amylose maize, which contains high amylose content from 40 to 70%; and (4) sugary maize, which contains lower starch content and higher accumulation of sucrose.[Citation3,Citation4]
Maize endosperm mutants have been reported to show varying carbohydrate compositions. These mutants show differences in amylose content, amylopectin and amylose structures, branching degree, chain length, and intermediate component percentage.[Citation5] The waxy gene encodes granule-bound starch synthase I (GBSSI), which is responsible for amylose synthesis in maize endosperm. Yuan et al. studied three waxy maize mutant starches (wx, duwx, and aewx) from two inbred lines, with results showing that higher enthalpy and gelatinization temperatures elicit higher amounts of longer chains.[Citation6] Mendez-Montealvoa et al. indicated that wxwx and duwx maize mutants exhibit distinct thermal and rheological properties in their native states and after β-amylolysis.[Citation1] Remarkable differences in starch accumulation, granule size distribution, starch microstructure, thermal properties, and kernel microstructure between ae wx and sbe1a ae wx maize mutants have been established.[Citation7]
Sweet maize, a maize mutant with high contents of soluble sugar, is a profitable cereal that is extensively used in the production of flour, bread, and vegetables. Two genes, namely, su1 and sh2, affect starch metabolism and have been extensively used for the development of sweet maize cultivars. White et al. studied the properties of sugary (su2/su2) maize starch and showed that sugary starch contains 35% amylose, exhibits a smaller granule size distribution than normal starch, and presents more suitable pasting properties for application in starch-thinned acidic food stuffs.[Citation8] The swelling power of sweet maize starch is significantly lower than that of normal maize starch (NMS).[Citation9] Super-sweet maize kernels contain decreased total carbohydrates at the maturation stage because of the high sugar content in the sh2sh2 mutant.[Citation10]
The starch properties of these two main types of maize cultivars (waxy and sweet) have been well studied.[Citation11,Citation12] However, comprehensive and systematical comparisons regarding the underlying starch-granule distribution mechanism in kernel endosperm and differences in morphology and physiochemical properties of starches from normal, waxy, and super-sweet maize have been rarely reported. The aim of the present study is to investigate differences in endosperm structure and physiochemical properties of starches from normal, waxy (wxwx), and super-sweet (sh2sh2) maize.
MATERIALS
Maize (Zea mays L.) cultivars used in this study included normal maize (NL988), waxy maize mutant (wxwx homozygote, YN1), and super-sweet maize mutant (sh2sh2 homozygote, CT135).[Citation13] Maize seeds were purchased from Lixiahe Regional Agricultural Research Institute, Jiangsu Province, China and were grown in the experimental fields of Yangzhou University (Key Laboratory of Crop Genetics and Physiology) in Jiangsu from April 2014 to August 2014. To determine the exact date of anthesis, the plants were tagged. Maize kernels were harvested at mature stage (45 days after anthesis) and used for structure observation and starch extraction.
METHODS
Observation of Endosperm Ultrastructure
Maize kernels were fractured longitudinally in the center of the kernel by hand using two pairs of pliers. The fractured kernels, with fractured surfaces facing upward, were adhered to the sample stage and sprinkled with gold at the fracture region. The structures of the caryopses were observed and photographed using a scanning electron microscope (SEM, XL-30, Philips, the Netherlands) equipped with a charge coupled device camera at 20 kV.
Starch Isolation
Maize kernels were steeped in distilled water at 25°C for 24 h and thoroughly ground in a mortar. The ground sample was filtered through four gauze layers and then sequentially through 100-, 200-, 300- and 400-mesh wire screens. The powder was then centrifuged at 4000 × g for 5 min to remove the yellow gel-like layer above the starch granules. The starch granules were collected, resuspended in 0.05 mol L–1 NaOH solution, and centrifuged at 4000 × g for 5 min to remove the protein. This step was repeated until the supernatant was clear and contained no protein. The starch was washed three times with water and twice with ethanol. Finally, samples were dried at room temperature (25°C) and then stored at –20°C until further use.
Determination of Total Starch, Amylose, and Soluble Sugar Contents
Maize kernels were dried at 70°C and ground in a mortar, and the ground sample was passed through 100-mesh wire screens. The powder was used for determining total starch and soluble sugar contents using the assay kit (QY1-1 [Comin Biotechnology Co., Ltd., China] and SY3-2 [Comin Biotechnology Co., Ltd., China], respectively). Amylose content of the isolated starch was determined using the assay kit (K-AMYL [Megazyme Ltd., Ireland]).
Morphological Observation
Starch granules were examined for the presence of birefringence using a polarizing light microscope. A sample was prepared from a mixture of 2 mg of starch granules and 1 mL of distilled water. The “Maltese cross” patterns of starch granules were observed under a polarizing light microscope (BX53, Olympus, Japan) equipped with a camera. The axial length of starch granules in the light microscope images was calculated using Image-pro Plus software (Version 6.0, Media Cybernetics, America). A total of 2000 samples of three types of starch granules were used for calculations.
Determination of Pasting and Thermal Properties
The pasting properties of starch were determined through a rapid viscosity analyzer (RVA, Newport Scientific, Narrabeen, Australia). Starch samples (1.96 g, dry weight) were suspended in 26.04 mL of deionized water and then heated from 50 to 95°C at a stirring rate 160 r/min. Peak viscosity (mPa.s), trough viscosity (mPa.s), breakdown (mPa.s) viscosity, final viscosity (mPa.s) viscosity, setback (mPa.s), pasting temperature (°C), and pasting time (min) were recorded. Starch thermal properties were determined using a differential scanning calorimeter (DSC 200-F3, Netzsch, Germany) following the method of Wei et al.[Citation14]
X-ray Diffraction (XRD) Analysis
Starches were stored in a sealed beaker with a saturated solution of NaCl maintained at a constant humidity (relative humidity = 75%) for 7 d at room temperature (25°C) prior to XRD. Starch XRD patterns were obtained on an X-ray power diffractometer (D8, Bruker, Germany) following the method of Wang et al.[Citation15] The diffractometer was operated at 200 mA and 40 kV. The scanning region of the diffraction angle (2θ) was 4 to 40° with a step size of 0.6 s. Image-Pro Plus and Adobe Photoshop CS5 were used to calculate the relative degree of crystallinity of the starches.
Fourier-Transform Infrared (FTIR) Spectroscopy Analysis
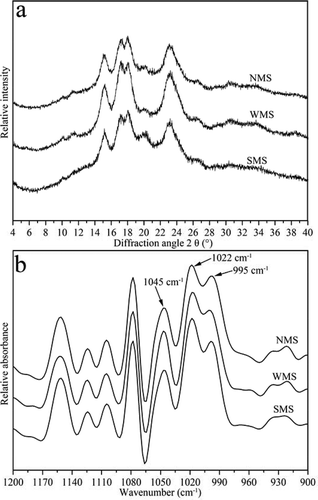
The starches (40% moisture) were added to a sampler in an FTIR spectrometer (Varian 7000, USA) equipped with an attenuated total reflectance (ATR) system. Sample spectra were recorded and corrected by a baseline in the region of 1200–800 cm–1 before deconvolution. The assumed line shape was Lorentzian with a half-width of 19 cm–1 and a resolution enhancement factor of 1.9.[Citation16] Infrared (IR) absorbance values at 1045, 1022, and 995 cm–1 were calculated from the deconvoluted spectra by recording the height of the absorbance bands from the baseline to the peak point.
Solid-State 13C Cross Polarization/Magic Angle Spinning-Nuclear Magnetic Resonance (13C CP/MAS NMR) Analysis
Solid-state 13C CP/MAS NMR analysis of starch was implemented on a spectrometer (Bruker Advance III 400 MHz, Bruker BioSpin International AG, Germany). Amorphous starch prepared in this study was isolated from rice kernel following the method of Atichokudomchai et al.[Citation17] The samples were spun at a rate of 7.5 kHz at room temperature in a 7 mm ZrO2 rotor. The C1 region of the ordered subspectra was peak-fitted using PeakFit software (version 4.12). Four individual peaks were distinguished at approximately 99.5, 100.4, 101.6, and 102.9 ppm according to the method described by Sivam et al.[Citation18] The relative proportions of amorphous, V-type single-helical, and double-helical components of starches were determined according to the method of Tan et al.[Citation19]
Statistical Analysis
Statistical analyses of data were conducted using SPSS Statistics (version 19.0, International Business Machines Corporation, USA). Means were compared using Fisher’s protected least significant difference (LSD) test at 0.05 probability significance level. Origin 8.0 and Adobe Photoshop CS5 were used to draw figures.
RESULTS AND DISCUSSION
Endosperm Ultrastructure
The maize caryopsis is composed of the endosperm, germ, and pericarp. Mature endosperm is the major component of the kernel and contains starch, proteins, and lipids.[Citation12] Two zones with different characteristics are found in the maize endosperm, namely, the vitreous and floury endosperms. Moreover, the proportion of these two zones in the endosperm greatly affects the quality and functionality of maize-based foods.[Citation20] Vitreous endosperm exhibits a hard and compact structure, whereas floury endosperm presents a soft and loose structure.[Citation21] Starch granules are polygonal in shape and rarely contain air space in the vitreous endosperm; in floury endosperm, starch granules are spherical in shape with a large amount of air space.[Citation12]
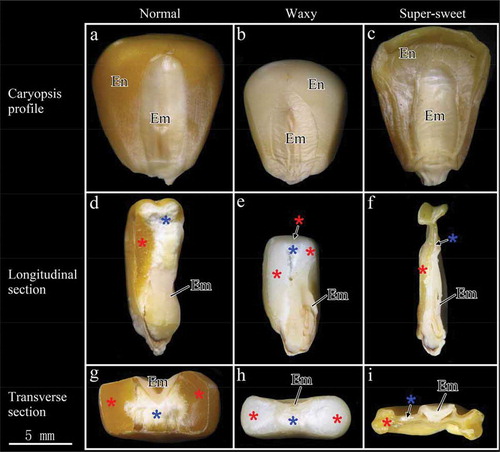
Normal, waxy, and super-sweet maize caryopsis profiles and endosperm structures are presented in . Normal maize was large in shape and showed yellow coloration (). Floury endosperm accounted for a large proportion of the total endosperm, whereas vitreous endosperm, which was only distributed on the lateral area of the kernel ( and ), accounted for only a minor proportion of the total endosperm. The kernel size of waxy maize was remarkably smaller than that of normal maize (). Floury endosperm accounted for a fraction of the total endosperm and was surrounded by vitreous endosperm positioned on the lateral and top regions of the kernel ( and ). The super-sweet maize kernel was seriously collapsed and appeared shrunken. This kernel exhibited semitransparent characteristics and primarily contained vitreous endosperm (, , and ).
FIGURE 1 Images of the kernel profile (a, b, c), longitudinal section (d, e, f), and transverse section (g, h, i) of normal, waxy, and super-sweet maize, respectively. En: endosperm; Em: embryo. Red and blue asterisks indicate vitreous endosperm and floury endosperm, respectively.
Distributions of starch granules in maize endosperm were clearly observed under SEM (). Nutrients transported from the endosperm cavity were transported to starchy endosperm cells by endosperm transfer cells.[Citation22] Larger volumes of the maize kernel elicited longer nutrient transport paths. This result signifies that small volumes of maize kernel are conducive to nutrient deposition, thereby forming a larger proportion of vitreous endosperm. This deduction may explain our results: normal maize with larger kernel volumes contained a higher proportion of floury endosperm, while super-sweet maize with smaller kernel volumes contained a higher proportion of vitreous endosperm.
FIGURE 2 Morphology of starch granules in vitreous and floury endosperm of normal, waxy, and super-sweet maize kernels: (a) Floury endosperm, normal maize; (b) vitreous endosperm, normal maize; (c) floury endosperm, waxy maize; (d) vitreous endosperm, waxy maize; (e) floury endosperm, super-sweet maize; and (f) vitreous endosperm, super-sweet maize. SG: starch granule; PB: protein body.
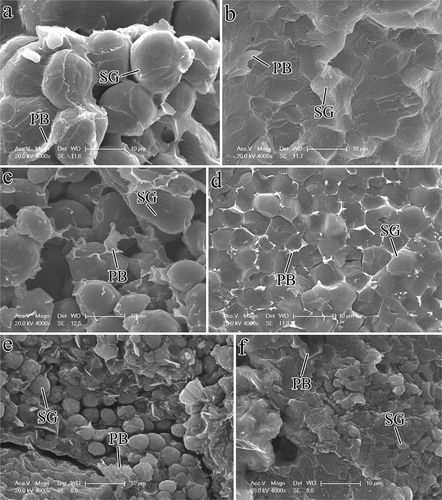
In normal and waxy maize floury endosperms, starch granules were larger in size and spherical in nature; these granules were surrounded by more protein bodies compared with those of the super-sweet maize (, , and ). In normal maize vitreous endosperm, starch granules were highly extruded by protein bodies that rendered polygonal granules and individual starch granules were difficult to distinguish (). For waxy maize, individual starch granules were easy to find in vitreous endosperm (). Starch granules in super-sweet maize vitreous endosperm were spherical and mixed with protein bodies; thus, these granules exhibited a smaller size compared with that in floury endosperm ().
Morphology, Size Distribution, and Content of Amylose, Total Starch, and Soluble Sugar
The morphologies of the starches captured by light and polarized microscopy are shown in . Normal maize starch (NMS) and waxy maize starch (WMS) were spherical, oval, or polyhedral in shape ( and ), whereas super-sweet maize starch (SMS) was completely oval in shape (). NMS showed typical distorted and dark “Maltese cross” patterns at the center of the granules (), and WMS exhibited strong birefringence patterns with the hila at the center of the granules (). Most of the SMS presented darker “Maltese cross” patterns and some birefringence patterns nearly completely disappeared (). The difference in “Maltese cross” patterns was attributed to the content of ordered amylopectin within the lamellar structure of the granules.[Citation23] Similar granule shapes and birefringence patterns have been reported in aewx and sbe1a ae wx maize mutants by Li et al.[Citation7]
FIGURE 3 Morphology of NMS, WMS, and SMS: (a, b) NMS, (c, d) WMS, and (e, f) SMS. Black arrows in (e) indicate starch granules with dark and dispersed Maltese cross patterns as observed under a polarized microscope (f).
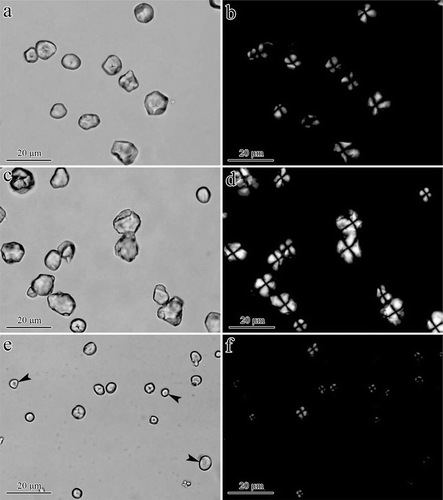
The three types of maize starch granules showed significant differences in size distribution and average size (). NMS showed the largest average size, whereas SMS exhibited the lowest. The amylose/total starch and soluble sugar contents of super-sweet maize were significantly higher than those of normal and waxy maize. However, super-sweet maize presented significantly lower total starch contents (30%) than normal maize (69%) and waxy maize (72%). This result was in accordance with a previous study that indicated a higher sucrose content and an equally lower content of total starch in the sh2 mutant compared with that in normal maize cultivars.[Citation24]
TABLE 1 Total starch, soluble sugar, and amylose contents of maize kernel and size distribution, crystallinity, and IR ratio of NMS, WMS, and SMS
XRD Spectra
Native starch granules were partially crystalline, and the degree of crystallinity ranged from 20 to 40%. Based on the XRD patterns, we classified the starch crystalline structure into three categories: A-type, B-type, and C-type.[Citation25] Relative degrees of starch crystallinity differ in amylose content, branching, length of amylopectin outer chains, and molecular mass. Native high-amylose maize starch showed a typical B-type crystalline structure with major reflections at approximately 5.7, 15.1, 17.1, 19.9, 22.1, and 24.0° 2θ.[Citation26,Citation27] Native NMS presented a typical A-type crystalline structure with major peaks at 15 and 23° and double peaks at 17 and 18°.[Citation28] The XRD patterns of NMS, WMS, and SMS are shown in . All three types of starches exhibited typical characteristics of the A-type crystalline structure. WMS showed stronger reflections at 15, 17, 18, and 23°. The peak intensity of this starch at 19.9° was primarily attributed to amylose-lipid complexes.[Citation29] SMS contained the highest amylose content (35%), and WMS consisted of approximately 100% amylopectin. This result may explain the fact that SMS showed strongest peak at 19.9°, followed by NMS and WMS. The relative degrees of crystallinities of the three types of starches are shown in . WMS showed the highest relative degree of crystallinity (31.4%), whereas SMS showed the lowest relative degree of crystallinity (22.7%). The degree of starch crystallinity has been reported to be negatively correlated with amylose content,[Citation30] which is in accordance with our results.
FTIR Spectra
The development of sampling devices, such as ATR-FTIR, combined with software for spectrum deconvolution, provides opportunities for studying short-range ordered structures in the external region of starch granules.[Citation16] FTIR is sensitive to variations in crystallinity, molecular chain conformation, and double helix structure. At wavenumbers from 1200 to 800 cm–1, three maximum absorbance peaks at approximately 1045, 1022, and 995 cm–1 were observed (see ). The bands at 1045 and 1022 cm–1 were attributed to the ordered and amorphous structures of starch, respectively.[Citation31] Absorbance at 995 cm–1 was attributed to the bending vibrations of C–OH bonds.[Citation32] IR ratios of 1045/1022 and 1022/995 cm–1 were used as convenient indices to evaluate the degree of ordered structure of the starches.[Citation16] The absorbances of these peaks were strongly associated with alterations in their macromolecular order.
The 1045/1022 and 1022/995 cm–1 IR ratios are listed in . Among the spectra, WMS showed the strongest absorbance at 1045 cm–1, whereas SMS showed the weakest. At 995 cm–1, WMS showed the weakest absorbance, whereas SMS showed the strongest. WMS showed the highest IR ratio of 1045/1022 cm–1 (0.72), followed by NMS (0.68) and SMS (0.64). These results indicate that WMS presents the largest proportion of ordered structures in the external region of starch granules, whereas SMS presents the least. This result is in accordance with XRD findings.
CP/MAS 13C NMR Spectra
CP/MAS 13C NMR spectroscopy is commonly used to study the structure and structural changes in different types of starches. All of the CP/MAS 13C NMR spectra obtained from 120 ppm to 50 ppm generated four main resonances peaks (103, 82, 73, and 62 ppm).[Citation33] The NMR spectra of native starch consist of amorphous and crystalline components.[Citation19] The NMR spectra of NMS, WMS, SMS, and amorphous starch are shown in . Four main resonances peaks (C1, C4, [C2, C3, and C5], and C6) were found. Differences were primarily observed at the C1 region, where NMS, WMS, and SMS occurred as triplets at approximately 99.5, 100.5, and 101.5 ppm. The peak at 102.9 ppm was presented as a shoulder on the downfield portion of the C1 region. Compared with NMS, WMS showed a weaker and narrower peak while SMS exhibited a stronger and wider peak at 102.9 ppm.
FIGURE 5 NMR spectrum of NMS, WMS, and SMS: (a) Native starch and amorphous starch spectra; (b) NMS and its subspectra; (c) WMS and its subspectra; and (d) SMS and its subspectra. Black arrows indicate individual peaks at 102.9, 101.5, 100.5, and 99.5 ppm in the C1 region.
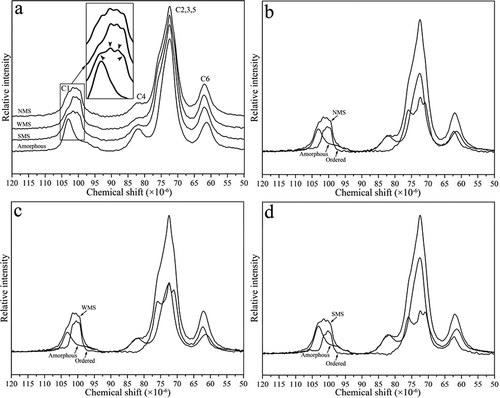
The third component reported for starch is the V-type single helix, which often includes a complexing agent in the helical channel.[Citation34] The NMR spectrum of native starch can be decomposed into amorphous and ordered subspectra. The peak at 102.9 ppm in the subspectra was coincident with V-type single-helical components, and peaks at 99.5, 100.5, and 101.5 ppm were consistent with double-helical components.[Citation33] According to the subtraction method, ordered subspectra can be separated from native starch spectra by subtracting the standard amorphous starch spectrum until no residual intensity remains at 84 ppm (a chemical shift of the native spectra with intensity attributed only to amorphous components).[Citation19] The ordered and amorphous subspectra of NMS, WMS, and SMS are shown in , , and , respectively, and the relative proportions of double-helical, single-helical, and amorphous components are listed in . WMS showed the weakest amorphous subspectra and the strongest ordered subspectra (). Therefore, WMS presents the lowest proportion of amorphous components (48%, ). By contrast, SMS presented the strongest amorphous and the weakest ordered subspectra (). Thus, SMS has the highest relative proportion of amorphous components observed (64.8%). In terms of crystalline components, NMS showed the highest proportion of single helices and SMS exhibited the lowest proportion of double helices (). Moreover, no single helical components were calculated in the WMS spectra. This V-type single helical component has only been observed in high-amylose starches and is attributed to complexation between lipids and a fraction of the amylose.[Citation35]
TABLE 2 Relative proportion of double-helix, single-helix, and amorphous components of NMS, WMS, and SMS
Pasting Properties
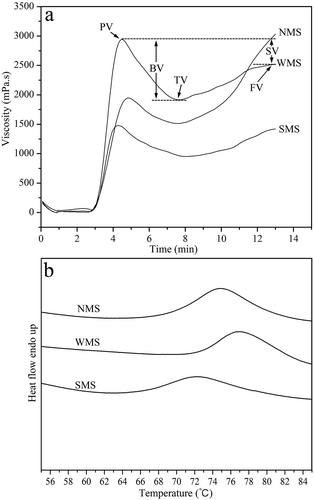
The RVA is an effective instrument for measuring the pasting properties of starch.[Citation36] RVA patterns of NMS, WMS, and SMS are provided in , and their pasting parameters are shown in . The peak, trough, breakdown, and final viscosities of NMS, WMS, and SMS showed significant differences. A previous study reported that breakdown viscosity is negatively correlated with amylose content.[Citation37,Citation38] Peak and trough viscosities were highest in WMS, followed by NMS and SMS. Breakdown viscosity was highest in WMS and lowest in NMS; the final viscosity and peak times were highest in NMS and lowest in SMS. Setback viscosity resulting from the rearrangement of amylose molecules leached from gelatinized starch during cooling was used to predict the retrogradation tendency of starch samples.[Citation30] NMS presented the highest setback viscosity, followed by SMS and WMS. The pasting temperature was highest in SMS and lowest in NMS. Differences in the pasting properties of starch from different cultivars may be due to various factors, such as amylose content, swelling power, granule size, amylose molecular size, and amylopectin chain length.[Citation37]
TABLE 3 Pasting properties of NMS, WMS, and SMS
Thermal Properties
The gelatinization properties of starch are usually measured through DSC and related to various factors, including the size, proportion, crystalline organization, and ultrastructure of starch granules.[Citation39] DSC patterns of NMS, WMS, and SMS are shown in , and their thermal parameters are listed in . Based on the patterns and calculated data, we observed that WMS presented the highest To, Tp, and Tc, whereas SMS presented the lowest corresponding values. This result agreed with previous results: increases in To, Tp, and Tc reflected more ordered crystallinities in the starches.[Citation40,Citation41] Singh et al. found that starches with higher contents of long-chain length amylopectins show higher gelatinization temperatures.[Citation42] WMS showed the highest ΔH, whereas SMS showed the lowest corresponding value; this parameter reflects a loss in double helical order during gelatinization as well as a decrease in amylose content.[Citation43] Starch with higher amylopectin contents could easily form more crystalline structures within granules; this type of starch also requires more energy to melt and uncoil the double helix structure during gelatinization.[Citation42]
TABLE 4 Thermal properties of NMS, WMS, and SMS
CONCLUSION
Floury and vitreous endosperms were found in the three types of maize kernels studied in this work. Normal maize contained a large proportion of floury endosperm, whereas waxy maize and super-sweet maize contained smaller proportions of floury endosperm. The starch granules in floury endosperm of normal maize and waxy maize were larger in size and spherical, whereas that of super-sweet maize were smaller. The starch granules in vitreous endosperm of normal maize and waxy maize were highly extruded and polygonal, whereas that of super-sweet maize were spherical. WMS showed the strongest birefringence patterns, while SMS showed the weakest birefringence. SMS showed the smallest average size and contained the lowest total starch content and highest amylose and soluble sugar contents. The three types of starch granules studied showed a typical A-type crystalline structure and exhibited different peak intensities at 15, 17, 18, and 19.9° 2 θ in the XRD patterns. The highest crystallinity was found in WMS, followed by NMS and SMS. WMS showed the highest IR ratio of 1045/1022 cm–1, whereas SMS showed the lowest. WMS and SMS respectively contained the largest and least proportions of short-range ordered structures in the external region of the granules. In the C1 region of the native starch NMR spectra, the three types of starch granules presented different resonances peak at 102.9 ppm. The starch granules also showed different peak intensities in the ordered and amorphous subspectra. SMS showed the largest proportion of amorphous components, followed by NMS and WMS. NMS showed the highest proportion of single-helix crystalline components, whereas SMS exhibited the lowest proportion of double-helical crystalline components. WMS showed the highest peak viscosity, trough viscosity, breakdown viscosity, gelatinization temperature (To, Tp, and Tc), and ΔH, whereas SMS showed the lowest corresponding values.
FUNDING
This work was supported by the National Natural Science Foundation of China (No. 31171482, 31270228, 31071341) and A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Additional information
Funding
REFERENCES
- Mendez-Montealvoa, G.; Wang, Y.J.; Campbell M. Thermal and Rheological Properties of Granular Waxy Maize Mutant Starches after β-Amylase Modification. Carbohydrate Polymers 2011, 83, 1106–1111.
- Singh, N.; Kaur, A.; Shevkani, K. Maize: Grain Structure, Composition, Milling, and Starch Characteristics. In Maize: Nutrition Dynamics and Novel Uses; Chaudhary, D.P.; Kumar, S.; Langyan, S.; Eds.; Springer: India, 2014; 65–76.
- Nelson, O.; Pan, D. Starch Synthesis in Maize Endosperm. Annual Review of Plant Physiology 1995, 46, 475–496.
- Singh, N.; Sandhu, K.S.; Kaur, M. Physicochemical Properties Including Granular Morphology, Amylose Content, Swelling and Solubility, Thermal, and Pasting Properties of Starches from Normal, Waxy, High Amylose, and Sugary Corn. Progress in Food Biopolymer Research 2005, 1, 43–54.
- Shannon, J.C.; Garwood, D.L. Genetics and Physiology of Starch Development. In Starch: Chemistry and Technology; Whistler, R.L.; BeMiller, J.N.; Paschall, E.F.; Eds.; Academic Press: New York, NY, 1984; 25–86.
- Yuan, R.C.; Thompson, D.B.; Boyer, C.D. Fine Structure of Amylopectin in Relation to Gelatinization and Retrogradation Behavior of Maize Starches from Three wx-Containing Genotypes in Two Inbred Lines. Cereal Chemistry 1993, 70, 81–89.
- Li, J.H.; Guiltinan, M.J.; Thompson, D.B. Mutation of the Maize sbe1a and ae genes Alters Morphology and Physical Behavior of wx-Type Endosperm Starch Granules. Carbohydrate Research 2007, 342, 2619–2627.
- White, P.J.; Pollak, L.M.; Johnson, L.A. Starch-Thickened Acidic Foodstuffs and Method of Preparation. US Patent, Washington, DC, 1994; 5, 356, 655 pp.
- Li, J.; Corke, H. Physicochemical Properties of Maize Starches Expressing Dull and Sugary-2 Mutants in Different Genetic Backgrounds. Journal of Agricultural and Food Chemistry 1999, 47, 4939–4943.
- Hossain, F.; Nepolean, T.; Vishwakarma, A.K.; Pandey, N.; Prasanna, B.M.; Gupta, H.S. Mapping and Validation of Microsatellite Markers Linked to sugary1 and shrunken2 Gene in Maize (Zea mays L.). Journal of Plant Biochemistry and Biotechnology 2015, 24, 135–142.
- Wang, B.; Wang, L.J.; Li, D.; Özkan, N.; Li, S.J.; Mao, Z. H. Rheological Properties of Waxy Maize Starch and Xanthan Gum Mixtures in the Presence of Sucrose. Carbohydrate Polymers 2009, 77, 472–481.
- Miao, M.; Li, R.; Jiang, B.; Cui, S.W.; Lu, K.; Zhang, T. Structure and Digestibility of Endosperm Water-Soluble α-Glucans from Different Sugary Maize Mutants. Food Chemistry 2014, 143, 156–162.
- Hao, X.Q.; Wu, Z.K. The Major Agronomic and Quality Characters in Double Recessive Sweet-Waxy Maize. Acta Agronomica Sinica 2003, 29, 321–329.
- Wei, C.X.; Qin, F.L.; Zhou, W.D.; Xu, B.; Chen, C.; Chen, Y.F.; Wang, Y.P.; Gu, M.H.; Liu, Q.Q. Comparison of the Crystalline Properties and Structural Changes of Starches from High-Amylose Transgenic Rice and Its Wild Type during Heating. Food Chemistry 2011, 128, 645–652.
- Wang, S.J.; Yu, J.L.; Zhu, Q.H.; Yu, J.G.; Jin, F.M. Granular Structure and Allomorph Position in C-Type Chinese Yam Starch Granule Revealed by SEM, 13C CP/MAS NMR and XRD. Food Hydrocolloids 2009, 23, 426–433.
- Sevenou, O.; Hill, S.E.; Farhat, I.A.; Mitchell, J.R. Organisation of the External Region of the Starch Granule as Determined by Infrared Spectroscopy. Internal Journal of Biological Macromolecules 2002, 31, 79–85.
- Atichokudomchai, N.; Varavinit, S.; Chinachoti, P. A study of Ordered Structure in Acid-Modified Tapioca Starch by 13C CP/MAS Solid-State NMR. Carbohydrate Polymers 2004, 58, 383–389.
- Sivam, A.S.; Waterhouse, G.I.N.; Zujovic, Z.D.; Perera, C.O.; Sun-Waterhouse, D. Structure and Dynamics of Wheat Starch in Breads Fortified with Polyphenols and Pectin: An ESEM and Solid-State CP/MAS 13C NMR Spectroscopic Study. Food and Bioprocess Technology 2013, 6, 110–123.
- Tan, I.; Flanagan, B.M.; Halley, P.J.; Whittaker, A.K.; Gidley, M.J. A Method for Estimating the Nature and Relative Proportions of Amorphous, Single, and Double-Helical Components in Starch Granules by 13C CP/MAS NMR. Biomacromolecules 2007, 8, 885–891.
- Rangel-Meza, E.; Muñoz-Orozco, A.; Vázquez-Carrillo, G.; Cuevas-Sánchez, J.; Merino-Castillo, J.; Miranda-Colín, S. Alkaline Cooking, Preparation and Quality of Corn Tortilla from Ecatlan, Puebla, México. Agrociencia 2004, 38, 53–61.
- Wang, D.; Eckhoff, S. R. Effect of Broken Corn Levels on Water Absorption and Steepwater Characteristics. Cereal Chemistry 2000, 77, 525–528.
- Becraft, P.W. Cell Fate Specification in the Cereal Endosperm. Seminars in Cell & Developmental Biology 2001, 12, 387–394.
- Shrestha, A.K.; Ng, C.S.; Lopez-Rubio, A.; Blazek, J.; Gilbert, E.P.; Gidley, M.J. Enzyme Resistance and Structural Organization in Extruded High Amylose Maize Starch. Carbohydrate Polymers 2010, 80, 699–710.
- Baird, R.E.; Huber, D.M.; Mullinix, B.G. The Mycobiota from Seeds of Shrunken-2 (sh2) Sweet Corn. Mycopathologia 1996, 132, 147−154.
- Morrison, W.R.; Law, R.V.; Snape, C.E. Evidence for Inclusion Complexes of Lipids with V-Amylose in Maize, Rice, and Oat Starches. Journal of Cereal Science 1993, 18, 107–109.
- Cheetham, N.W.H.; Tao, L. Variation in Crystalline Type with Amylase Content in Maize Starch Granules: An X-Ray Powder Diffraction Study. Carbohydrate Polymers 1998, 36, 277–284.
- Zhang, B.; Huang, Q.; Luo, F.X.; Fu, X. Structural Characterizations and Digestibility of Debranched High-Amylose Maize Starch Complexed with Lauric Acid. Food Hydrocolloids 2012, 28, 174–181.
- Chang, F.D.; He, X.W.; Huang, Q. The Physicochemical Properties of Swelled Maize Starch Granules Complexed with Lauric Acid. Food Hydrocolloids 2013, 32, 365–372.
- Singh, N.; Inouchi, N.; Nishinari, K. Structural, Thermal, and Viscoelastic Characteristics of Starches Separated from Normal, Sugary, and Waxy Maize. Food Hydrocolloids 2006, 20, 923–935.
- Gao, H.M.; Cai, J.W.; Han, W.L.; Huai, H.Y.; Chen, Y.F.; Wei, C.X. Comparison of Starches Isolated from Three Different Trapa Species. Food Hydrocolloids 2014, 37, 174–181.
- Van Soest, J.J.G.; Tournoisa, H.; Wit, D.; Vliegenthart, J.F.G. Short-Range Structure in (Partially) Crystalline Potato Starch Determined with Attenuated Total Reflectance Fourier-Transform IR Spectroscopy. Carbohydrate Research 1995, 279, 201–214.
- Shingel, K.I. Determination of Structural Peculiarities of Dexran, Pullulan, and Gamma-Irradiated Pullulan by Fourier-Transform IR Spectroscopy. Carbohydrate Research 2002, 337, 1445–1451.
- Fan, D.; Ma, W.R.; Wang, L.Y.; Huang, J.L.; Zhang, F.M.; Zhao, J.X.; Zhang, H.; Chen, W. Determining the effects of microwave heating on the ordered structures of rice starch by NMR. Carbohydrate Polymers 2013, 92, 1395–1401.
- Snape, C.E.; Morrison, W.R.; Maroto-Valer, M.M.; Karkalas, J.; Pethrick, R.A. Solid State 13C NMR Investigation of Lipid Ligands in V-Amylose Inclusion Complexes. Carbohydrate Polymers 1998, 36, 225–237.
- Morell, M.K.; Kosar-Hashemi, B.; Cmiel M.; Samuel, M.S.; Chandler, P.; Rahman, S.; Buleon, A.; Batey, I.L.; Li, Z. Barley sex 6 Mutants Lack Starch Synthase lla Activity and Contain a Starch with Novel Properties. Plant Journal 2003, 34, 172–184.
- Blazek, J.; Copeland, L. Pasting and Swelling Properties of Wheat flour and Starch in Relation to Amylose Content. Carbohydrate Polymers 2008, 71, 380–387.
- Singh, N.; Kaur, L.; Ezekiel, R.; Guraya, H.S. Microstructural, Cooking, and Textural Characteristics of Potato (Solanum tuberosum L.) Tubers in Relation to Physicochemical and Functional Properties of Their flours. Journal of the Science of Food and Agriculture 2005, 85, 1275–1284.
- Ali, T.M.; Hasnain, A. Morphological, Physicochemical, and Pasting Properties of Modified White Sorghum (Sorghum bicolor) Starch. International Journal of Food Properties 2014, 17, 523−535.
- Lindeboom, N.; Chang, P.R.; Tyler, R.T. Analytical, Biochemical, and Physicochemical Aspects of Starch Granule Size, with Emphasis on Small Granule Starches: A Review. Starch 2004, 56, 89–99.
- Wongsagonsup, R.; Varavinit, S.; BeMiller, J.N. Increasing Slowly Digestible Starch Content of Normal and Waxy Maize Starches and Properties of Starch Products. Cereal Chemistry 2008, 85, 738–745.
- Gujral, H.S.; Sharma, P.; Kaur H.; Singh, J. Physiochemical, Pasting, and Thermal Properties of Starch Isolated from Different Barley Cultivars. International Journal of Food Properties 2013, 16, 1494−1506.
- Singh, S.; Singh, N.; Isono, N.; Noda, T. Relationship of Granule Size Distribution and Amylopectin Structure with Pasting, Thermal, and Retrogradation Properties in Wheat Starch. Journal of Agricultural and Food Chemistry 2010, 58, 1180–1188.
- Matveev, Y.I.; van Soest, J.J.G.; Nieman, C.; Wasserman, L.A.; Protserov, V.A.; Ezernitskaja, M.; Yuryev, V.P. The Relationship Between Thermodynamic and Structural Properties of Low and High Amylose Maize Starches. Carbohydrate Polymers 2001, 44, 151–160.